Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19
Abstract
:1. Introduction
1.1. Breath Analysis and Diseases
1.2. Gas Sensing Techniques
1.3. Selected Disease for Demonstration: COVID-19
1.4. Intelligent Healthcare Products
1.5. Our Approach
2. Materials and Methods
2.1. Gas Biomarker Sensitive Solutions Preparation
2.2. Application to Surgical Facemasks
2.3. Breathalyzer
3. Results and Discussion
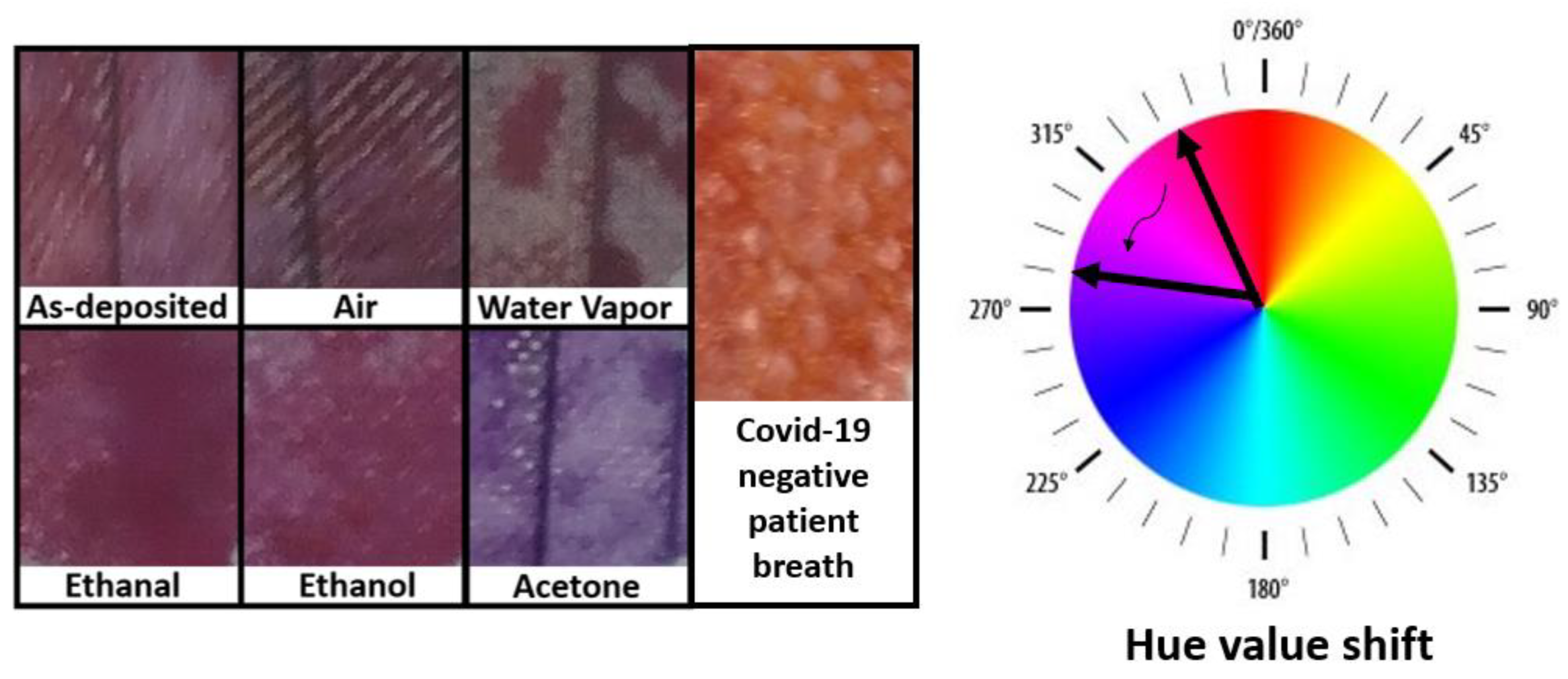
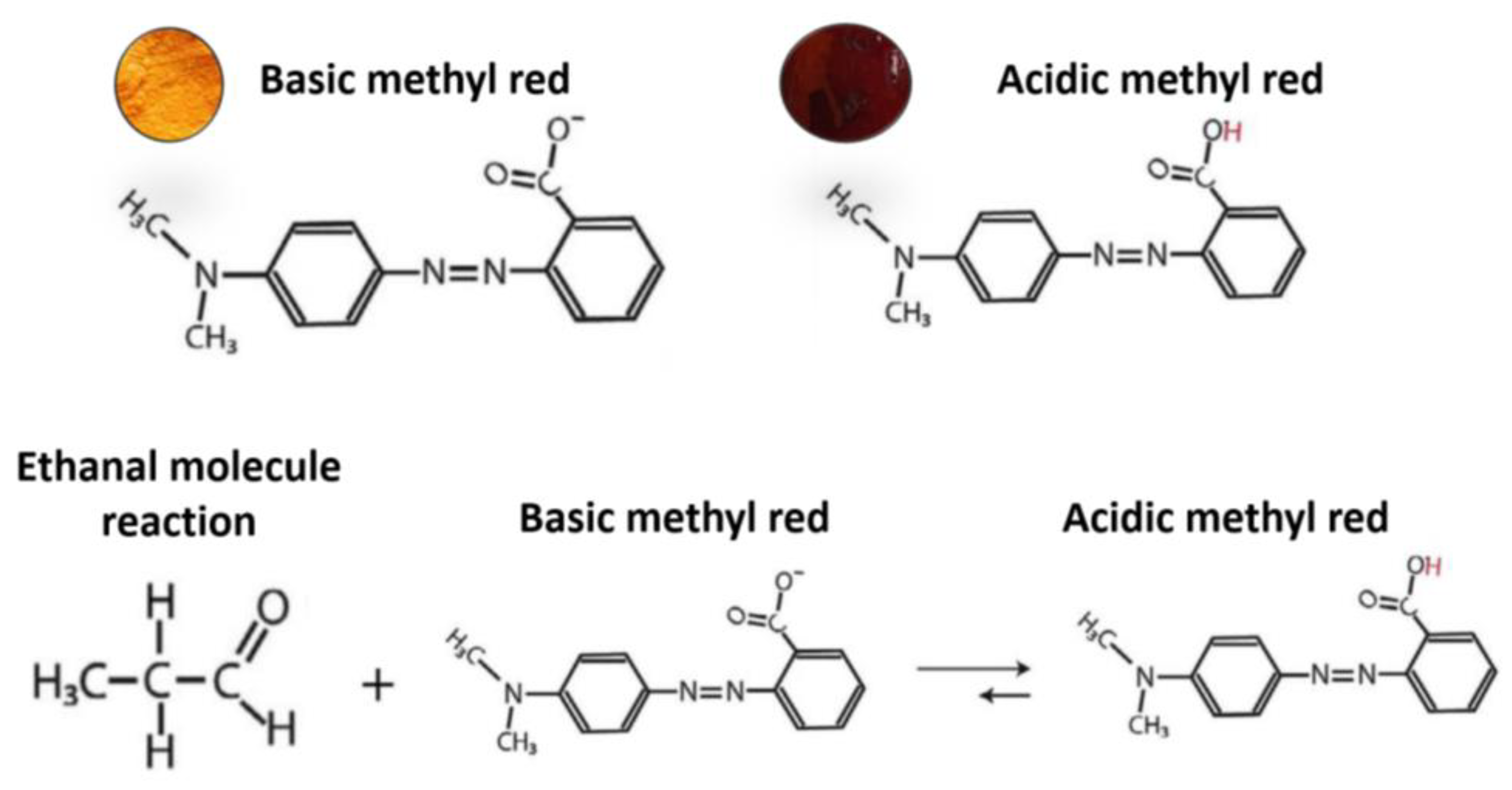
3.1. Solutions Reactivity and Selectivity
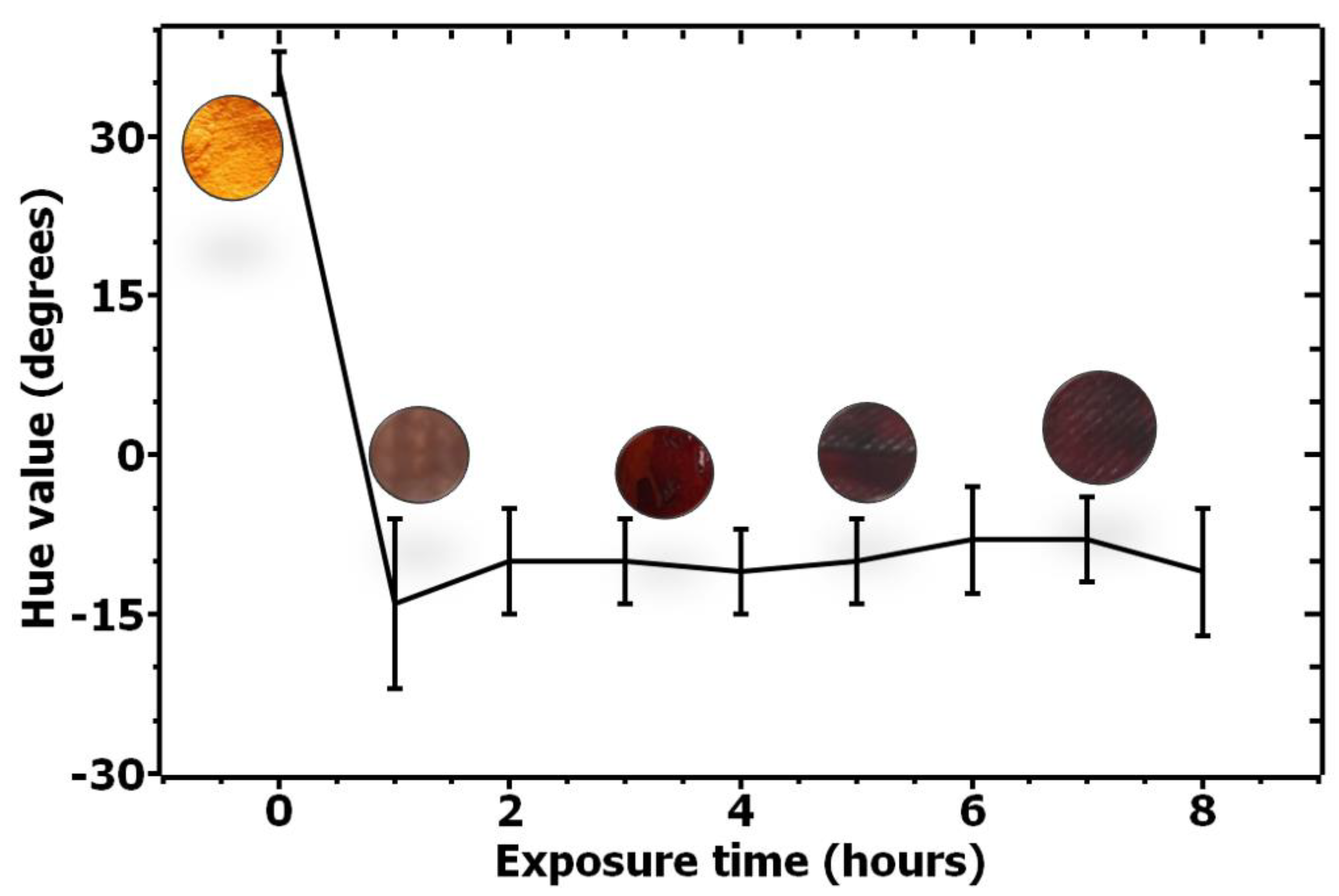
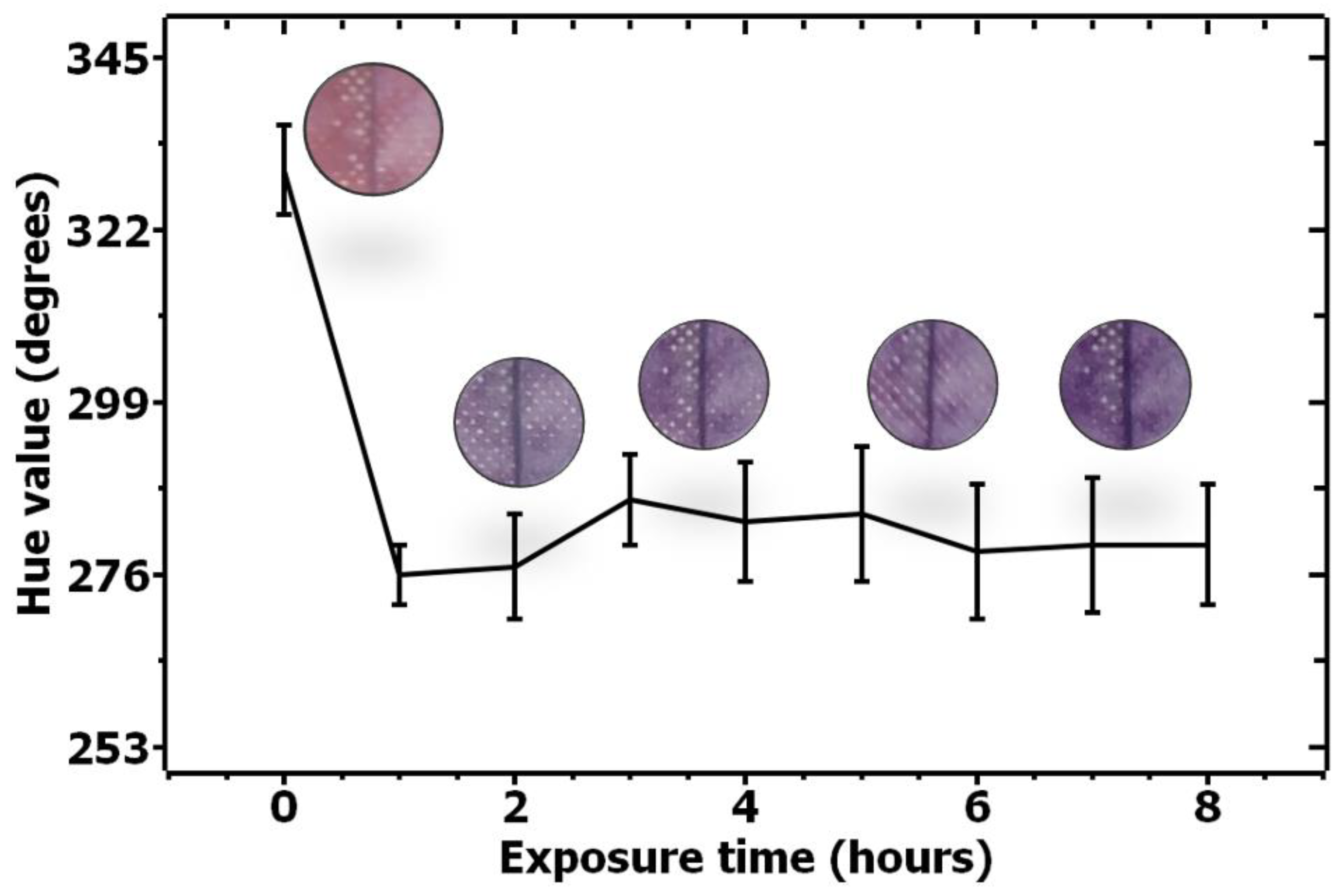
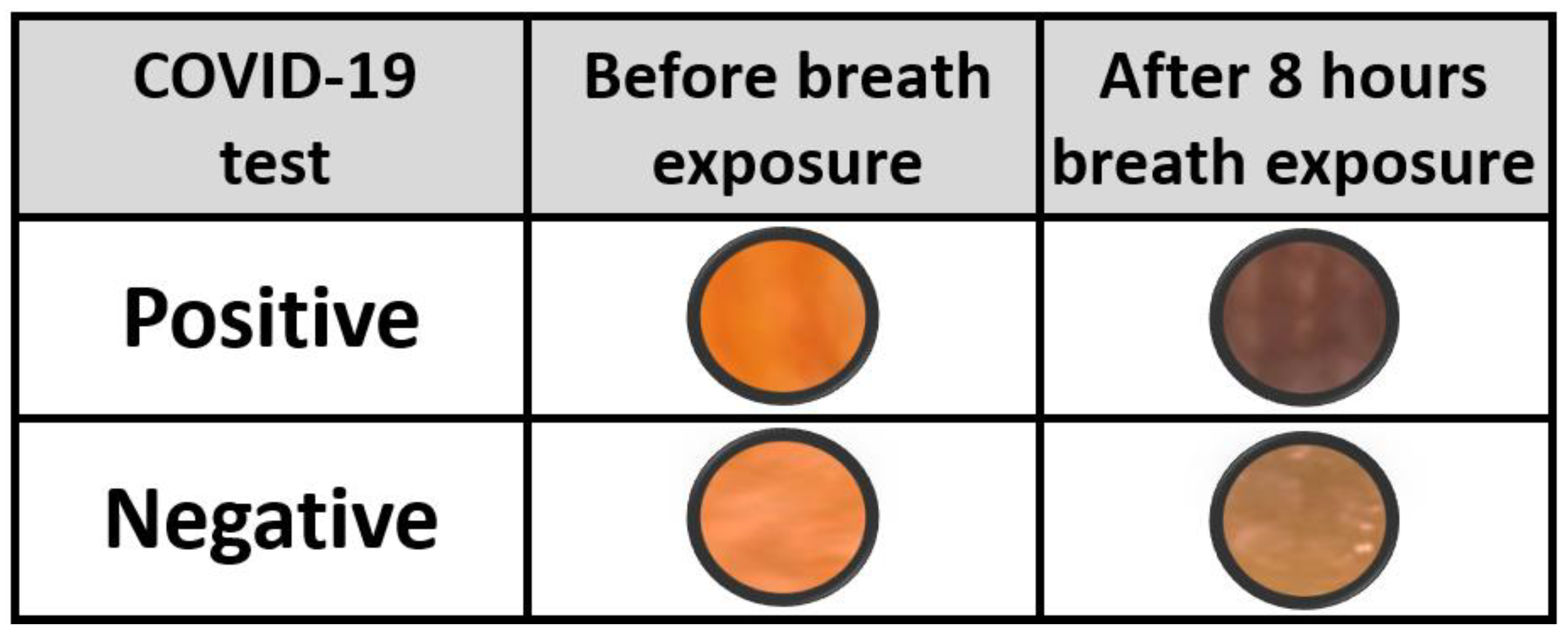
3.2. COVID-19 Patients Breath Exposure
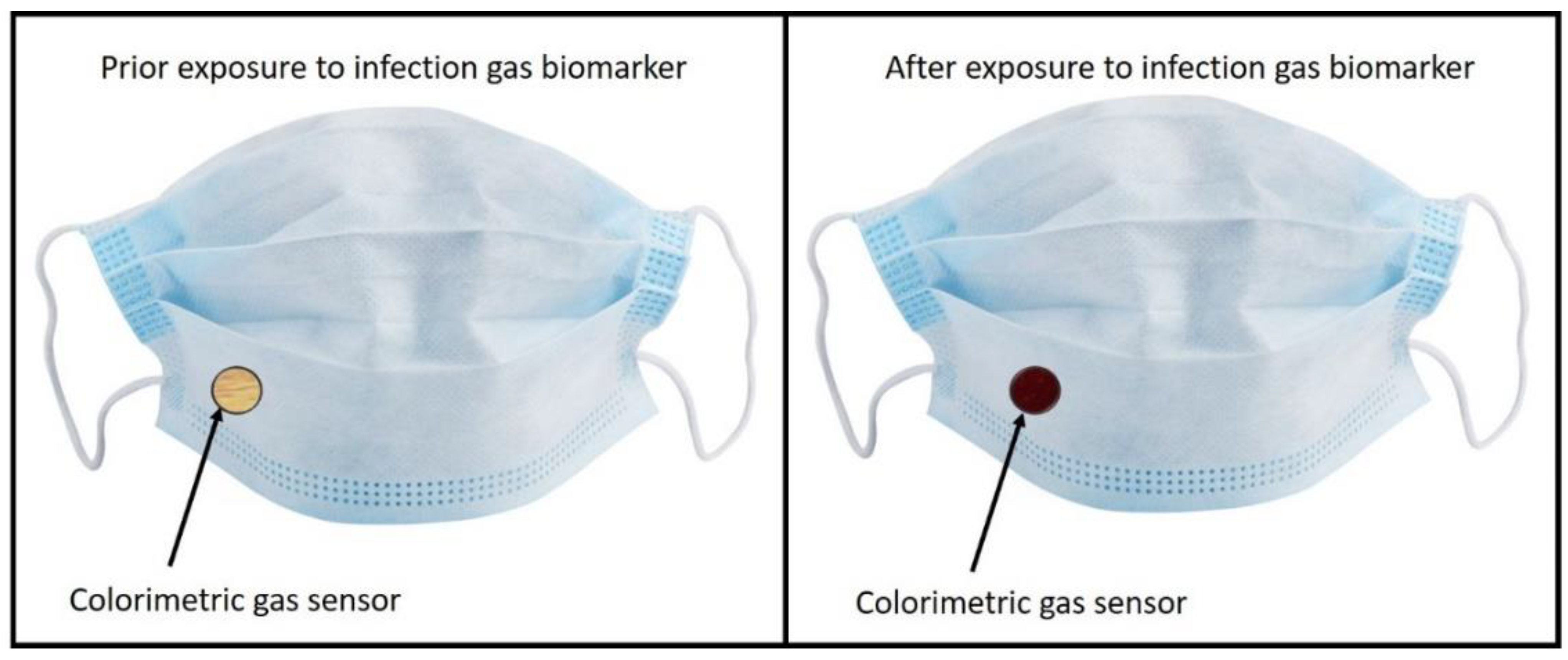
3.3. Technological Outlook: Facemasks with Infection Indicators
3.4. Technological Outlook: Breathalyzer
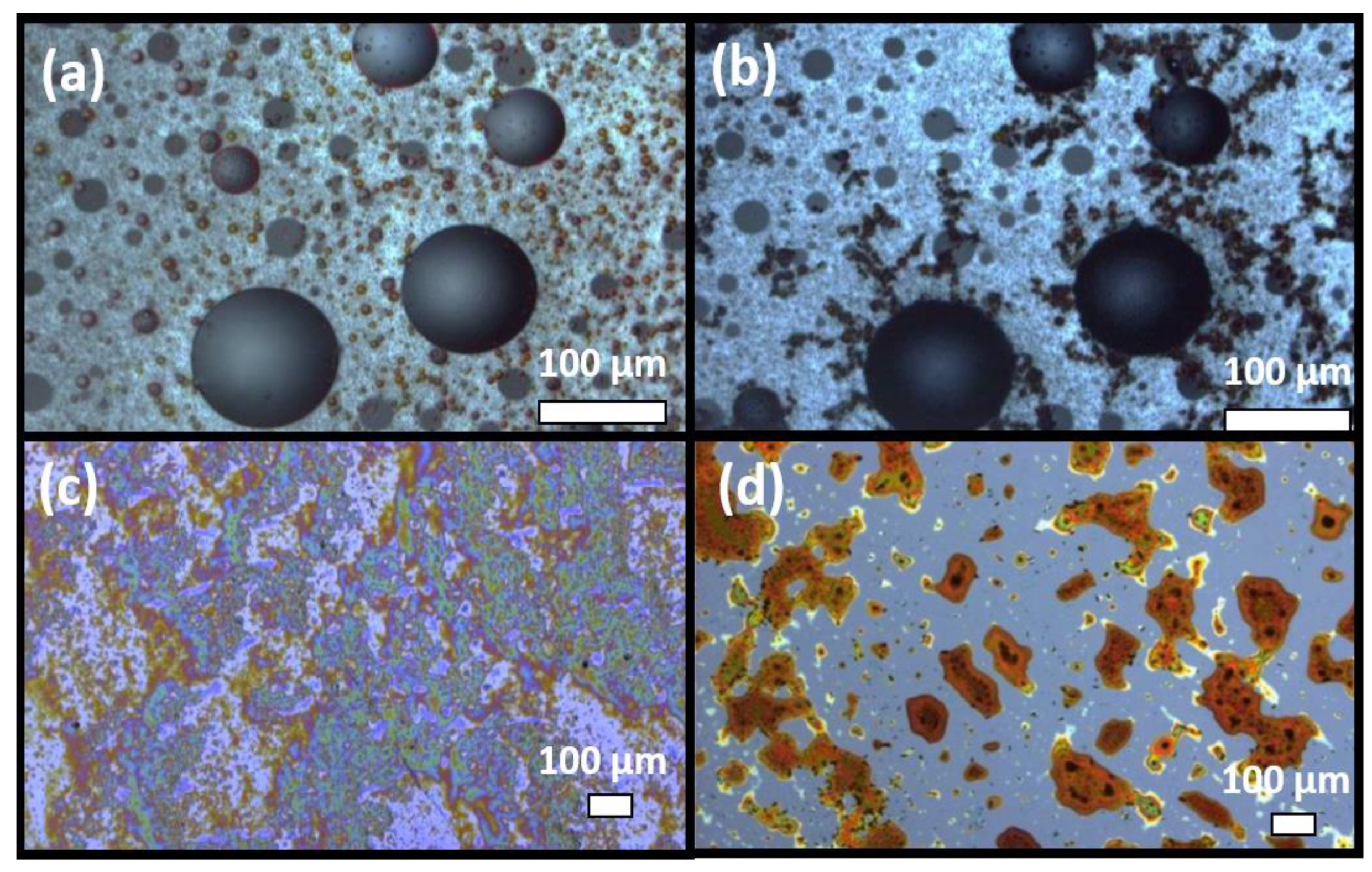
3.5. Microscopic Investigation of Coated Sensing Element upon Ethanal Exposition
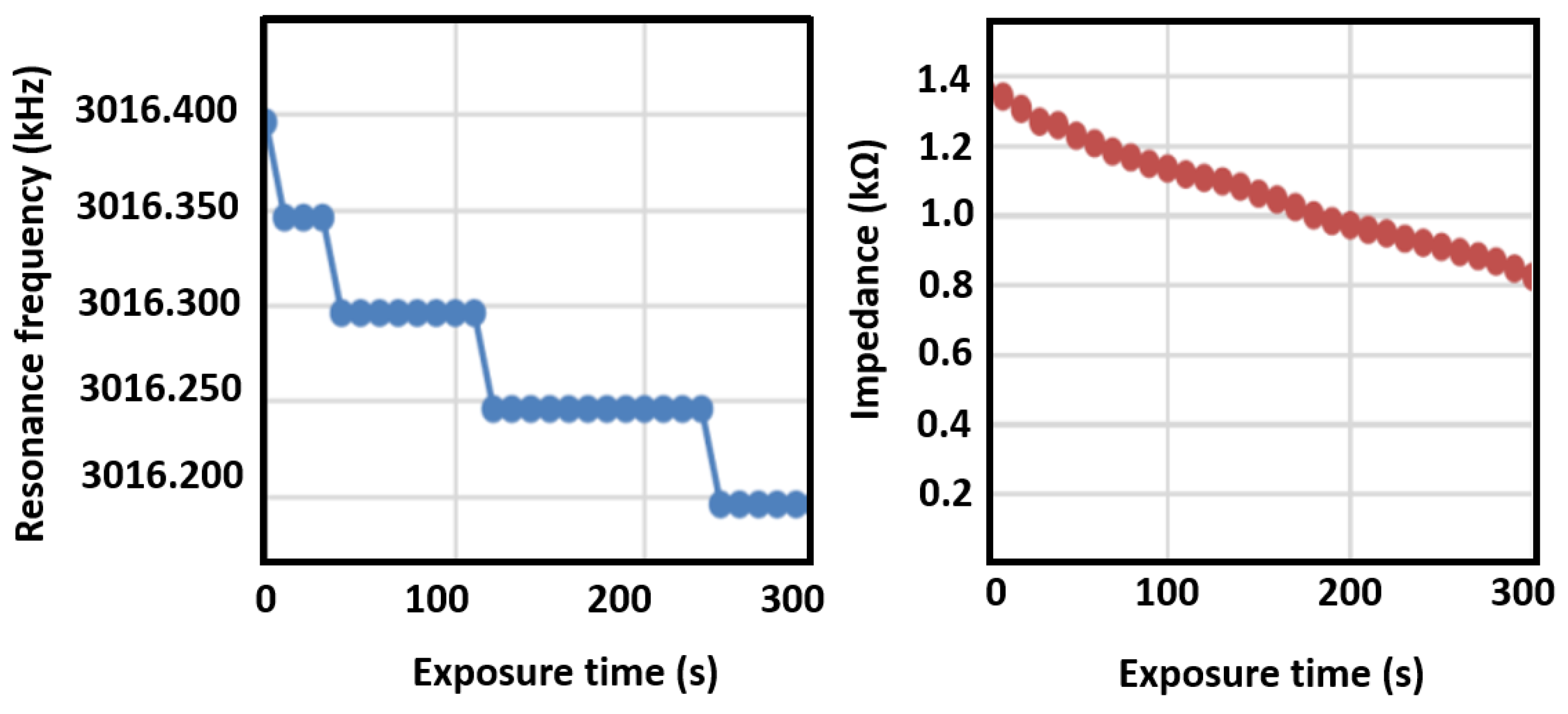
3.6. Breathalyzer Electronic Response to Ethanal Exposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M. Breath tests in medicine. Sci. Am. 1992, 267, 74–79. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Barnes, P.J.; Chowdhury, B.; Kharitonov, S.A.; Magnussen, H.; Page, C.P.; Postma, D.; Saetta, M. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 174, 6–14. [Google Scholar] [CrossRef]
- Wang, C.; Sahay, P. Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits. Sensors 2009, 9, 8230–8262. [Google Scholar] [CrossRef]
- Brindicci, C.; Kharitonov, S.A.; Ito, M.; Elliott, M.W.; Hogg, J.C.; Barnes, P.J.; Ito, K. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Krisher, S.; Riley, A.; Mehta, K. Designing breathalyser technology for the developing world: How a single breath can fight the double disease burden. J. Med. Eng. Technol. 2014, 38, 156–163. [Google Scholar] [CrossRef]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; et al. Rapid “breath-print” of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS ONE 2013, 8, e59658. [Google Scholar]
- Arasaradnam, R.P.; Covington, J.A.; Harmston, C.; Nwokolo, C.U. Review article: Next generation diagnostic modalities in gastroenterology--gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 2014, 39, 780–789. [Google Scholar] [CrossRef]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-destructive monitoring of apple ripeness using an aldehyde sensitive colorimetric sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Prabhakar, A.; Qin, X.; Forzani, E.S.; Tao, N. Colorimetric Sensor for Online Accurate Detection of Breath Acetone. ACS Sens. 2021, 6, 450–453. [Google Scholar] [CrossRef]
- Ebralidze, I.; Laschuck, N.; Poisson, J.; Zenkina, O. Colorimetric Sensors and Sensor Arrays. Nanomaterials Design for Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-814505-0. [Google Scholar]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef]
- Boisen, A.; Dohn, S.; Keller, S.S.; Schmid, S.; Tenje, M. Cantilever-like micromechanical sensors. Rep. Prog. Phys. 2011, 74, 036101. [Google Scholar] [CrossRef]
- Preumont, A. Vibration Control of Active Structures: An Introduction; Springer: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Younis, M.I. MEMS Linear and Nonlinear Statics and Dynamics; Springer: New York, NY, USA; Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK, 2011. [Google Scholar]
- Korsa, M.; Domingo, J.M.C.; Nsubuga, L.; Hvam, J.; Niekiel, F.; Lofink, F.; Rubahn, H.-G.; Adam, J.; de Oliveira Hansen, R. Optimizing piezoelectric cantilever design for electronic nose applications. Chemosensors 2020, 8, 114. [Google Scholar] [CrossRef]
- Alexi, N.; Hvam, J.; Lund, B.L.W.; Nsubuga, L.; Hansen, R.D.; Lofink, F.; Byrne, D.; Leisner, J. Cadaverine as a freshness indicator of microbial and Quality Index Method (QIM) quality of chilled Yellowfin Tuna (Thunnus albacares) steaks: The potential of novel biosensor technology for shelf life prediction. Food Control 2020, 125, 107958. [Google Scholar] [CrossRef]
- Costa, C.A.B.; Grazhdan, D.; Fiutowski, J.; Nebling, E.; Blohm, L.; Lofink, F.; de Oliveira Hansen, R. Meat and fish freshness evaluation by functionalized cantilever-based biosensors. Microsyst. Technol. 2019, 26, 867–871. [Google Scholar] [CrossRef]
- Wang, Y.; Sobolewska, E.K.; Fiutowski, J.; Rubahn, H.G.; de Oliveira Hansen, R.; Albers, J.; Hansen, R.D.O. Functionalizing micro-cantilevers for meat degradation measurements. In Proceedings of the 2016 Symposium on Design, Test, Integration and Packaging of MEMS and MOEMS, Budapest, Hungary, 30 May—2 June 2016; pp. 151–154. [Google Scholar]
- Wang, Y.; Costa CA, B.; Sobolewska, E.K.; Fiutowski, J.; Brehm, R.; Albers, J.; de Oliveira Hansen, R. Micro-cantilevers for optical sensing of biogenic amines. Microsyst. Technol. 2016, 24, 363–369. [Google Scholar] [CrossRef]
- Mamou, D.; Nsubuga, L.; Lisboa Marcondes, T.; Høegh, S.O.; Hvam, J.; Niekiel, F.; Lofink, F.; Rubahn, H.-G.; de Oliveira Hansen, R. Surface Modification Enabling Reproducible Cantilever Functionalization for Industrial Gas Sensors. Sensors 2021, 21, 6041. [Google Scholar] [CrossRef]
- Bashir, M.F.; Ma, B.; Shahzad, L. A brief review of socio-economic and environmental impact of COVID-19. Air Qual. Atmos. Health 2020, 13, 1403–1409. [Google Scholar] [CrossRef]
- Parihar, A.; Ranjan, P.; Sanghi, S.; Srivastava, A.; Khan, R. Point-of-Care Biosensor-Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio Mater. 2020, 3, 11, 7326–7343. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Bal, J.; Seo, S.K.; Chong, C.-K.; Lee, J.H.; Park, H. Development and Clinical Evaluation of an Immunochromatography-Based Rapid Antigen Test (GenBody™ COVAG025) for COVID-19 Diagnosis. Viruses 2021, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; He, Y.; Qi, Y.; Chen, J.; Ma, Y.; Liu, F.; Lai, K.; Zhang, Y.; Jiang, L.; et al. A new and rapid approach for detecting COVID-19 based on S1 protein fragments. Clin. Transl. Med. 2020, 10, e90. [Google Scholar] [CrossRef]
- Longhitano, Y.; Zanza, C.; Romenskaya, T.; Saviano, A.; Persiano, T.; Leo, M.; Piccioni, A.; Betti, M.; Maconi, A.; Pindinello, I.; et al. Single-Breath Counting Test Predicts Non-Invasive Respiratory Support Requirements in Patients with COVID-19 Pneumonia. J. Clin. Med. 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—A feasibility study. EClinicalMedicine 2020, 29, 100609. [Google Scholar] [CrossRef]
- Chen, H.; Qi, X.; Zhang, L.; Li, X.; Ma, J.; Zhang, C.; Yao, M. COVID-19 screening using breath-borne volatile organic compounds. J. Breath Res. 2021, 15, 047104. [Google Scholar] [CrossRef]
- Available online: www.abenanova.com (accessed on 22 April 2022).
- Available online: www.donutrobotics.com/c-mask (accessed on 22 April 2022).
- Nowrin, A.; Afroz, S.; Rahman, M.S.; Mahmud, I.; Cho, Y.-Z. Comprehensive Review on Facemask Detection Techniques in the Context of COVID-19. IEEE Access 2021, 9, 106839–106864. [Google Scholar] [CrossRef]
- Available online: www.medgadget.com/2021/01/face-mask-sensor-to-detect-covid-19.html (accessed on 22 April 2022).
- Gao, Z.; Lou, Z.; Chen, S.; Li, L.; Jiang, K.; Fu, Z.; Han, W.; Shen, G. Fiber gas sensor-integrated smart face mask for room-temperature distinguishing of target gases. Nano Res. 2018, 11, 511–519. [Google Scholar] [CrossRef]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ethanol and acetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 61–68. [Google Scholar] [CrossRef]
- Uebelacker, M.; Lachenmeier, D.W. Quantitative Determination of Acetaldehyde in Foods Using Automated Digestion with Simulated Gastric Fluid Followed by Headspace Gas Chromatography. J. Autom. Methods Manag. Chem. 2011, 2011, 907317. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, Z.-M.; Blaser, A.F.; Kavianynejad, K.; de Torrella, C.G.M.G.; Nsubuga, L.; Mishra, Y.K.; Rubahn, H.-G.; de Oliveira Hansen, R. Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19. Chemosensors 2022, 10, 167. https://doi.org/10.3390/chemosensors10050167
Török Z-M, Blaser AF, Kavianynejad K, de Torrella CGMG, Nsubuga L, Mishra YK, Rubahn H-G, de Oliveira Hansen R. Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19. Chemosensors. 2022; 10(5):167. https://doi.org/10.3390/chemosensors10050167
Chicago/Turabian StyleTörök, Zoltan-Mihály, Arthur Frederic Blaser, Kiana Kavianynejad, Carlos Gonzalo Moya Gual de Torrella, Lawrence Nsubuga, Yogendra Kumar Mishra, Horst-Günter Rubahn, and Roana de Oliveira Hansen. 2022. "Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19" Chemosensors 10, no. 5: 167. https://doi.org/10.3390/chemosensors10050167
APA StyleTörök, Z.-M., Blaser, A. F., Kavianynejad, K., de Torrella, C. G. M. G., Nsubuga, L., Mishra, Y. K., Rubahn, H.-G., & de Oliveira Hansen, R. (2022). Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19. Chemosensors, 10(5), 167. https://doi.org/10.3390/chemosensors10050167








