Abstract
In order to gradually reduce automobile exhaust pollution and improve fuel quality, the NOx sensor, which can be monitored in real time in an automobile engine’s electronic control system, has become an indispensable part of the automobile lean burn system. In these types of NOx sensors, Au-doped platinum electrodes have received great attention due to their selectivity towards NO. However, the reaction process of NO gas on the Au-doped platinum electrode in the sensor and the possible regulation mechanism is still unclear. In this paper, the density functional theory (DFT) was used to analyze the effect of Au-doped Pt electrodes on the performance of nitrogen oxide sensors in automobiles. Firstly, the adsorption energies of NO molecules on pure Pt and Au/Pt surfaces were compared. The adsorption and dissociation of NO on Pt substrates doped with Au monomers, dimers, and trimers were investigated. These results showed that Au can effectively weaken the adsorption energy of NO molecules on a Pt surface. It was noted that with the increase in the number of Au atoms on the surface of Pt(111), the adsorption capacity of NO molecules on the alloy surface becomes weaker. When observing the transition state of NO decomposition on three different alloy surfaces, the study showed that the activation energy and reaction heat of NO dissociation increased. It further showed that doping with Au increased the activation energy of NO decomposition, thereby effectively inhibiting the decomposition of NO.
1. Introduction
In recent years, a rapid increase in automobile production and the demand for better consumer products has been observed, leading to a drastic impact on the environment due to exhaust emissions from automobiles and factories. NOx is among the major contributors of air pollution owing to its highly toxic nature. It is very dangerous for human health [1], as well as the environment [2], ecology [3], and social economy [4]. Therefore, governments’ emission regulations for automobile exhaust are becoming more and more stringent. To further reduce vehicle exhaust emissions and improve fuel efficiency, the sensor that can monitor the real-time NOx in automobile engines and electronic control systems has become an indispensable part of the lean burn control system. The current-mode NOx sensor is the only commercial NOx sensor that has been used in automobile selective catalytic reduction systems (SCR) [5]. Its detection principle is explained in the following text. There are two kinds of gas-sensing electrodes in the structure of the current-mode NOx sensor, as shown in Figure 1. One is the main pump electrode, and the other is the auxiliary pump electrode, which is located in the front middle of the sensor. Because the concentration of the O2 automobile exhaust is usually measured in terms of percentage of total contents, it causes great interference when measuring the ppm of NOx. Therefore, the purpose of the main pump electrode and the auxiliary pump electrode is to pump O2 out of the tail gas under the operation of external voltage, reducing its concentration to the level of 1 × 10−6 without decomposing NOx. The other is the measuring pump electrode, in which the remaining NOx in the tail gas is reduced to N2 and O2− at the measuring pump electrode under the operation of external voltage. Under the action of an electric field, O2− migrates through the solid electrolyte YSZ to the external anode to form a current. According to Faraday’s first law [6], the concentration of NOx in the gas can be obtained by measuring the current.
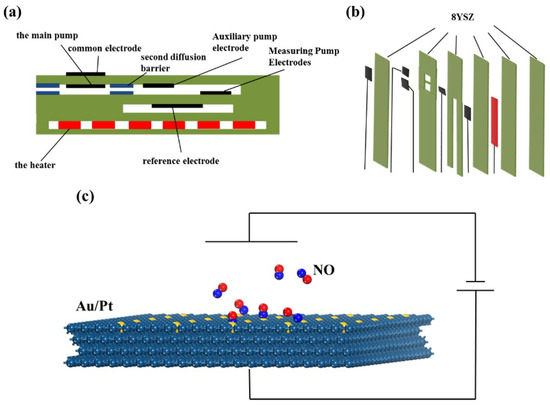
Figure 1.
(a,b) Schematic diagram of the structure and (c) the molecular simulation structure of the nitrogen oxide sensor.
Because the precious metal Pt has better stability and oxygen catalytic activity than other electrode materials [7,8,9,10,11], it is often used as the oxygen pump electrode material for the main pump electrode and auxiliary pump electrode. However, the main pump electrode and auxiliary pump electrode should not only pump oxygen, but also ensure that other gases except oxygen (especially NO) do not decompose; thus, a certain amount of Au is added to reduce the catalytic activity of Pt [12]. For years, Pt was considered a reductive catalyst to NO [13,14,15,16,17]. Therefore, the surface science of NO on Pt has always attracted much attention [18,19,20,21,22,23]. Tang et al. calculated the chemisorption of NO on the surfaces of Pt(111), Rh/Pt(111), and Pd/Pt(111) alloys using the first-principles density functional theory (DFT) [24]. Zhanpeisov et al. used the combined basis set to calculate the interaction of NO and Br with the Pt(111) surface at the B3LYP level and simulated the interaction with the bilayer Pt(111) cluster model. The interaction between Br and Pt is well explained, and the three adsorption modes of NO on the Pt(111) surface are explained [25]. Rachel B. Getman et al. calculated the energy, charge distribution, and vibrational spectra of the steady and metastable adsorption of NO, NO2, and NO3 on the Pt(111) surface, indicating the possible adsorption states of nitrogen oxides on the Pt(111) surface [26]. Huang et al. studied the formulation of non-activated electrode paste for the NOx sensor. By adjusting the content of the pore-forming agent and Au in the formula of the Pt electrode, the paste managed to meet the requirements of the main pump electrode and auxiliary pump electrode of the NOx sensor [27]. Ovesson et al. carried out a kinetic Monte Carlo simulation using the parameters of density functional theory and found that the conversion of NO to NO2 is due to a strong oxygen–platinum bond; the intrinsic NO+O→NO2 reaction on Pt(111) is inhibited (endothermic) rather than promoted. Pt can be an efficient oxidation catalyst only under sufficient oxidation chemical potential [28]. Although many studies associate the adsorption and reaction of NO on the alloy surface with the composition and structure of the alloy surface, there are few reports on the adsorption and decomposition of NO on the Pt(111) surface doped with different proportions of Au.
Therefore, the density functional theory (DFT) is proposed to analyze the surface structure of Pt-based alloy catalysts doped with different amounts of Au. The adsorption and decomposition transition state of NO on Au/Pt(111) surfaces were studied, and we analyzed the difference in the charge density of NO after the addition of Au.
2. Materials and Methods
When theoretical calculations are carried out to build surface models, the actual catalyst structure is often set up, and the construction is based on real information such as high-exposure or high-activity crystal planes in the catalyst. Pt crystals (111), (200), and (220), as well as other crystal planes, are generally exposed more [29]. Therefore, in order to find their guiding potential for practical significance, this paper mainly calculates on the basis of (111), (200), and (220) crystal planes.
The VASP software package was used for calculations. The GGA-PBE exchange correlation function and projected plane wave (PAW) methods were used to simulate the electron–ion interaction. The surface Brillouin zone used a (3 × 3 × 1) k-point mesh, and the plane wave cutoff energy of 450 eV was selected to sample the Brillouin zone and the expanded plane wave. When the force change was less than 0.02 eV·Å−1 and the energy difference was less than 1 × 10−5 eV, it was determined that the structure had reached the convergence condition. CI-NEB and dimer methods were used to search the transition state (TS) until the force difference was less than 0.05 eV·Å−1, and the transition state was determined by confirming that there was a unique imaginary frequency in the reaction coordinate state.
The structure of bulk Pt was calculated within a primitive face-centered cubic (fcc) cell. The lattice parameter was calculated to be 3.9654 Å, in excellent agreement with previous reports [30]. The calculated lattice parameter exceeded the experimental value by 0.042 Å [31], consistent with the general tendency of the GGA to overestimate internuclear distances. The pure Pt(111), (200), and (220) surfaces were first simulated by constructing a four-layer (2 × 2) supercell. Then, Au single atoms, dimers, and trimers were placed on the surface of the metal Pt crystal face, respectively, i.e., Ausingle/Pt(200), Ausingle/Pt(220), Ausingle/Pt(111), Audimer/Pt(111), and Autrimer/Pt(111), as well as Au single atoms and dimer- and trimer-embedded Pt surfaces. The vacuum was set to 1.5 nm to ensure that the plates adjacent to the z-axis did not interact with each other. The bottom two layers remained fixed, while the other two layers allowed for complete relaxation.
As shown in Figure 2, after constructing the surface model of the Au/Pt(111) alloy with different structural units, NO molecules were placed on the surface of each model, and all possible adsorption sites were considered. Then, the structure was optimized to determine the lowest energy adsorption configuration and adsorption energy Eads. The calculation formula of adsorption energy is as follows:
where Etotal represents the total energy of the system after adsorption of NO molecules, Eslab represents the energy of the Pt surface before adsorption of NO molecules, and ENO represents the energy of free gas molecules of NO. The negative value of Eads indicates that the adsorption process releases energy, and the greater the energy release, the stronger the adsorption capacity.
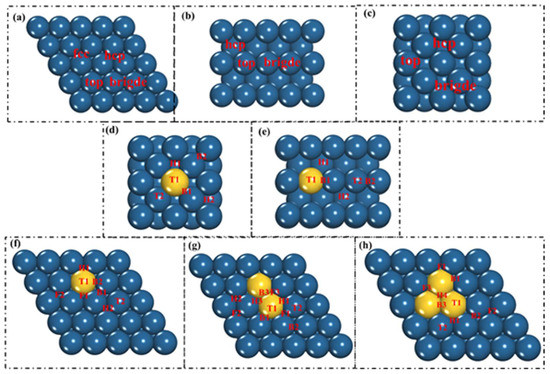
Figure 2.
Possible adsorption sites of NO on (a) Pt(111), (b) Pt(220), (c) Pt(200); Au single-atom doped (d) Pt(200), (e) Pt(220), (f) Pt(111) surfaces; and (g) Au dimer, (h) Au trimer Pt(111) doped. Steel blue and yellow represent Pt and Au atoms, respectively.
In addition, the reaction heat ΔE and activation energy Ea are calculated as follows:
In Equations (2) and (3), EIS represents the energy of the initial state, ETS represents the energy of the transition state, and EFS represents the energy of the final state.
3. Results and Discussion
3.1. Effects of Au Single-Atom Doping on Pt with Different Crystal Planes on NO Adsorption Energy
As shown in Figure 2, when NO adsorption took place on the Pt(111) surface, the NO adsorption calculation was performed on the fcc, hcp, bridge, and top active sites using the vertical adsorption model. It can be seen from Table 1 that the corresponding NO adsorption energies are −2.44 eV, −2.32 eV, −2.23 eV, and −1.75 eV, respectively. On the surface of Pt(200), the adsorption energies of NO on the top and bridge sites are not much different, and slightly smaller on hcp sites. On the Pt(220) surface, the adsorption energy of NO on the bridge site is the largest and the smallest on the hcp site. It can be seen that the adsorption energy of NO on the three surfaces is greatly affected by the surface structure.

Table 1.
Adsorption energies of NO on adsorption sites on Pt(111), Pt(200), and Pt(220) surfaces.
When Au/Pt(111) adsorbs NO, as shown in Figure 2, the vertical adsorption model is used for adsorption at the T1, T2, B1, B2, H1, H2, F1, and F2 active sites. The adsorption sites of Au/Pt (200) and Au/Pt (220) are B1, B2, H1, H2, T1, and T2. It can be seen from Table 1 and Table 2 that when the NO molecule is closer to Au, its adsorption capacity is weakened. The small energy gap indicates that NO does not have an excessive energy barrier when the configuration changes on the Pt surface. In addition, the adsorption energies of NO on each active site on the Au/Pt surface were lower than those on the corresponding active sites on the pure Pt surface. It is preliminarily indicated that with the doping of Au in Pt, the catalytic ability of the Pt surface for NO is weakened.

Table 2.
Adsorption energies of Au single atoms for NO on Pt(111), Pt(200), and Pt(220) surfaces.
In addition, previous studies have shown that the exposed area of the Pt(111) surface is larger [29]. Research is also extensive [32,33]. Therefore, in the following sections, we mainly calculate and analyze the adsorbates on the (111) surface.
3.2. Effects of Au Single-Atom, Dimer, and Trimer Doping on Pt surface on NO Adsorption Energy
Table 3 shows the stable adsorption of NO on several adsorption sites. From the information above, it can be seen that the adsorption energy of the NO molecule on Ausingle/Pt(111) is larger at the F2, H2, and B2 sites. It can be seen from the table that when Audimer/Pt adsorbs NO molecules, they are adsorbed on the active sites of T1, T2, B2, B3, H2, and F2, respectively. The corresponding adsorption energies are: −0.33 eV, −1.23 eV, −1.59 eV, −0.26 eV, −1.83 eV, and −1.82 eV. The adsorption energies on the active sites of H2 and F2 are larger, followed by the sites of T2 and B2, and the sites of T1 and B3 are smaller. On Audimer/Pt(111), the T1 and B3 sites have a greater influence on the adsorption energy of NO in the alloy. When Autrimer/Pt(111) adsorbs NO molecules, it is adsorbed on the T1, T2, B2, B3, H2, H4, and F2 active sites, respectively. The corresponding adsorption energies are: −0.30 eV, −1.31 eV, −1.77 eV, −0.21 eV, −1.49 eV, −0.31 eV, and −1.69 eV. The adsorption energies on the active sites of B2 and F2 are larger; the sites of T2 and H2 are the last; and the sites of T1, B3, and H4 are smaller. It can be seen that the adsorption capacity of the alloy surface is weakest when the NO molecule forms bonds with the Au atoms only on the surface of the alloy.

Table 3.
Adsorption sites and adsorption energies of NO molecules on Audimer/Pt(111) and Autrimer/Pt(111) surfaces.
In summary, when the NO molecule is adsorbed closer to the Au atom, the adsorption energy on the corresponding adsorption site becomes smaller. This indicates that Au atoms have a certain weakening effect on the adsorption of NO molecules on the surface of Pt(111). Furthermore, when observing the adsorption energies of the corresponding adsorption sites of NO molecules on different surfaces, such as H2, the adsorption energies of NO molecules on the Ausingle/Pt, Audimer/Pt, and Autrimer/Pt surfaces are −2.30 eV, −1.83 eV, and −1.49 eV, respectively. With the increase in the number of Au atoms on the Pt surface, the adsorption capacity of NO molecules becomes weaker and weaker. This also shows that Au can passivate Pt, which reduces the adsorption reaction of NO on the surface of the alloy electrode to a certain extent. In fact, it has also been experimentally proven that the addition of Au to Pt can weaken the catalytic performance of the alloy for NO [27]. This characteristic can be used to develop NOx sensors with very good selectivity.
To illustrate the relationship between NO and surfaces, the adsorption sites of NO on the surface of alloy catalysts with different structures were determined, as shown in Figure 3. The analysis results show that the electrons in the NO molecules adsorbed on the surface are mainly distributed on the O atoms. The total charge density was calculated to further study the interaction of gas molecules and Au/Pt(111). As shown in Figure 4, electron transfer between the three surfaces and NO molecules was observed, and the number of electron transfers on Audimer/Pt(111) and Autrimer/Pt(111) was less than that on Ausingle/Pt(111), especially on Autrimer/Pt(111). This result implies that the interaction between NO and Autrimer/Pt(111) is smaller than that of the other two alloy surfaces, and further indicates that Autrimer/Pt(111) is less gas-sensitive to NO.
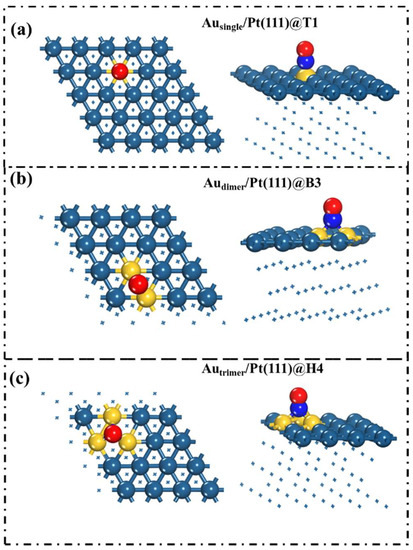
Figure 3.
Stable adsorption site diagrams on (a) Ausingle/Pt(111), (b) Audimer/Pt(111), and (c) Autrimer/Pt(111) surfaces. Red represents O atoms, blue represents N atoms.
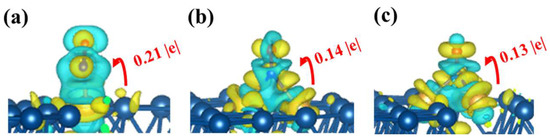
Figure 4.
Charge density difference of the NO intermediates for (a) Ausingle/Pt(111), (b) Audimer/Pt(111), and (c) Autrimer/Pt(111). Isosurface level is 0.0012 e/Å3.
3.3. NO Dissociation Reaction on the Au/Pt(111) Surfaces
In the NO dissociation reaction on the surfaces of Ausingle/Pt(111), Audimer/Pt(111), and Autrimer/Pt(111), the initial reactant NO was first adsorbed at the T1, B3, and H4 sites. N and O atoms are generated by the dissociation of NO. As shown in Figure 5 and Figure 6, NO dissociates into N and O atoms on the surface of Ausingle/Pt(111), which are adsorbed at the F1 and H1 sites, respectively. This step is required to overcome the activation energy barrier of 2.27 eV, and the reaction heat is 0.16 eV. On Audimer/Pt(111), NO dissociates into N and O atoms, which are adsorbed at the F3 and H1 sites, respectively. In this step, the activation energy barrier of 3.22 eV needs to be overcome, and the reaction heat is 1.28 eV. On Autrimer/Pt(111), NO dissociates into N and O atoms, which are adsorbed at the H4 and H1 sites, respectively. In this step, the activation energy barrier of 4.56 eV needs to be overcome, and the reaction heat is 1.51 eV.
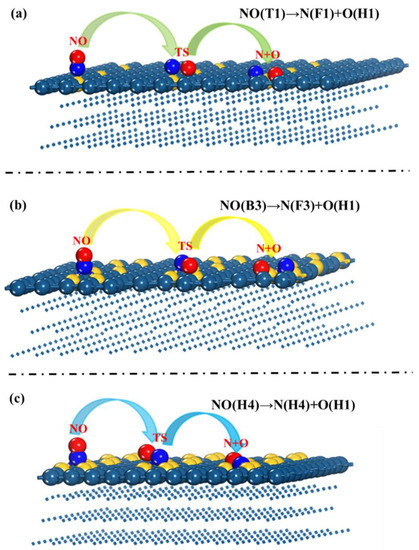
Figure 5.
Structural diagram of NO decomposition on (a) Ausingle/Pt(111), (b) Audimer/Pt(111), and (c) Autrimer/Pt(111) surfaces.
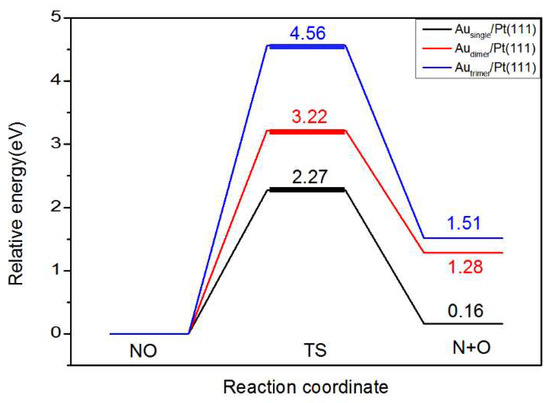
Figure 6.
Decomposition potential energy diagram of NO on Ausingle/Pt(111), Audimer/Pt(111), and Autrimer/Pt(111) surfaces.
The decomposition processes of NO on Au/Pt(111) surfaces with different structural units are compared. In the process of NO molecular decomposition, the two bonds between N and O are not easy to break, and the surface needs to absorb a lot of energy to decompose, so the activation energy of NO decomposition is higher. When NO is adsorbed on the surface of Au/Pt(111), the decrease in the charge transfer between Au and N atoms can strengthen the force between N and O, thus inhibiting the decomposition of NO. With the increase in the doping amount of Au atoms on the surface of Pt, the potential barrier for NO decomposition increases gradually, and the energy barrier of NO decomposition on the Autrimer/Pt(111) surface is the highest, as shown in Figure 6. The reaction heat of the Autrimer/Pt(111) surface after decomposition is also the highest. In short, doping Au can improve the activation energy of NO decomposition and can effectively inhibit the decomposition of NO so that the oxygen-pumping process occurs preferentially on the electrode. There are also experimental data in previous studies showing that doping Pt with a certain proportion of Au can increase the activation energy of NO during surface dissociation [34,35]. This is also consistent with our calculated results.
4. Conclusions
By combining density functional theory (DFT) calculations with micro-reaction kinetic analysis, the adsorption properties of NO on different crystal planes of Au doped on Pt substrates were investigated. Furthermore, the adsorption and dissociation of NO on Pt(111) by Au single atoms, dimers, and trimers were analyzed. The adsorption and dissociation of NO on Pt(111) aggregates and the effect of different amounts of Au on the performance of Pt electrodes were clarified. The results show that with the increase in the number of Au atoms on the surface of Pt(111), the adsorption capacity of NO on the surface of the alloy decreases, thereby reducing the gas sensitivity of the electrode to NO. In addition, the interaction force between NO and Au atoms is reduced, and the activation energy of NO decomposition is increased, which can effectively inhibit the decomposition of NO and make the oxygen-pumping process preferentially occur on the electrode. In conclusion, these results are expected to provide guidance for the design of future high-performance NOx sensor electrode materials.
Author Contributions
Conceptualization: J.Z. and Z.T.; methodology: Y.W., S.Z. and Y.L; software: Y.L.; validation: Y.L., H.H. and X.J.; formal analysis: Y.L.; investigation: Y.L.; resources: J.Z. and Q.Z.; data curation: Y.L.; writing—original draft preparation: Y.L.; writing—review and editing: Y.L.; visualization: Y.L.; supervision: W.Z.; project administration: J.J.; funding acquisition: J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Nos. 61971251), the China Postdoctoral Science Foundation (Grant No. 2019M663474), the Natural Science Foundation of Zhejiang (Grant No. LGG22F010017, LY18F010009), and the Natural Science Foundation of Ningbo (Grant No. 2018A610002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J.; Yoon, B.Y.; Park, C.O.; Lee, W.J.; Lee, C.B. Sensing behavior and mechanism of mixed potential NOx sensors using NiO, NiO (+ YSZ) and CuO oxide electrodes. Sens. Actuators B Chem. 2009, 135, 516–523. [Google Scholar] [CrossRef]
- Guo, X. Zirconia Solid Electrolyte and Its Application in Oxygen Sensor; Huazhong University of Science and Technology: Wuhan, China, 1992. [Google Scholar]
- Cheng, C.; Wang, J.X.; Jian, J.W.; Zou, J. Research on the diffused hole of limiting current oxygen sensor fabricated by screen printing method. Transducer Microsyst. Technol. 2019, 38, 11–14. [Google Scholar]
- Gao, J.Y.; Zou, J.; Zhang, D.X.; Jian, J. Affection of Oxygen to the Sensing Behavior of NOx Sensor. Chin. J. Sens. Actuators 2010, 23, 1215–1219. [Google Scholar]
- Wang, G.W.; Li, H.P.; Xu, L.P.; Zhang, L.; Wang, R.P. Study of the Pt/YSZ electrode sintering technics. J. Funct. Mater. 2008, 39, 1997–2001+2004. [Google Scholar]
- Lv, S.; Zhang, Y.; Jiang, L.; Zhao, L.; Wang, J.; Liu, F.; Wang, C.; Yan, X.; Sun, P.; Wang, L.; et al. Mixed potential type YSZ-based NO2 sensors with efficient three-dimensional three-phase boundary processed by electrospinning. Sens. Actuators B Chem. 2022, 354, 131219. [Google Scholar] [CrossRef]
- Burch, R.; Daniells, S.T.; Hu, P. N2O and NO2 formation on Pt(111): A density functional theory study. J. Chem. Phys. 2002, 117, 2902–2908. [Google Scholar] [CrossRef]
- Santana, J.A.; Ishikawa, Y. DFT calculations of the electrochemical adsorption of sulfuric acid anions on the Pt(100) and Pt(100) surfaces. Electrocatalysis 2020, 11, 86–93. [Google Scholar] [CrossRef]
- Su, M.; Dong, J.C.; Le, J.-B.; Zhao, Y.; Yang, W.M.; Yang, Z.L.; Li, J.F.; Attard, G.; Liu, G.; Cheng, J.; et al. In Situ Raman Study of CO Electrooxidation on Pt (hkl) Single-Crystal Surfaces in Acidic Solution. Angew. Chem. Int. Ed. 2020, 59, 23554–23558. [Google Scholar] [CrossRef] [PubMed]
- Zinola, C.F. On the kinetics and mechanism of simultaneous CO and NO oxidations on polyoriented and Pt nanoparticles. Int. J. Hydrogen Energy 2020, 45, 1453–1465. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Song, Y.; Guo, C.; Hu, P. Quantitative Studies of the Coverage Effects on Microkinetic Simulations for NO Oxidation on Pt(111). J. Phys. Chem. C 2019, 123, 27594–27602. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, K.G.; Darby, M.T.; Stamatakis, M. Adlayer structure and lattice size effects on catalytic rates predicted from KMC simulations: NO oxidation on Pt(111). J. Chem. Phys. 2018, 149, 184701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J. A computational study on the electrified Pt(100) surface by the cluster model. Phys. Chem. Chem. Phys. 2019, 21, 6112–6125. [Google Scholar] [CrossRef] [PubMed]
- Zorko, M.; Farinazzo Bergamo Dias Martins, P.; Connell, J.G.; Lopes, P.P.; Markovic, N.M.; Stamenkovic, V.R.; Strmcnik, D. Improved rate for the oxygen reduction reaction in a sulfuric acid electrolyte using a Pt(111) surface modified with melamine. ACS Appl. Mater. Interfaces 2021, 13, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Liu, L.; Boronat, M.; Arenal, R.; Concepcion, P.; Corma, A. Low-temperature catalytic NO reduction with CO by subnanometric Pt clusters. ACS Catal. 2019, 9, 11530–11541. [Google Scholar] [CrossRef] [Green Version]
- Souda, R.; Aizawa, T. Crystallization kinetics of thin water films on Pt(100): Effects of oxygen and carbon-monoxide adspecies. Phys. Chem. Chem. Phys. 2019, 21, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Grasso, S.; Di Loreto, M.V.; Arienzo, A.; Gallo, V.; Sabatini, A.; Zompanti, A.; Pennazza, G.; De Gara, L.; Antonini, G.; Santonico, M. Microbiological Risk Assessment of Ready-to-Eat Leafy Green Salads via a Novel Electrochemical Sensor. Chemosensors 2022, 10, 134. [Google Scholar] [CrossRef]
- Clayborne, A.; Chun, H.J.; Rankin, R.-B.; Greeley, J. Elucidation of pathways for NO electroreduction on Pt(100) from first principles. Angew. Chem. 2015, 127, 8373–8376. [Google Scholar] [CrossRef]
- Zagalo, P.M.; Ribeiro, P.A.; Raposo, M. Effect of Applied Electrical Stimuli to Interdigitated Electrode Sensors While Detecting 17α-Ethinylestradiol in Water Samples. Chemosensors 2022, 10, 114. [Google Scholar] [CrossRef]
- Zheng, J.; Ivashenko, O.; Fjellvåg, H.; Groot, I.M.; Sjåstad, A.-O. Roadmap for Modeling RhPt/Pt(100) Catalytic Surfaces. J. Phys. Chem. C 2018, 122, 26430–26437. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Frey, K.; Schneider, W.F. Binary approach to ternary cluster expansions: NO–O–vacancy system on Pt(100). J. Phys. Chem. C 2017, 121, 7344–7354. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Chen, D.; Miao, J.; Zhi, X.; Deng, S.; Lin, S.; Jin, H.; Cui, D. Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors 2021, 9, 227. [Google Scholar] [CrossRef]
- Xia, M.; Yue, R.; Chen, P.; Wang, M.; Jiao, T.; Zhang, L.; Li, L. Density functional theory investigation of the adsorption behaviors of SO2 and NO2 on a Pt(100) surface. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 266–270. [Google Scholar] [CrossRef]
- Tang, H.; Trout, B.L. NO chemisorption on Pt(100), Rh/Pt(100), and Pd/Pt(100). J. Phys. Chem. B 2005, 109, 17630–17634. [Google Scholar] [CrossRef] [PubMed]
- Zhanpeisov, N.U.; Fukumura, H. Theoretical DFT Study on the Interaction of NO and Br2 with the Pt(100) Surface. J. Chem. Theory Comput. 2006, 2, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Getman, R.B.; Schneider, W.F. DFT-based characterization of the multiple adsorption modes of nitrogen oxides on Pt(100). J. Phys. Chem. C 2007, 111, 389–397. [Google Scholar] [CrossRef]
- Huang, H.; Xie, G.; Wang, X.; Yin, L.; Peng, Z. Study of inactivated electrode paste used for zro_2-based no_x sensor. J. Wuhan Univ. Sci. Technol. 2012, 35, 41–43. [Google Scholar]
- Ovesson, S.; Lundqvist, B.I.; Schneider, W.F.; Bogicevic, A. NO oxidation properties of Pt(100) revealed by ab initio kinetic simulations. Phys. Rev. B 2005, 71, 115406. [Google Scholar] [CrossRef]
- Zhang, Q. Localization of Pt Catalysts with Different Configurations and their Adsorption Processes of CO and O2: A First-Principle Investigation; South China University of Technology: Guangzhou, China, 2020. [Google Scholar]
- Ge, Q.; King, D.A. Energetics, geometry and spin density of NO chemisorbed on Pt {111}. Chem. Phys. Lett. 1998, 285, 15–20. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystallography Open Database. Available online: http://crystallography.net/cod/ (accessed on 28 April 2022).
- Burns, A.-R.; Stechel, E.-B.; Jennison, D.-R. Rotational dynamics and electronic energy partitioning in the electron-stimulated desorption of NO from Pt(100). J. Vac. Sci. Technol. A Vac. Surf. Film. 1988, 6, 895–898. [Google Scholar] [CrossRef]
- Xue, M.; Jia, J.; Wu, H. Density functional theory study on the adsorption and decomposition of CO on Ni-and Pt-Au(1 1 1) bimetallic surfaces. Comput. Theor. Chem. 2021, 1205, 113439. [Google Scholar] [CrossRef]
- Hibino, T.; Inoue, T.; Sano, M. Electrochemical reduction of NO by alternating current electrolysis using yttria-stabilized zirconia as the solid electrolyte: Part I. Characterizations of alternating current electrolysis of NO. Solid State Ion. 2000, 130, 19–29. [Google Scholar] [CrossRef]
- Cao, L.J. The Structural Properties of Bimetallic Nanoparticles Au-Pt; Beijing University of Chemical Technology: Beijing, China, 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).