Couplants in Acoustic Biosensing Systems
Abstract
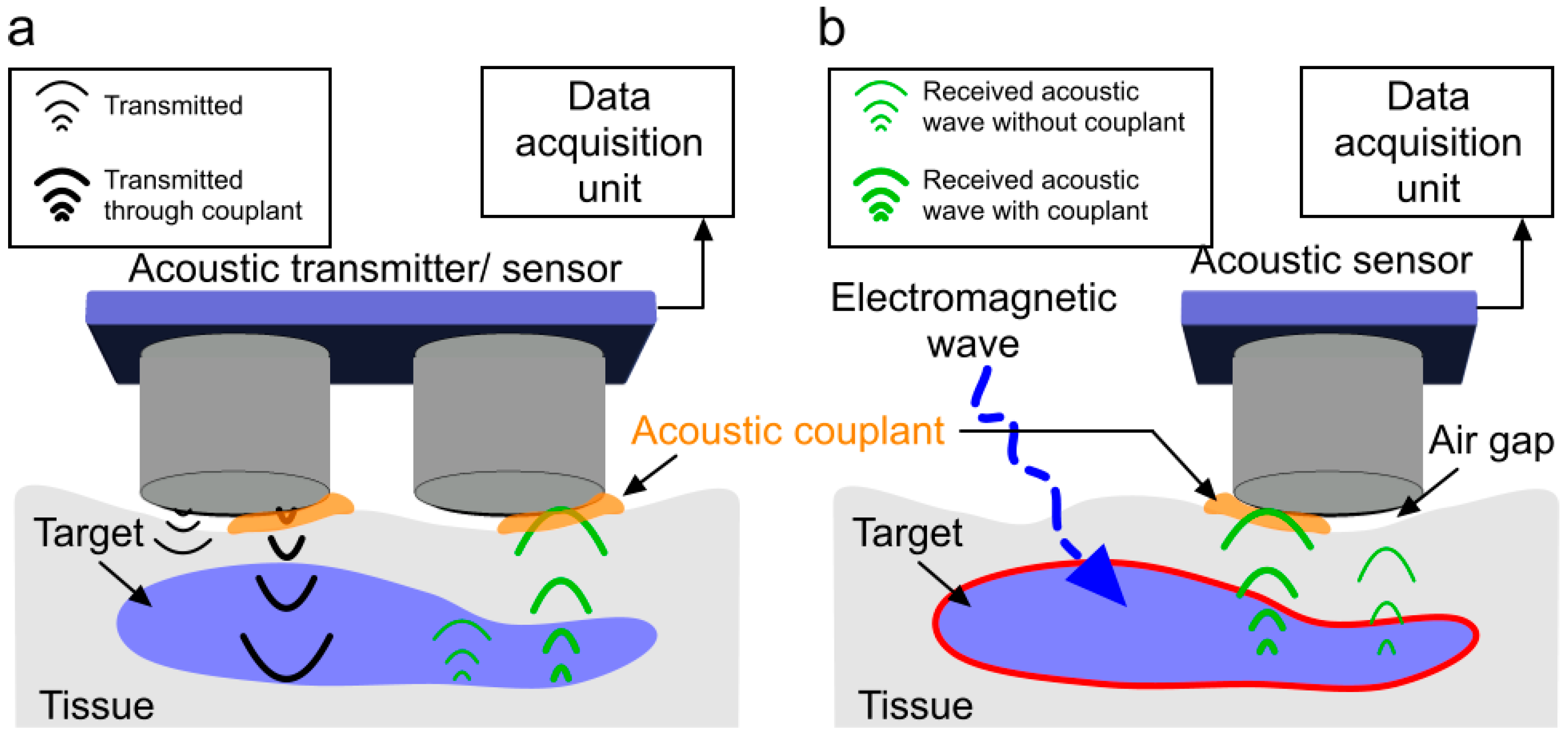
1. Introduction
2. Material Collection Method
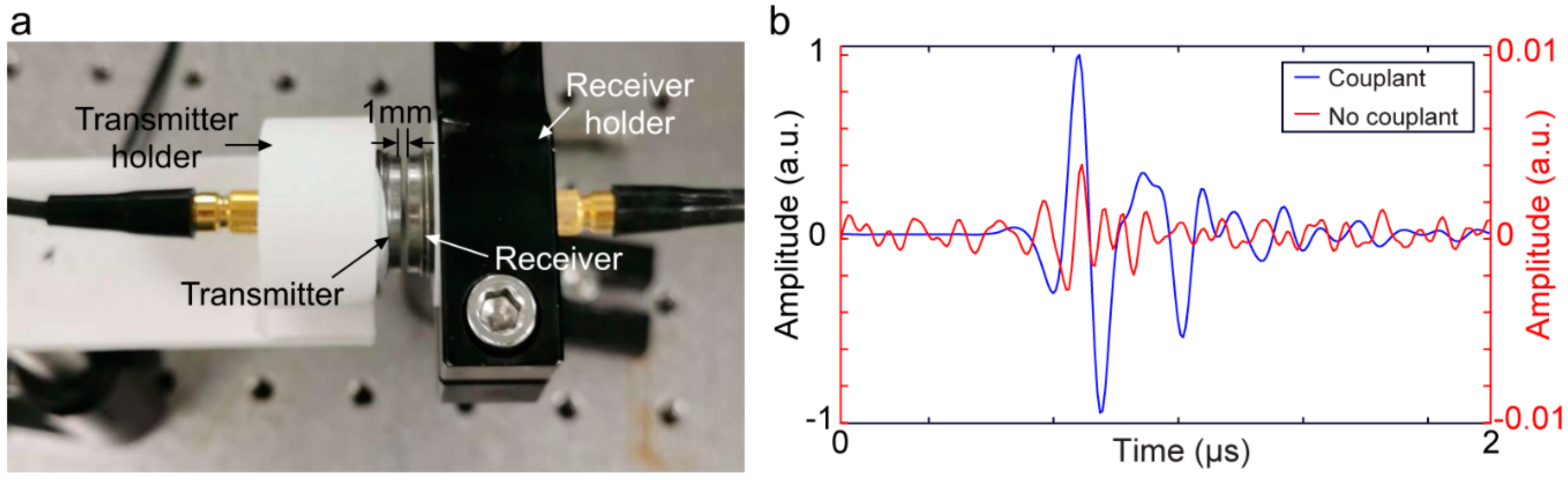
3. Acoustic Coupling Agents
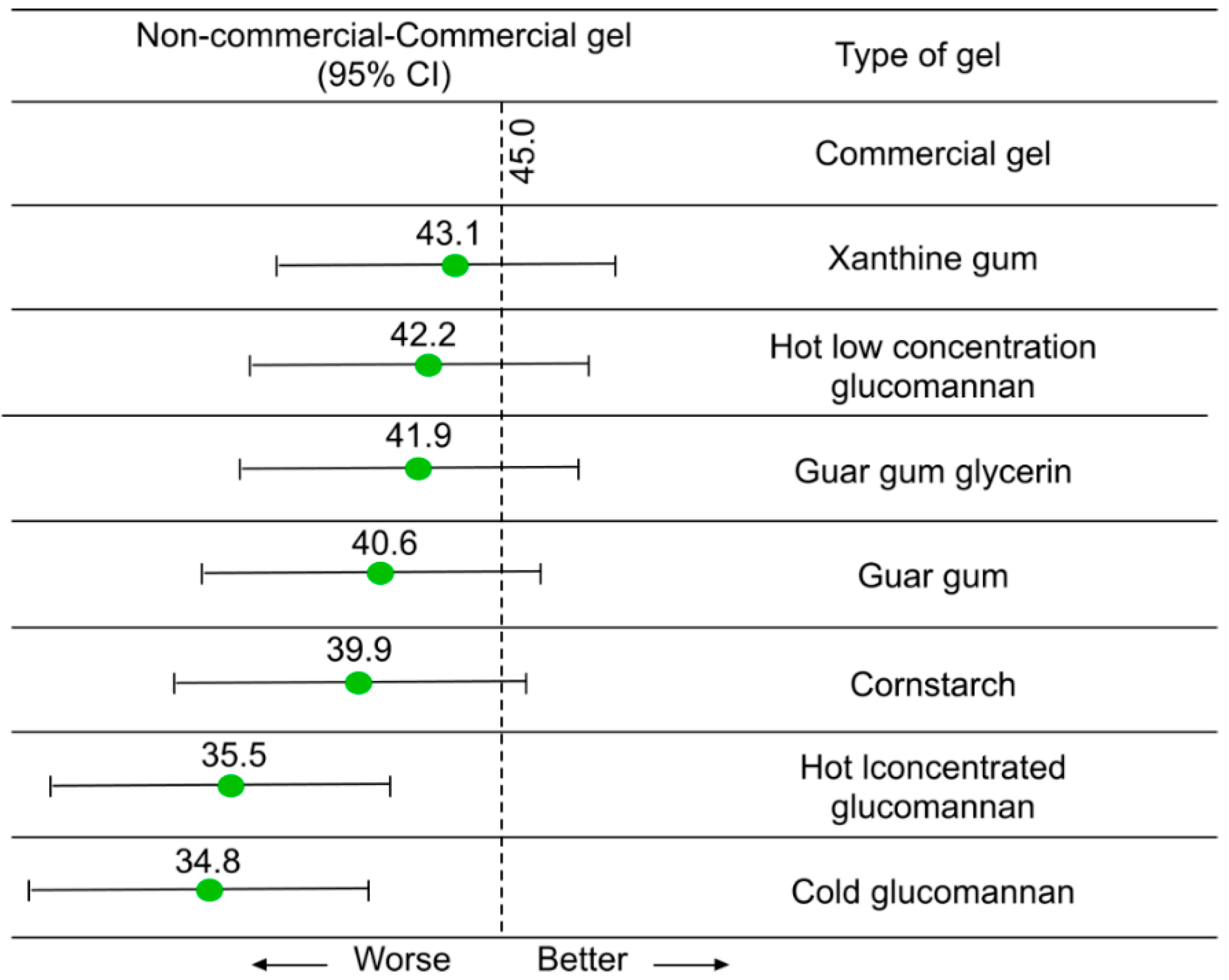
3.1. Liquid/Gel-Based Couplants
3.2. Dry Couplants
3.3. Semi-Dry Acoustic Couplants
| Housing Material | Cross-Linking Material | Actual Couplant | Form Factor | Ref |
|---|---|---|---|---|
| Hydrophilic block copolymer | Biocompatible liquid | Gel | Sheath type membrane | [97] |
| Polyalkylene glycol plasticizer, water, ammonium acetate, magnesium acetate | Gel | Adhesive patch | [98] | |
| Polypropylene | - | polyurethane gel | Flexible plate | [101] |
| Mylar | - | Water/gel | Flexible membrane | [103] |
| Silicone | polyurethane, latex, and rubber | Degassed water | Rigid container | [104] |
| Cellulose | glycerin and water/oil | Gel | Gel pad | [105] |
| Sylgard 184 | zinc oxide | Gel | Membrane | [106] |
4. Coupling Mechanism within Ultrasound Transducer
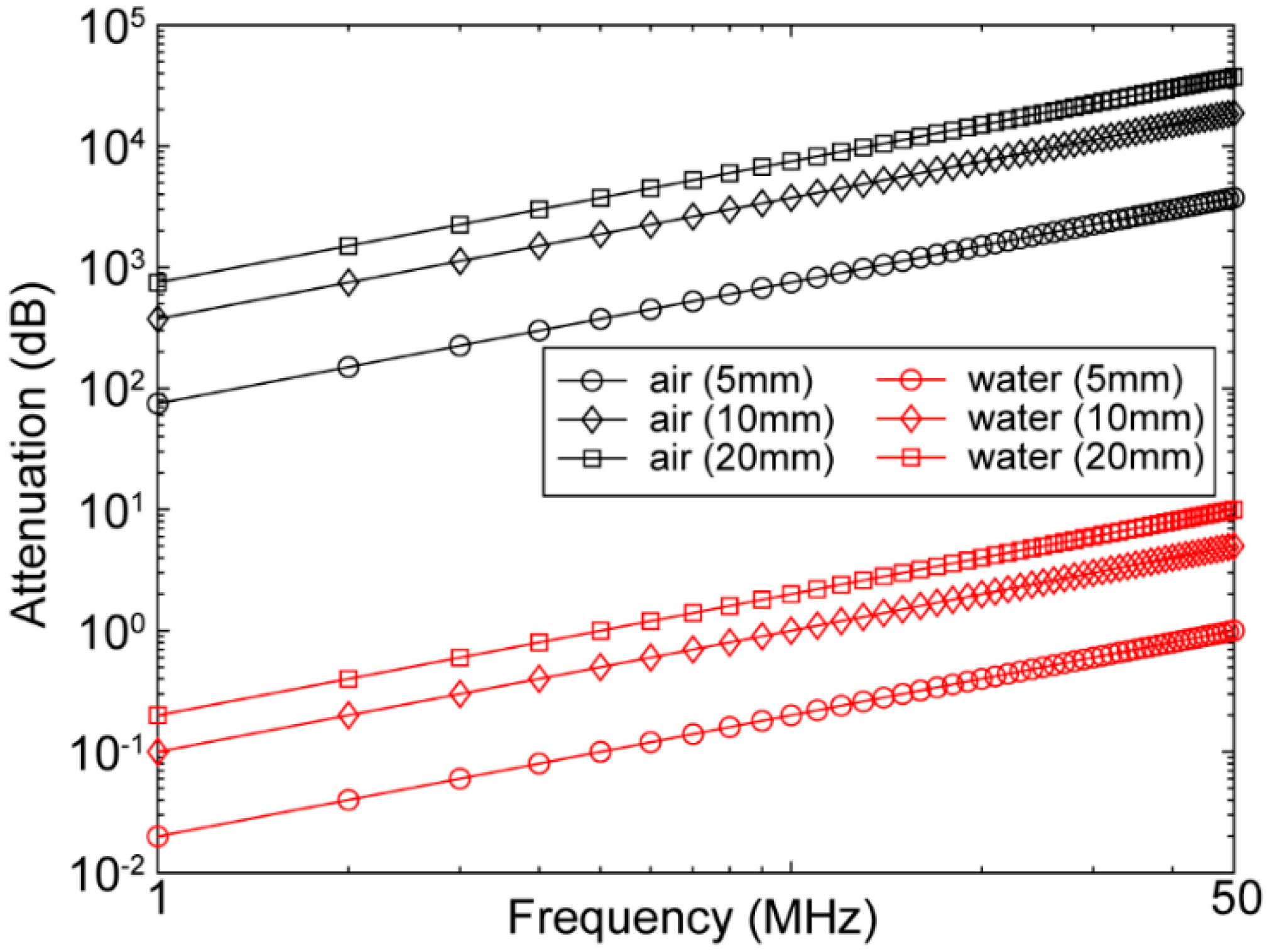
5. Air Couplant (for Air-Coupled Transducers)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vigneshvar, S.; Sudhakumari, C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications—An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J. Biosensor technology for detecting biological warfare agents: Recent progress and future trends. Anal. Chim. Acta 2006, 559, 137–151. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Bhardwaj, T. Review on biosensor technologies. Int. J. Adv. Res. Eng. Technol. 2015, 6, 36–62. [Google Scholar]
- Pohanka, M.; Skládal, P. Electrochemical biosensors—Principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar] [CrossRef]
- Ünlü, M.S.; Chiari, M.; Özcan, A. Introduction to the special issue of optical biosensors. Nanophotonics 2017, 6, 623–625. [Google Scholar] [CrossRef][Green Version]
- Manwar, R.; Kratkiewicz, K.; Avanaki, K. Overview of ultrasound detection technologies for photoacoustic imaging. Micromachines 2020, 11, 692. [Google Scholar] [CrossRef]
- Wang, M.; Zarafshani, A.; Samant, P.; Merrill, J.; Li, D.; Xiang, L. Feasibility of Electroacoustic Tomography: A Simulation Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 889–897. [Google Scholar] [CrossRef]
- Zarafshani, A.; Zheng, B.; Xiang, L. Electroacoustic tomography system using ultra-short electric filed excitation source induced acoustic signals. J. Acoust. Soc. Am. 2019, 145, 1920–1921. [Google Scholar] [CrossRef]
- Kellnberger, S.; Rosenthal, A.; Myklatun, A.; Westmeyer, G.G.; Sergiadis, G.; Ntziachristos, V. Magnetoacoustic sensing of magnetic nanoparticles. Phys. Rev. Lett. 2016, 116, 108103. [Google Scholar] [CrossRef]
- Xu, Y.; He, B. Magnetoacoustic tomography with magnetic induction (MAT-MI). Phys. Med. Biol. 2005, 50, 5175. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, C.; Wang, L.V. Effects of acoustic heterogeneities on transcranial brain imaging with microwave-induced thermoacoustic tomography. Med. Phys. 2008, 35, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Time-domain reconstruction for thermoacoustic tomography in a spherical geometry. IEEE Trans. Med. Imaging 2002, 21, 814–822. [Google Scholar] [PubMed]
- Wang, L.V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef]
- Wang, L.V.; Gao, L. Photoacoustic microscopy and computed tomography: From bench to bedside. Annu. Rev. Biomed. Eng. 2014, 16, 155–185. [Google Scholar] [CrossRef]
- Manwar, R.; Li, X.; Mahmoodkalayeh, S.; Asano, E.; Zhu, D.; Avanaki, K. Deep learning protocol for improved photoacoustic brain imaging. J. Biophotonics 2020, 13, e202000212. [Google Scholar] [CrossRef]
- Kratkiewicz, K.; Manwar, R.; Zafar, M.; Mohsen Ranjbaran, S.; Mozaffarzadeh, M.; de Jong, N.; Ji, K.; Avanaki, K. Development of a stationary 3D photoacoustic imaging system using sparse single-element transducers: Phantom study. Appl. Sci. 2019, 9, 4505. [Google Scholar] [CrossRef]
- Matchynski, J.I.; Manwar, R.; Kratkiewicz, K.J.; Madangopal, R.; Lennon, V.A.; Makki, K.M.; Reppen, A.L.; Woznicki, A.R.; Hope, B.T.; Perrine, S.A. Direct measurement of neuronal ensemble activity using photoacoustic imaging in the stimulated Fos-LacZ transgenic rat brain: A proof-of-principle study. Photoacoustics 2021, 24, 100297. [Google Scholar] [CrossRef]
- Mozaffarzadeh, M.; Mahloojifar, A.; Nasiriavanaki, M.; Orooji, M. Eigenspace-based minimum variance adaptive beamformer combined with delay multiply and sum: Experimental study. In Proceedings of the Photonics in Dermatology and Plastic Surgery 2018, San Francisco, CA, USA, 27 January–1 February 2018; p. 1046717. [Google Scholar]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-resolution photoacoustic tomography of resting-state functional connectivity in the mouse brain. Proc. Natl. Acad. Sci. USA 2014, 111, 21–26. [Google Scholar] [CrossRef]
- Zafar, M.; Manwar, R.; Avanaki, K. High-fidelity compression for high-throughput photoacoustic microscopy systems. J. Biophotonics 2022, 15, e202100350. [Google Scholar] [CrossRef]
- Mahmoodkalayeh, S.; Zarei, M.; Ansari, M.A.; Kratkiewicz, K.; Ranjbaran, M.; Manwar, R.; Avanaki, K. Improving vascular imaging with co-planar mutually guided photoacoustic and diffuse optical tomography: A simulation study. Biomed. Opt. Express 2020, 11, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Manwar, R.; Kratkiewicz, K.; Avanaki, K. Investigation of the effect of the skull in transcranial photoacoustic imaging: A preliminary ex vivo study. Sensors 2020, 20, 4189. [Google Scholar] [CrossRef] [PubMed]
- Manwar, R.; Benavides Lara, J.; Prakash, R.; Ranjbaran, S.M.; Avanaki, K. Randomized Multi-Angle Illumination for Improved Linear Array Photoacoustic Computed Tomography in Brain. J. Biophotonics 2022, e202200016. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Omidi, P.; Nasiriavanaki, M. Resting-state functional connectivity measurement in the mouse brain using a low cost photoacoustic computed tomography. In Proceedings of the Laser Science, Rochester, NY, USA, 16–20 October 2016; p. JW4A.62. [Google Scholar]
- Mohammadi, L.; Behnam, H.; Tavakkoli, J.; Avanaki, K. Skull acoustic aberration correction in photoacoustic microscopy using a vector space similarity model: A proof-of-concept simulation study. Biomed. Opt. Express 2020, 11, 5542–5556. [Google Scholar] [CrossRef]
- Mohammadi, L.; Behnam, H.; Tavakkoli, J.; Avanaki, M. Skull’s photoacoustic attenuation and dispersion modeling with deterministic ray-tracing: Towards real-time aberration correction. Sensors 2019, 19, 345. [Google Scholar] [CrossRef]
- Kratkiewicz, K.; Manwar, R.; Zhou, Y.; Mozaffarzadeh, M.; Avanaki, K. Technical considerations in the Verasonics research ultrasound platform for developing a photoacoustic imaging system. Biomed. Opt. Express 2021, 12, 1050–1084. [Google Scholar] [CrossRef]
- Hariri, A.; Bely, N.; Chen, C.; Nasiriavanaki, M. Towards ultrahigh resting-state functional connectivity in the mouse brain using photoacoustic microscopy. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2016, San Francisco, CA, USA, 14–17 February 2016; p. 97085A. [Google Scholar]
- Manwar, R.; Islam, M.T.; Ranjbaran, S.M.; Avanaki, K. Transfontanelle photoacoustic imaging: Ultrasound transducer selection analysis. Biomed. Opt. Express 2022, 13, 676–693. [Google Scholar] [CrossRef]
- Avanaki, K.; Gelovani, J.G. Ultrasound and Multispectral Photoacoustic Systems and Methods for Brain and Spinal Cord Imaging through Acoustic Windows. U.S. Patent 16/566,212, 12 March 2020. [Google Scholar]
- Panchal, R.; Horton, L.; Poozesh, P.; Baqersad, J.; Nasiriavanaki, M. Vibration analysis of healthy skin: Toward a noninvasive skin diagnosis methodology. J. Biomed. Opt. 2019, 24, 015001. [Google Scholar] [CrossRef]
- Mahmoodkalayeh, S.; Kratkiewicz, K.; Manwar, R.; Shahbazi, M.; Ansari, M.A.; Natarajan, G.; Asano, E.; Avanaki, K. Wavelength and pulse energy optimization for detecting hypoxia in photoacoustic imaging of the neonatal brain: A simulation study. Biomed. Opt. Express 2021, 12, 7458–7477. [Google Scholar] [CrossRef]
- Tang, S.; Nguyen, D.; Zarafshani, A.; Ramseyer, C.; Zheng, B.; Liu, H.; Xiang, L. X-ray-induced acoustic computed tomography with an ultrasound transducer ring-array. Appl. Phys. Lett. 2017, 110, 103504. [Google Scholar] [CrossRef]
- Xiang, L.; Tang, S.; Ahmad, M.; Xing, L. High resolution X-ray-induced acoustic tomography. Sci. Rep. 2016, 6, 26118. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Xiang, L.; Yousefi, S.; Xing, L. Theoretical detection threshold of the proton-acoustic range verification technique. Med. Phys. 2015, 42, 5735–5744. [Google Scholar] [CrossRef] [PubMed]
- Kellnberger, S.; Assmann, W.; Lehrack, S.; Reinhardt, S.; Thirolf, P.; Queiros, D.; Sergiadis, G.; Dollinger, G.; Parodi, K.; Ntziachristos, V. Ionoacoustic tomography of the proton Bragg peak in combination with ultrasound and optoacoustic imaging. Sci. Rep. 2016, 6, 29305. [Google Scholar] [CrossRef]
- Lerch, R. Finite element analysis of piezoelectric transducers. In Proceedings of the IEEE 1988 Ultrasonics Symposium Proceedings, Chicago, IL, USA, 2–5 October 1988; pp. 643–654. [Google Scholar]
- Loveday, P.W. Analysis of piezoelectric ultrasonic transducers attached to waveguides using waveguide finite elements. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Chhiba, L.; Zaher, B.; Sidqui, M.; Marzak, A. Glucose Sensing for Diabetes Monitoring: From Invasive to Wearable Device. In Proceedings of the Third International Conference on Smart City Applications, Casablanca, Morocco, 2–4 October 2019; pp. 350–364. [Google Scholar]
- Chen, L.; Neethirajan, S. A homogenous fluorescence quenching based assay for specific and sensitive detection of influenza virus A hemagglutinin antigen. Sensors 2015, 15, 8852–8865. [Google Scholar] [CrossRef]
- Murugaiyan, S.B.; Ramasamy, R.; Gopal, N.; Kuzhandaivelu, V. Biosensors in clinical chemistry: An overview. Adv. Biomed. Res. 2014, 3, 67. [Google Scholar]
- Jolly, M.R.; Prabhakar, A.; Sturzu, B.; Hollstein, K.; Singh, R.; Thomas, S.; Foote, P.; Shaw, A. Review of non-destructive testing (NDT) techniques and their applicability to thick walled composites. Procedia CIRP 2015, 38, 129–136. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of ultrasound in medicine. Acta Inform. Med. 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Webb, A.G. Introduction to Biomedical Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside out; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Adamowski, J.C.; Andrade, M.A.; Perez, N.; Buiochi, F. A large aperture ultrasonic receiver for through-transmission determination of elastic constants of composite materials. In Proceedings of 2008 IEEE Ultrasonics Symposium, Beijing, China, 2–5 November 2008; pp. 1524–1527. [Google Scholar]
- Li, M.; Hayward, G. Ultrasound nondestructive evaluation (NDE) imaging with transducer arrays and adaptive processing. Sensors 2012, 12, 42–54. [Google Scholar] [CrossRef]
- Trachenko, K.; Monserrat, B.; Pickard, C.; Brazhkin, V. Speed of sound from fundamental physical constants. Sci. Adv. 2020, 6, eabc8662. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.M.; Institute, B.M.; Foundation, N.S.; Health, B.O.R. Interaction of Ultrasound and Biological Tissues; US Bureau of Radiological Health: Des Moines, IA, USA, 1972. [Google Scholar]
- Casarotto, R.A.; Adamowski, J.C.; Fallopa, F.; Bacanelli, F. Coupling agents in therapeutic ultrasound: Acoustic and thermal behavior. Arch. Phys. Med. Rehabil. 2004, 85, 162–165. [Google Scholar] [CrossRef]
- Fahy, F. 7—Sound Absorption and Sound Absorbers. In Foundations of Engineering Acoustics; Fahy, F., Ed.; Academic Press: London, UK, 2001; pp. 140–180. [Google Scholar] [CrossRef]
- Kinsler, L.; Frey, A.; Coppens, A.; Sanders, J. The acoustic wave equation and simple solutions. In Fundamentals of Acoustics; Wiley: Hoboken, NJ, USA, 2000; pp. 113–148. [Google Scholar]
- Kozaczka, E.; Grelowska, G. Theoretical model of acoustic wave propagation in shallow water. Pol. Marit. Res. 2017, 24, 48–55. [Google Scholar] [CrossRef]
- Hanyga, A.; Seredyńska, M. Power-law attenuation in acoustic and isotropic anelastic media. Geophys. J. Int. 2003, 155, 830–838. [Google Scholar] [CrossRef]
- Azhari, H. Appendix A: Typical acoustic properties of tissues. In Basics of Biomedical Ultrasound for Engineers; Wiley-IEEE Press: Hoboken, NJ, USA, 2010. [Google Scholar]
- Aaslid, R. Cerebral autoregulation and vasomotor reactivity. In Handbook on Neurovascular Ultrasound; Karger Publishers: Basel, Switzerland, 2006; Volume 21, pp. 216–228. [Google Scholar]
- Amin, V.R. Ultrasonic Attenuation Estimation for Tissue Characterization. Master’s Thesis, Iowa State University, Ames, IA, USA, 1989. [Google Scholar]
- Bourne, S.; Newborough, M.; Highgate, D. Novel solid contact ultrasonic couplants based on hydrophilic polymers. In Proceedings of the 15th World Conference on NDT, Roma, Italy, 15–21 October 2000. [Google Scholar]
- Bindal, V. Water-based couplants for general purpose use for ultrasonic NDT applications. J. Sci. Ind. Res. 2000, 59, 935–939. [Google Scholar]
- Klucinec, B.; Scheidler, M.; Denegar, C.; Domholdt, E.; Burgess, S. Transmissivity of coupling agents used to deliver ultrasound through indirect methods. J. Orthop. Sports Phys. Ther. 2000, 30, 263–269. [Google Scholar] [CrossRef]
- Online Database: Acoustic Properties of Liquids. ONDA Corporation. Available online: http://www.ondacorp.com/images/Liquids.pdf (accessed on 1 April 2022).
- Zhang, Y. Measuring Acoustic Attenuation of Polymer Materials Using Drop Ball Test. Master’s Thesis, Embry-Riddle Aeronautical University, Daytona Beach, FL, USA, 2013. [Google Scholar]
- Desai, S.; Jones, J. Ultrashield Devices and Methods for Use in Ultrasonic Procedures. U.S. Patent 62/285,758, 9 November 2015. [Google Scholar]
- Burns, J.M. Development and Characterisation of a Fibre-Optic Acoustic Emission Sensor. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2012. [Google Scholar]
- Montecalvo, D.A.; Rolf, D. Solid Multipurpose Ultrasonic Biomedical Couplant Gel in Sheet Form and Method. U.S. Patent 5,522,878, 4 June 1996. [Google Scholar]
- Yochev, B.; Kutzarov, S.; Ganchev, D.; Staykov, K. Investigation of ultrasonic properties of hydrophilic polymers for dry-coupled inspection. In Proceedings of the European Conference on Non-Destructive Testing, Berlin, Germany, 1–7 January 2006; pp. 25–29. [Google Scholar]
- Muradali, D.; Gold, W.; Phillips, A.; Wilson, S. Can ultrasound probes and coupling gel be a source of nosocomial infection in patients undergoing sonography? An in vivo and in vitro study. Am. J. Roentgenol. 1995, 164, 1521–1524. [Google Scholar] [CrossRef]
- Nyholt, J.; Langlois, G.N. Dry-Coupled Permanently Installed Ultrasonic Sensor Linear Array. U.S. Patent 8,408,065, 2 April 2013. [Google Scholar]
- Watson, T. Ultrasound Gels and Coupling Agents. Electrotherapy on the Web. Available online: http://www.electrotherapy.org/modality/ultrasound-gels-and-coupling-agents#Ultrasound%20Transmissivity%20through%20Coupling%20Agents%20(Gels)%20in%20the%20UK (accessed on 1 April 2022).
- Poltawski, L.; Watson, T. Relative transmissivity of ultrasound coupling agents commonly used by therapists in the UK. Ultrasound Med. Biol. 2007, 33, 120–128. [Google Scholar] [CrossRef]
- Webpage: Couplant Selection Chart. Echo Ultrasonics. Available online: https://www.echoultrasonics.com/products/ (accessed on 1 April 2022).
- Online Database: Ultrasonic Testing Couplant. Magnaflux. Available online: https://www.magnaflux.com/Magnaflux/Products/Ultrasonic-Couplants.htm (accessed on 1 April 2022).
- Online Database: Ultrasonic Couplants. Oympus Corporation. Available online: https://www.olympus-ims.com/en/applications/ultrasonic-couplant/ (accessed on 1 April 2022).
- Dickson, J. Dry coupling ultrasonic method of inspection on composite and metallic honeycomb panels on aircraft structure. In Proceedings of the Non-Destructive Testing. Tenth World Conference, Moscow, Russia, 22–28 August 1982; pp. 216–221. [Google Scholar]
- Çetin, M.İ. Effect of Solid Couplants Made of Hydrophilic Polymers in Ultrasonic Testing. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2003. [Google Scholar]
- Mitchell, P. Tool and Manufacturing Engineers Handbook: Plastic Part Manufacturing; Society of Manufacturing Engineers: Southfield, MI, USA, 1996; Volume 8. [Google Scholar]
- Mitchell, B.S. An Introduction to Materials Engineering and Science for Chemical and Materials Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Vondráček, P.; Doležel, B. Biostability of medical elastomers: A review. Biomaterials 1984, 5, 209–214. [Google Scholar] [CrossRef]
- Ali, M.R.; Salit, M.S.; Jawaid, M.; Mansur, M.R.; Manap, M.F.A. Polyurethane-Based Biocomposites. In Polyurethane Polymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 525–546. [Google Scholar]
- Sanabria, S.J.; Mueller, C.; Neuenschwander, J.; Niemz, P.; Sennhauser, U. Air-coupled ultrasound as an accurate and reproducible method for bonding assessment of glued timber. Wood Sci. Technol. 2011, 45, 645–659. [Google Scholar] [CrossRef]
- Norman, J. Ultrasonic dry coupling through tissue. Can. Acoust. 2015, 43. [Google Scholar]
- Mojabi, P. Ultrasound Tomography: An Inverse Scattering Approach. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2014. [Google Scholar]
- Ginzel, E.; Ginzel, R.; Brothers, G. Ultrasonic Properties of a New Low Attenuation Dry Couplant Elastomer. Available online: https://www.ndt.net/article/ginzel/ginzel.htm (accessed on 1 April 2022).
- Burke, M.; Smith, J.; Carroll, N.; Townend, D.; Porter, D.; Hoskins, P. Acoustic properties of butadiene and silicone elastomers at megahertz frequencies. Plast. Rubber Compos. 2009, 38, 343–348. [Google Scholar] [CrossRef]
- Bhadwal, N.; Torabi Milani, M.; Coyle, T.; Sinclair, A. Dry Coupling of Ultrasonic Transducer Components for High Temperature Applications. Sensors 2019, 19, 5383. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, R. Sound Transfer—Coupling Media Innovation Polymers. Available online: http://www.innovationpolymers.ca/assets/pdfs/material_property_overview.pdf (accessed on 1 April 2022).
- Ginzel, E.; Macneil, R.; Ginzel, R.; Zuber, M.; Sinclar, A. Acoustic Properties of the Elastomeric Materials Aqualene™ and ACE™. e-J. Nondestruct. Test. (NDT) 2015, 20, 1–2. [Google Scholar]
- Andrėkutė, K. Development of the Stable Ultrasound Phantoms for Superficial Human Tissue Investigation. Available online: https://nam04.safelinks.protection.outlook.com/?url=http%3A%2F%2Fbiomed.ktu.lt%2Findex.php%2FBME%2Farticle%2Fview%2F428&data=05%7C01%7Crmanwar%40uic.edu%7Cbe9f5c0f87ef4370fcc908da3009c1a5%7Ce202cd477a564baa99e3e3b71a7c77dd%7C0%7C0%7C637875116147137752%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=a64EBhQH1zyneUgzuMhvxcUyFhEMDQ9qSRN7zZ68HRA%3D&reserved=0 (accessed on 1 April 2022).
- Meimani, N.; Abani, N.; Gelovani, J.; Avanaki, M.R. A numerical analysis of a semi-dry coupling configuration in photoacoustic computed tomography for infant brain imaging. Photoacoustics 2017, 7, 27–35. [Google Scholar] [CrossRef]
- Mathur, A.M.; Moorjani, S.K.; Scranton, A.B. Methods for synthesis of hydrogel networks: A review. J. Macromol. Sci. Part C Polym. Rev. 1996, 36, 405–430. [Google Scholar] [CrossRef]
- Kálal, J. Water sensitive chemically cross-linked gels. In Chemistry and Technology of Water-Soluble Polymers; Springer: Berlin/Heidelberg, Germany, 1983; pp. 71–80. [Google Scholar]
- Luprano, V.; Montagna, G.; Maffezzoli, A. A study of the water sorption kinetic of Poly (HEMA) hydrogels by SLAM and FT-IR measurements. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1996, 43, 948–955. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Luprano, A.; Montagna, G.; Nicolais, L. Ultrasonic characterization of water sorption in poly (2-hydroxyethyl methacrylate) hydrogels. J. Appl. Polym. Sci. 1998, 67, 823–831. [Google Scholar] [CrossRef]
- Smith, L.L. Adhesive Hydrophilic Membranes as Couplants in Ultrasound Imaging Applications. U.S. Patent 6,719,699, 13 April 2004. [Google Scholar]
- Larson, M.J.; Rutter, J.W.; Smith, L.L. Coupling Sheath for Ultrasound Transducers. U.S. Patent 6,039,694, 21 March 2000. [Google Scholar]
- Sieverding, D.L. Hydrophilic, Elastomeric, Pressure-Sensitive Adhesive. U.S. Patent 4,699,146, 13 October 1987. [Google Scholar]
- Richardson, P.C.; Stevens, A.L. Ultrasonic Transducer Coupling Member. U.S. Patent 4,459,854, 17 July 1984. [Google Scholar]
- Bourne, S.J. Novel Hydrophilic Polymer Couplant for Application in Ultrasonic Non Destructive Testing. Ph.D. Thesis, Cranfield University, Bedford, UK, 2001. [Google Scholar]
- Shikinami, Y.; Tsuta, K.; Taniguchi, M.; Boutani, H. Liquid Segment Polyurethane Gel and Couplers for Ultrasonic Diagnostic Probe Comprising the Same. U.S. Patent 5,039,774, 13 August 1991. [Google Scholar]
- Vinograd, A.M.; Fasina, A.; Dean, A.J.; Shofer, F.; Panebianco, N.L.; Lewiss, R.E.; Gupta, S.; Rao, A.R.; Henwood, P.C. Evaluation of Noncommercial Ultrasound Gels for Use in Resource-Limited Settings. J. Ultrasound Med. 2019, 38, 371–377. [Google Scholar] [CrossRef]
- Murdock, D.M. Flexible Ultrasound Coupling System. U.S. Patent 4,059,098, 22 November 1977. [Google Scholar]
- Jahnke, R.C.; Bertolina, J.A.; Roberts, W.W.; Cain, C.A.; Teofilovic, D.; Davison, T.W. Disposable Acoustic Coupling Medium Container. U.S. Patent 9,061,131, 23 June 2015. [Google Scholar]
- Pretlow III, R.A. Coupling Pad for Use with Medical Ultrasound Devices. U.S. Patent 5,782,767, 21 July 1998. [Google Scholar]
- Norman, J. Development of a Dry Coupling Material for Ultrasonic Transcutaneous Energy Transfer. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2017. [Google Scholar]
- Rathod, V.T. A review of acoustic impedance matching techniques for piezoelectric sensors and transducers. Sensors 2020, 20, 4051. [Google Scholar] [CrossRef]
- Chapelon, J.-Y.; Cathignol, D.; Cain, C.; Ebbini, E.; Kluiwstra, J.-U.; Sapozhnikov, O.A.; Fleury, G.; Berriet, R.; Chupin, L.; Guey, J.-L. New piezoelectric transducers for therapeutic ultrasound. Ultrasound Med. Biol. 2000, 26, 153–159. [Google Scholar] [CrossRef]
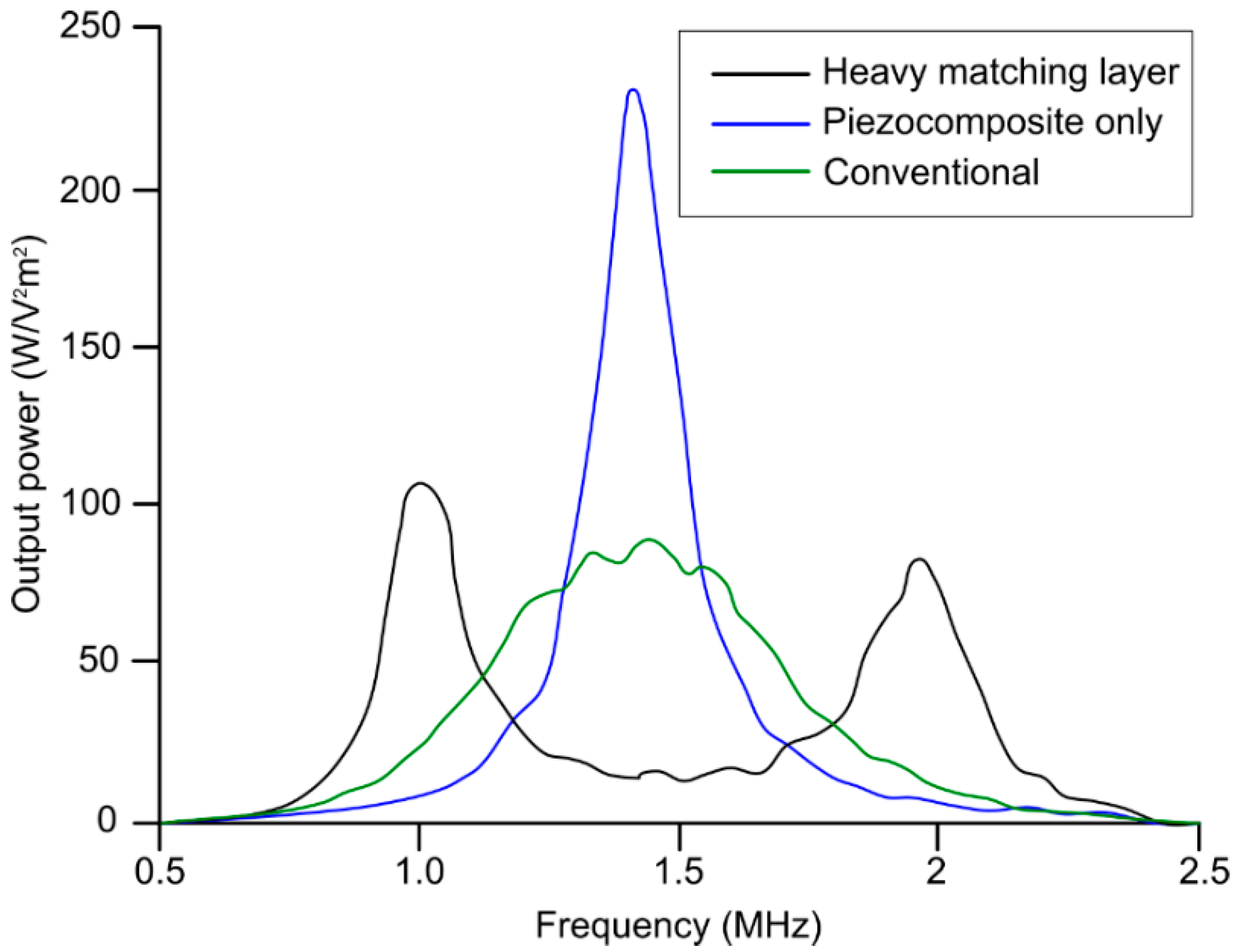
- Zaini, Z.; Osuga, M.; Jimbo, H.; Yasuda, J.; Takagi, R.; Yoshizawa, S.; Umemura, S.-I. Study on heavy matching layer transducer towards producing second harmonics. Jpn. J. Appl. Phys. 2016, 55, 07KF15. [Google Scholar] [CrossRef]
- Bovtun, V.; Döring, J.; Bartusch, J.; Beck, U.; Erhard, A.; Yakymenko, Y. Ferroelectret non-contact ultrasonic transducers. Appl. Phys. A 2007, 88, 737–743. [Google Scholar] [CrossRef]
- Rupitsch, S.J.; Lerch, R.; Strobel, J.; Streicher, A. Ultrasound transducers based on ferroelectret materials. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 69–80. [Google Scholar] [CrossRef]
- Billson, D.; Hutchins, D. Development of novel piezoelectric ultrasonic transducers for couplant-free non-destructive testing. Br. J. Non-Destr. Test. 1993, 35, 705–709. [Google Scholar]
- Drinkwater, B.; Cawley, P. An ultrasonic wheel probe alternative to liquid coupling. Insight 1994, 36, 430–433. [Google Scholar]
- Drinkwater, B.; Cawley, P. The practical application of solid coupled ultrasonic transducers. Mater. Eval. 1996, 55, 2041–2044. [Google Scholar]
- Silk, M.G. Ultrasonic Transducers for Nondestructive Testing; U.S. Department of Energy: Washington, DC, USA, 1984. [Google Scholar]
- Shenk, T. Ultrasonic Transducers. In Airducer Catalog; Corporation, A.T., Ed.; Airmar Technlogy Corporation: Milford, NH, USA, 2018; Available online: www.airmar.com (accessed on 1 April 2022).
| Biosensing Technique | Advantage | Disadvantage | Sensitivity | Selectivity |
|---|---|---|---|---|
| Electrochemical | - easy to integrate - label free - low cost | - mostly in-vitro - poor stability - time consuming | Low | High |
| Electromagnetic | - high resolution - can be non-invasive - real-time detection | - limited penetration - complex instrumentation - affected by environmental factors | High | Average |
| Acoustic | - label free - non-invasive - high dynamic range - deeper penetration | - limited resolution - bulky | High | Low |
| Type | Liquid/Gel-Based | Dry | Semi-Dry |
|---|---|---|---|
| Form factor |  |  |  |
| Material | Longitudinal Speed (m/s) | Density (g/cm3) | Acoustic Impedance (MRayl) | Attenuation (dB/cm·MHz) |
|---|---|---|---|---|
| Water | 1480 | 1 | 1.5 | 0.0022 |
| Air | 344 | 0.00125 | 0.00001 | 7.5 |
| Blood | 1570 | 1.26 | 1.61 | 0.087 |
| Brain | 1550 | 1.087 | 1.58 | 0.87 |
| Fat | 1450 | 0.870 | 1.38 | 0.61 |
| Liver | 1590 | 1.06 | 1.69 | 0.9 |
| Kidney | 1570 | 1.05 | 1.65 | 1 |
| Heart | 1570 | 1.045 | 1.64 | 2 |
| Eye lens | 1525 | 1.04 | 1.72 | 2 |
| Muscle | 1580 | 1.065 | 1.58 | 0.7~1.4 |
| Bone | 3500 | 1.9 | 7.80 | 8.7 |
| Material | Longitudinal Speed (m/s) | Density (g/cm3) | Acoustic Impedance (MRayl) | Attenuation (dB/cm·MHz) |
|---|---|---|---|---|
| Glycerin | 1930 | 1.26 | 2.42 | 0.25 |
| Ethylene glycol | 1626 | 1.087 | 1.8 | 0.34 |
| Oil | 1753 | 0.870 | 1.51 | 0.15~0.5 |
| Gel | 1390–1620 | 0.98–1.03 | 1.45–1.60 | <0.05 |
| Water at 20 °C | 1473 | 1 | 1.48 | 0.002 |
| Type | Material | Longitudinal Speed (m/s) | Density (g/cm3) | Acoustic Impedance (MRayl) | Attenuation @ 5MHz (dB/cm) |
|---|---|---|---|---|---|
| Thermoplastic | PVC (soft) | 2270 | 1.36 | 3.27 | 11.2 |
| PTFE | 1390 | 2.17 | 3 | 3.9 | |
| UHMWP | 2364 | 0.91 | 2.33 | 8 | |
| Polypropylene | 2740 | 0.92 | 2.4 | 5.1 | |
| Polycarbonate | 2300 | 1.22 | 2.75 | 23.2 | |
| PMMA (clear) | 2750 | 1.20 | 2.32 | 11.3 | |
| Nylon 6-6 | 2600 | 1.314 | 2.9 | 12.9 | |
| Thermoset | Polyester, | 2290 | 1.21 | 2.86 | 10–20 |
| Epoxy | 2360 | 1.15 | 2.86 | 15–20 | |
| Elastomer | Polyurethane | 2090 | 0.941 | 2.36 | 27.6–100 |
| Polystyrene | 2400 | 1.21 | 2.52 | 1.8 | |
| Butadiene | 1567 | 0.95 | 1.49 | 1 | |
| Silicone | 1041 | 0.99 | 1.04 | 0.71 |
| Material | Type | Acoustic Speed (m/s) | Attenuation (dB/mm @5MHz) | Hardness (Shore A) | Feature |
|---|---|---|---|---|---|
| Aqualene 200 | Thermoset | 1589 | −0.22 | 40 | Soft, flexible |
| ACE 400 | Thermoplastic | 1541 | −0.99 | 40 | Low temperature |
| Aqualink | Thermoplastic | 1489 | 0.44 | 5 | Conforming, Clear, Supersoft |
| Aquasilox | Silicone based | 1001 | −0.80 | 23 | High temperature |
| AquaCyan | Urethane | 1589 | −3.33 | 90 | High abrasion, tough |
| Material | Longitudinal Speed (m/s) | Density (g/cm3) | Acoustic Impedance (MRayl) |
|---|---|---|---|
| Parylene | 1100 | 2.35 | 2.58 |
| Gold | 19,700 | 3.24 | 63.8 |
| Aluminum | 6320 | 2.70 | 17 |
| Glass | 5900 | 7.70 | 45 |
| Perspex | 5000 | 3.00 | 15 |
| Anodic aluminum oxide epoxy | 2350 | 1.06 | 2.5 |
| High density polyehylene | 3460 | 2.75 | 9.5 |
| Syntactic foam | 2339 | 0.95 | 2.2 |
| Epotek 301 | 2486 | 0.70 | 1.75 |
| Teflon | 2800 | 2.30 | 6.4 |
| Acrylonitrile-butadiene-styrene | 2300 | 1.22 | 2.8 |
| Polysulfone | 2510 | 1.06 | 2.7 |
| Mylar | 2740 | 0.92 | 2.4 |
| Transducer Diameter (mm) | Center Frequency (kHz) | Sensing Range (m) |
|---|---|---|
| 205 | 19.5 | 0.8–40 |
| 106 | 30 | 0.8–25 |
| 77.5 | 41 | 0.35–15 |
| 77.5 | 50 | 0.30–10 |
| 76.2 | 75 | 0.25–7 |
| 25 | 125 | 0.20–3 |
| 16 | 200 | 0.12–2 |
| 13 | 228 | 0.10–1.5 |
| 12 | 300 | 0.05–0.5 |
| Parameters | Liquid/Gel | Dry | Semi-Dry | Air-Coupled |
|---|---|---|---|---|
| Impedance mismatch | + | ++++ | ++ | ++++ |
| Attenuation | + | ++++ | ++ | ++++ |
| Biodegradability | ++++ | + | ++ | + |
| Flexibility | ++++ | + | +++ | ++++ |
| Adhesion | ++ | + | +++ | - |
| Cost | ++ | + | +++ | +++ |
| Complexity (Develop) | + | +++ | ++++ | ++++ |
| Complexity (Usage) | + | ++ | +++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manwar, R.; Saint-Martin, L.; Avanaki, K. Couplants in Acoustic Biosensing Systems. Chemosensors 2022, 10, 181. https://doi.org/10.3390/chemosensors10050181
Manwar R, Saint-Martin L, Avanaki K. Couplants in Acoustic Biosensing Systems. Chemosensors. 2022; 10(5):181. https://doi.org/10.3390/chemosensors10050181
Chicago/Turabian StyleManwar, Rayyan, Loїc Saint-Martin, and Kamran Avanaki. 2022. "Couplants in Acoustic Biosensing Systems" Chemosensors 10, no. 5: 181. https://doi.org/10.3390/chemosensors10050181
APA StyleManwar, R., Saint-Martin, L., & Avanaki, K. (2022). Couplants in Acoustic Biosensing Systems. Chemosensors, 10(5), 181. https://doi.org/10.3390/chemosensors10050181





