Digital Detection of Olive Oil Rancidity Levels and Aroma Profiles Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Machine Learning Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Description
2.2. Gas Chromatography/Mass Spectroscopy
2.3. Near-Infrared Spectroscopy
2.4. Electronic Nose
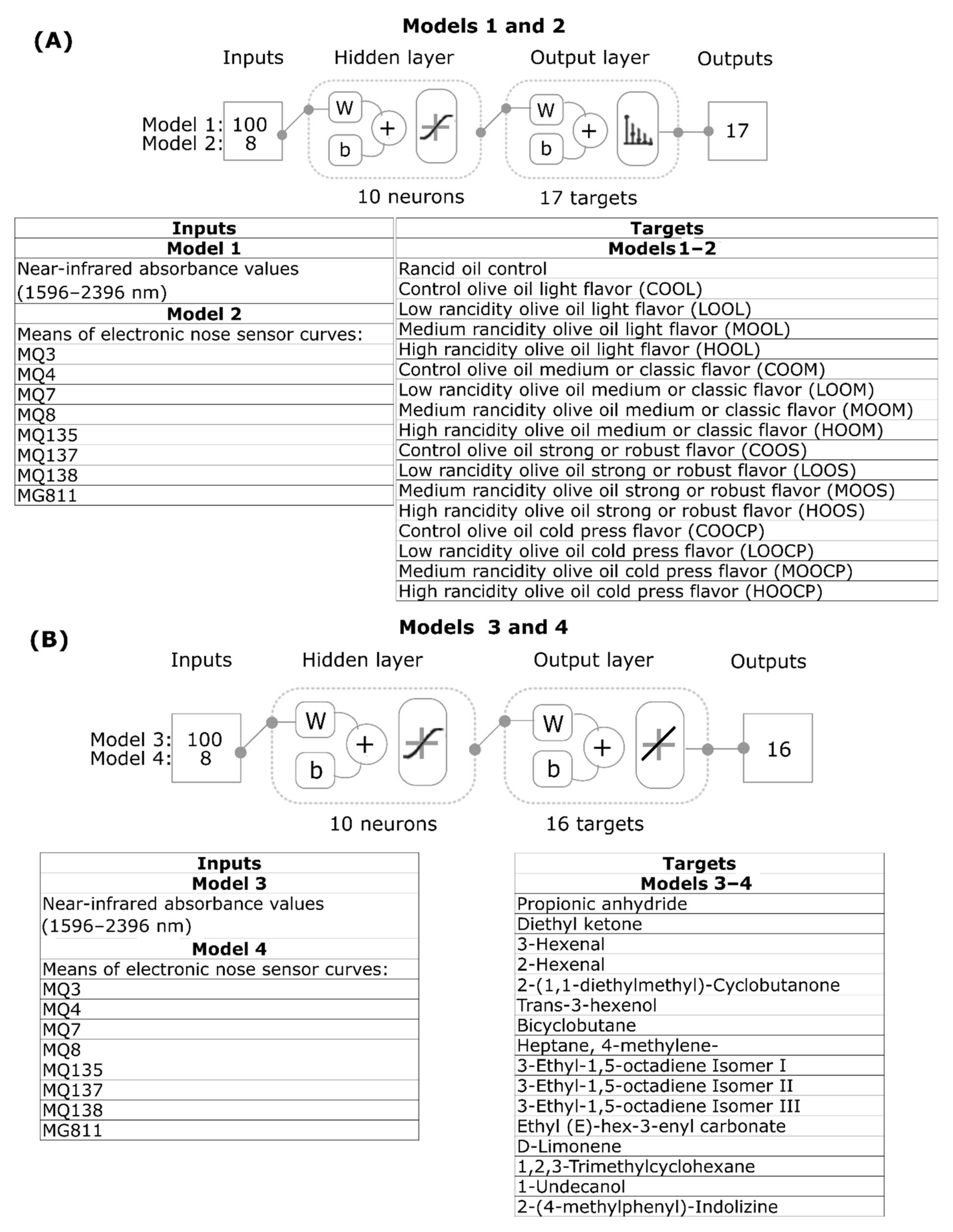
2.5. Statistical Analysis and Machine Learning Modeling
3. Results and Discussion
3.1. Volatile Aromatic Compounds from GC-MS
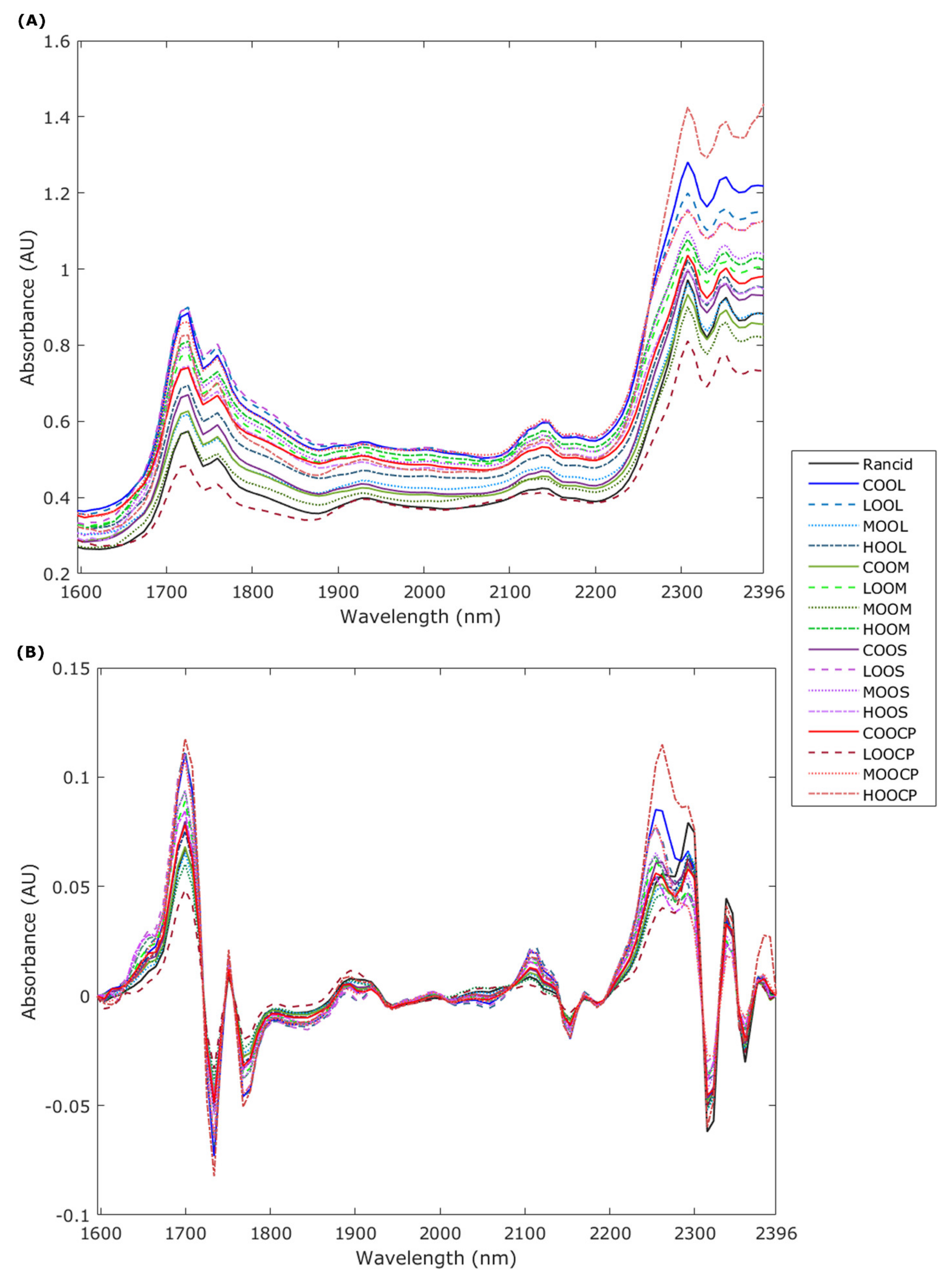
3.2. Near-Infrared Spectroscopy
3.3. Electronic Nose
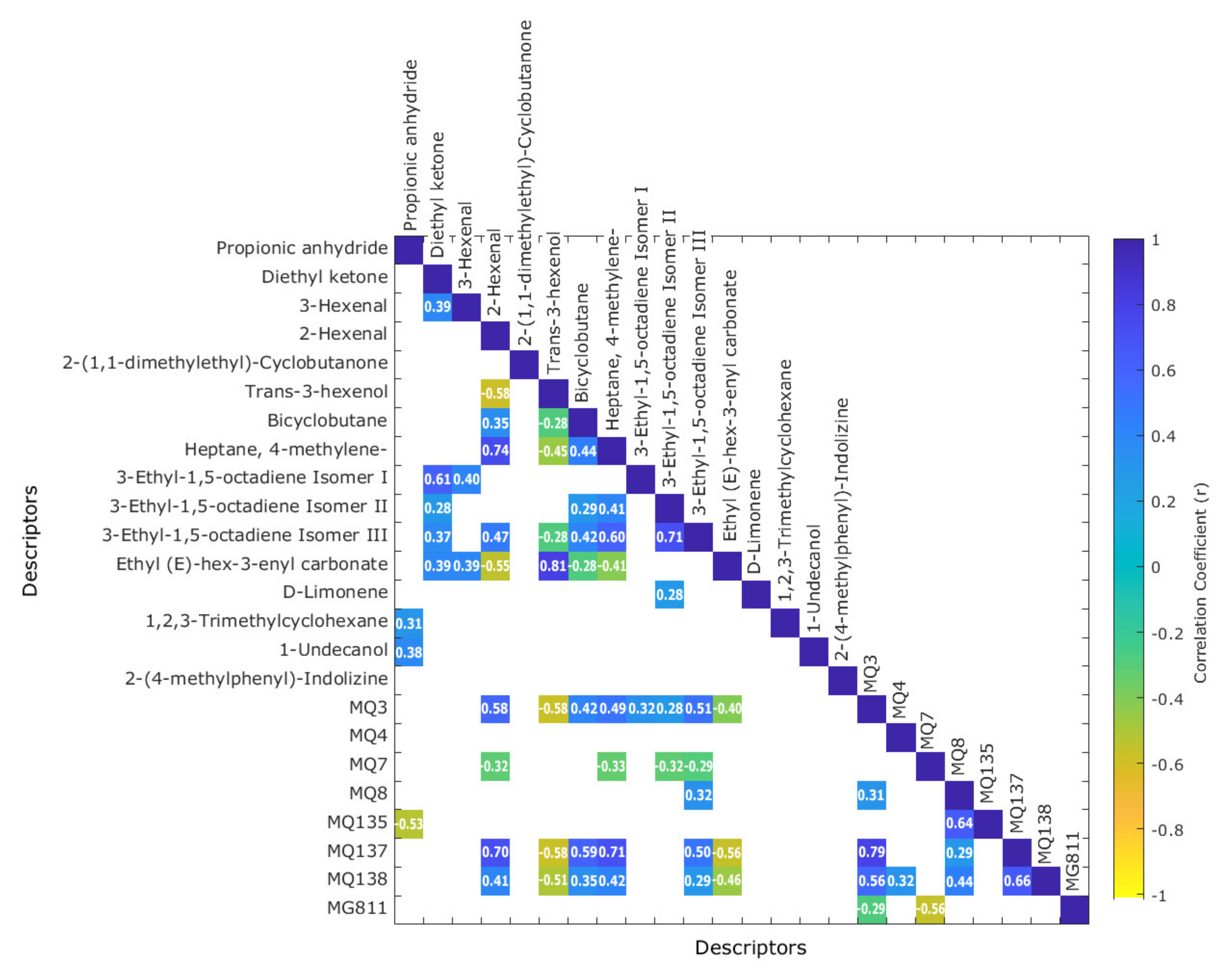
3.4. Correlations between Volatile Aromatic Compounds (GC-MS) and E-Nose Gas Sensors
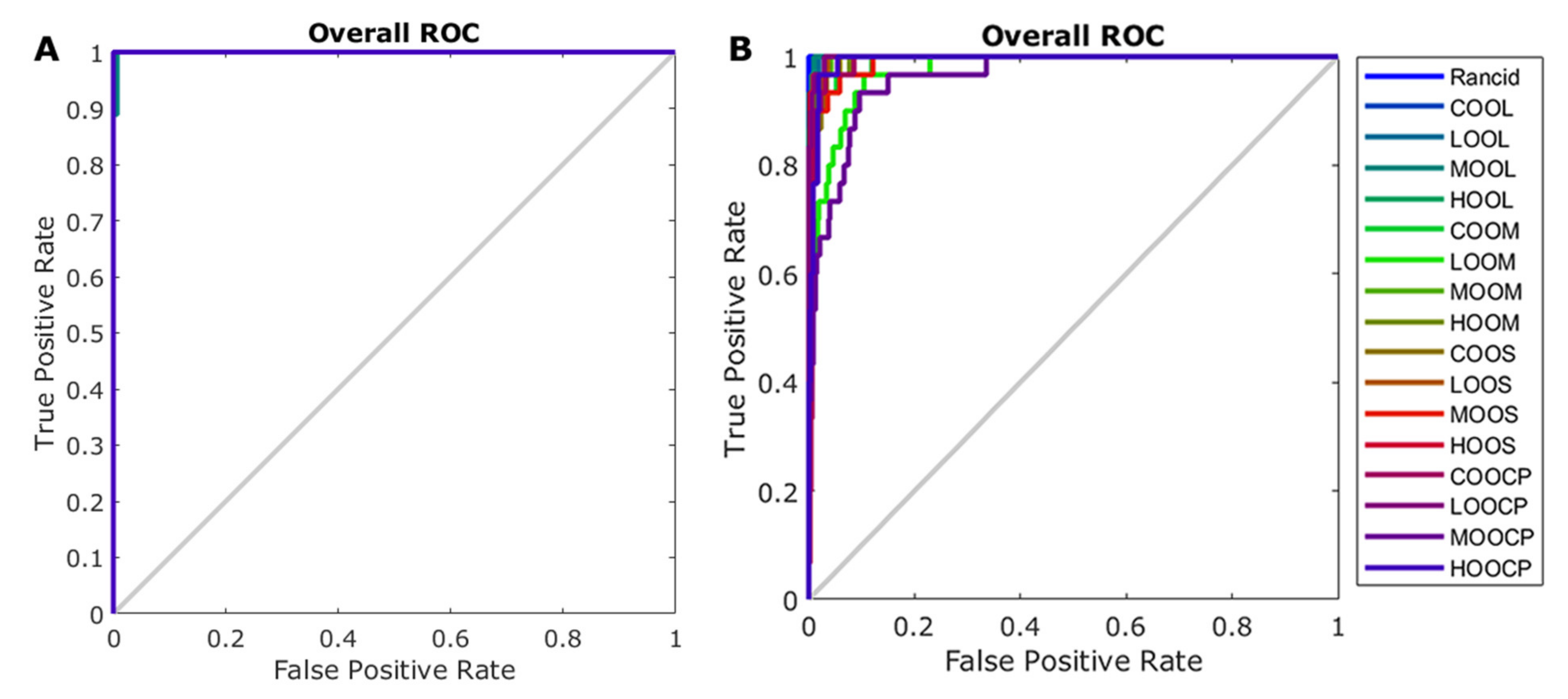
3.5. Machine Learning Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbandeh, M. Consumption of Olive Oil Worldwide from 2012/13 to 2020/21. Available online: https://www-statista-com.eu1.proxy.openathens.net/statistics/940491/olive-oil-consumption-worldwide/#statisticContainer (accessed on 8 February 2022).
- Teres, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.; Poiana, M. Packaging and storage of olive oil. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; InTechOpen: London, UK, 2012; pp. 201–222. [Google Scholar]
- Savarese, M.; Caporaso, N.; Parisini, C.; Paduano, A.; De Marco, E.; Sacchi, R. Application of an electronic nose for the evaluation of rancidity and shelf life in virgin olive oil. In Proceedings of the Electronic International Interdisciplinary Conference, Virtual, 2–6 September 2013; pp. 361–366. [Google Scholar]
- Harwood, J.; Aparicio, R. Handbook of Olive Oil: Analysis and Properties; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Gámbaro, A.; Ellis, A.C.; Raggio, L. Virgin olive oil acceptability in emerging olive oil-producing countries. Food Nutr. Sci. 2013, 4, 37230. [Google Scholar] [CrossRef][Green Version]
- Aparicio, R.; Morales, M.T.; García-González, D.L. Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Morales, M.; Rios, J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Barthel, G.; Grosch, W. Peroxide value determination—Comparison of some methods. J. Am. Oil Chem. Soc. 1974, 51, 540–544. [Google Scholar] [CrossRef]
- Morales, M.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Christy, A.A.; Kasemsumran, S.; Du, Y.; Ozaki, Y. The detection and quantification of adulteration in olive oil by near-infrared spectroscopy and chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Milinovic, J.; Garcia, R.; Rato, A.E.; Cabrita, M.J. Rapid Assessment of Monovarietal Portuguese Extra Virgin Olive Oil's (EVOO's) Fatty Acids by Fourier-Transform Near-Infrared Spectroscopy (FT-NIRS). Eur. J. Lipid Sci. Technol. 2019, 121, 1800392. [Google Scholar] [CrossRef]
- Lerma-García, M.; Cerretani, L.; Cevoli, C.; Simó-Alfonso, E.; Bendini, A.; Toschi, T.G. Use of electronic nose to determine defect percentage in oils. Comparison with sensory panel results. Sens. Actuators B Chem. 2010, 147, 283–289. [Google Scholar] [CrossRef]
- Cano Marchal, P.; Sanmartin, C.; Satorres Martínez, S.; Gómez Ortega, J.; Mencarelli, F.; Gámez García, J. Prediction of fruity aroma intensity and defect presence in virgin olive oil using an electronic nose. Sensors 2021, 21, 2298. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F. Assessment of beer quality based on foamability and chemical composition using computer vision algorithms, near infrared spectroscopy and machine learning algorithms. J. Sci. Food Agric. 2018, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a Low-Cost Electronic Nose and Machine Learning Modelling to Assess Coffee Aroma Profile and Intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Torrico, D.; Dunshea, F.; Fuentes, S. Development of Artificial Neural Network Models to Assess Beer Acceptability Based on Sensory Properties Using a Robotic Pourer: A Comparative Model Approach to Achieve an Artificial Intelligence System. Beverages 2019, 5, 33. [Google Scholar] [CrossRef]
- Martins, N.; Jiménez-Morillo, N.T.; Freitas, F.; Garcia, R.; Da Silva, M.G.; Cabrita, M.J. Revisiting 3D van Krevelen diagrams as a tool for the visualization of volatile profile of varietal olive oils from Alentejo region, Portugal. Talanta 2020, 207, 120276. [Google Scholar] [CrossRef] [PubMed]
- Kaftan, A.; Elmaci, Y. Aroma characterization of virgin olive oil from two Turkish olive varieties by SPME/GC/MS. Int. J. Food Prop. 2011, 14, 1160–1169. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Dynamic headspace/GC–MS to control the aroma fingerprint of extra-virgin olive oil from the same and different olive varieties. Food Control. 2012, 25, 684–695. [Google Scholar] [CrossRef]
- García-Vico, L.; Belaj, A.; Sánchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile compound profiling by HS-SPME/GC-MS-FID of a core olive cultivar collection as a tool for aroma improvement of virgin olive oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- The Good Scents Company. The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/data/rw1038291.html (accessed on 3 September 2021).
- Da Costa, J.R.O.; Dal Bosco, S.M.; Ramos, R.C.d.S.; Machado, I.C.K.; Garavaglia, J.; Villasclaras, S.S. Determination of volatile compounds responsible for sensory characteristics from Brazilian extra virgin olive oil using HS-SPME/GC-MS direct method. J. Food Sci. 2020, 85, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Ciurczak, E.W.; Igne, B.; Workman, J., Jr.; Burns, D.A. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Borghi, F.T.; Santos, P.C.; Santos, F.D.; Nascimento, M.H.; Correa, T.; Cesconetto, M.; Pires, A.A.; Ribeiro, A.V.; Lacerda, V., Jr.; Romao, W. Quantification and classification of vegetable oils in extra virgin olive oil samples using a portable near-infrared spectrometer associated with chemometrics. Microchem. J. 2020, 159, 105544. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cayuela, J.A.; García, J.F. Nondestructive measurement of squalene in olive oil by near infrared spectroscopy. LWT 2018, 88, 103–108. [Google Scholar] [CrossRef]
- Pineda, M.; Rojas, M.; Gálvez-Valdivieso, G.; Aguilar, M. The origin of aliphatic hydrocarbons in olive oil. J. Sci. Food Agric. 2017, 97, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M. The effect of cultivar and harvest season on the n-alkane and the n-alkene composition of virgin olive oil. Eur. Food Res. Technol. 2021, 247, 25–36. [Google Scholar] [CrossRef]
- Moret, S.; Populin, T.; Conte, L.S. Mineral paraffins in olives and olive oils. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 499–506. [Google Scholar]
- Fountoulakis, M.; Drakopoulou, S.; Terzakis, S.; Georgaki, E.; Manios, T. Potential for methane production from typical Mediterranean agro-industrial by-products. Biomass Bioenergy 2008, 32, 155–161. [Google Scholar] [CrossRef]
- Del Alamo, R.R.; Fregapane, G.; Aranda, F.; Gómez-Alonso, S.; Salvador, M. Sterol and alcohol composition of Cornicabra virgin olive oil: The campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem. 2004, 84, 533–537. [Google Scholar] [CrossRef]

| Volatile Aromatic Compound | Functional Group | Aroma * |
|---|---|---|
| Propionic anhydride | Anhydride | Pungent |
| Diethyl ketone | Ketone | Ethereal/Acetone |
| 3-Hexenal | Aldehyde | Green/Leafy/Apple/Melon |
| 2-Hexenal | Aldehyde | Green/Almond/Leafy/Apple/Plum |
| 2-(1,1-dimethylethyl)-Cyclobutanone | Cyclic Ketone | NR |
| Trans-3-hexenol | Alcohol | Green/Leafy/Floral/Oily/Earthy |
| Bicyclobutane | Cycloalkane | NR |
| Heptane, 4-methylene- | Hydrocarbon/Alkene | NR |
| 3-Ethyl-1,5-octadiene Isomer I | Hydrocarbons/Alkadiene | Musty |
| 3-Ethyl-1,5-octadiene Isomer II | Hydrocarbons/Alkadiene | Musty |
| 3-Ethyl-1,5-octadiene Isomer III | Hydrocarbons/Alkadiene | Musty |
| Ethyl (E)-hex-3-enyl carbonate | Carbonate ester | NR |
| D-Limonene | Monoterpene | Citrus/Orange/Fresh/Sweet |
| 1,2,3-Trimethylcyclohexane | Cycloalkane | NR |
| 1-Undecanol | Alcohol | Waxy/Fresh/Rose/Soapy/Citrus |
| 2-(4-methylphenyl)-Indolizine | Heterocyclic aromatic | NR |
| Stage | Samples | Accuracy | Error | Performance (Model 1: MSE; Model 2: Cross-Entropy) |
|---|---|---|---|---|
| Model 1 Inputs: Near-infrared absorbance values (Classification) | ||||
| Training | 107 | 100% | 0.0% | <0.01 |
| Testing | 46 | 91.3% | 8.7% | 0.01 |
| Overall | 153 | 97.4% | 2.6% | - |
| Model 2 Inputs: electronic nose voltage values (Classification) | ||||
| Training | 356 | 89.0% | 11.0% | 0.02 |
| Validation | 77 | 79.2% | 20.8% | 0.04 |
| Testing | 77 | 81.8% | 18.2% | 0.04 |
| Overall | 510 | 86.5% | 13.5% | - |
| Stage | Samples | Observations | Correlation Coefficient (R) | Slope | Performance (MSE) |
|---|---|---|---|---|---|
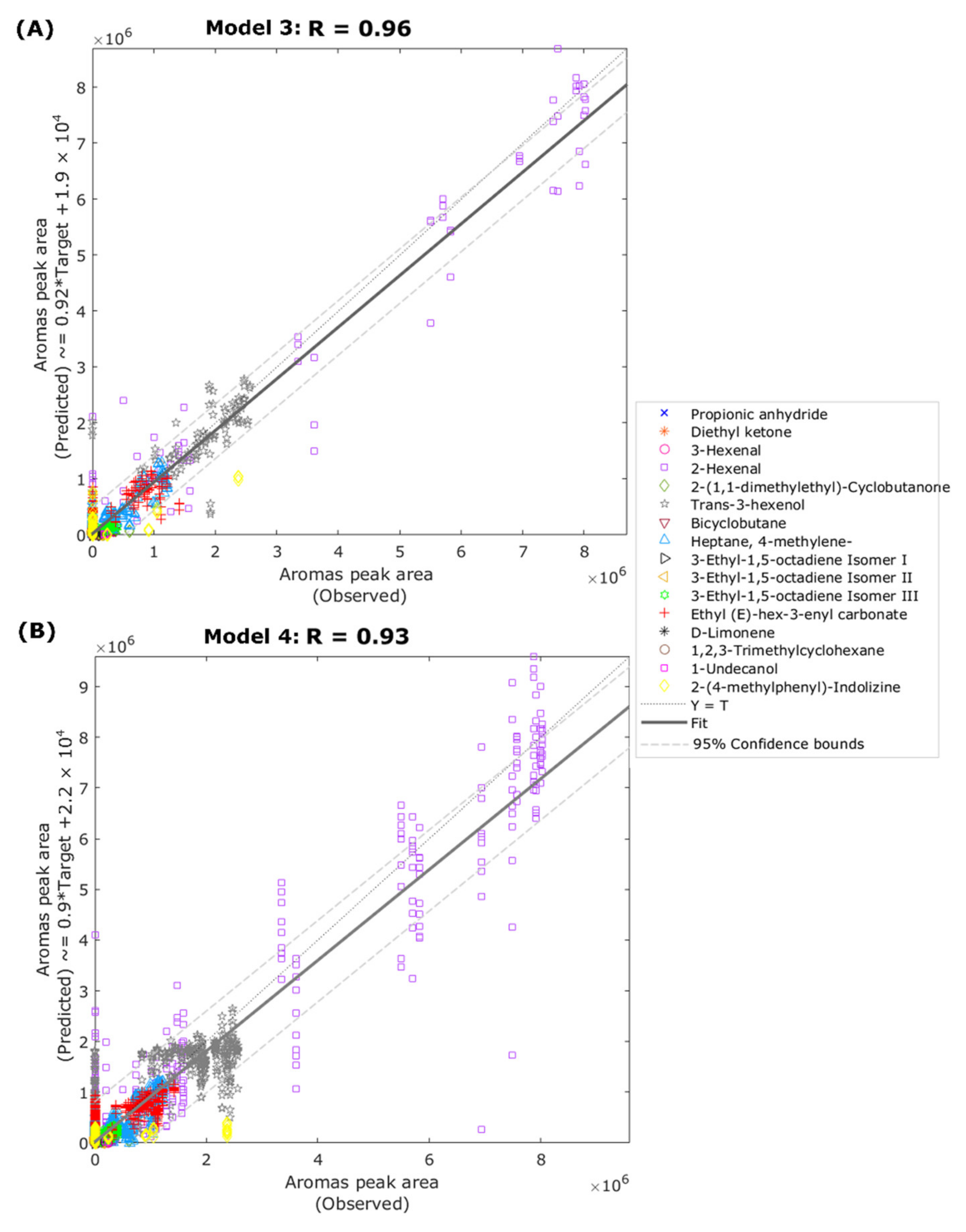
| Model 3 Inputs: Near-infrared absorbance values (Regression) | |||||
| Training | 107 | 1712 | 0.98 | 0.96 | 2.29 × 1010 |
| Validation | 23 | 368 | 0.91 | 0.89 | 13.83 × 1010 |
| Testing | 23 | 368 | 0.94 | 0.84 | 12.69 × 1010 |
| Overall | 153 | 2448 | 0.96 | 0.92 | - |
| Model 4 Inputs: electronic nose voltage values (Regression) | |||||
| Training | 357 | 5712 | 0.95 | 0.90 | 7.45 × 1010 |
| Testing | 153 | 2448 | 0.90 | 0.88 | 16.98 × 1010 |
| Overall | 510 | 8160 | 0.93 | 0.90 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Viejo, C.; Fuentes, S. Digital Detection of Olive Oil Rancidity Levels and Aroma Profiles Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Machine Learning Modelling. Chemosensors 2022, 10, 159. https://doi.org/10.3390/chemosensors10050159
Gonzalez Viejo C, Fuentes S. Digital Detection of Olive Oil Rancidity Levels and Aroma Profiles Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Machine Learning Modelling. Chemosensors. 2022; 10(5):159. https://doi.org/10.3390/chemosensors10050159
Chicago/Turabian StyleGonzalez Viejo, Claudia, and Sigfredo Fuentes. 2022. "Digital Detection of Olive Oil Rancidity Levels and Aroma Profiles Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Machine Learning Modelling" Chemosensors 10, no. 5: 159. https://doi.org/10.3390/chemosensors10050159
APA StyleGonzalez Viejo, C., & Fuentes, S. (2022). Digital Detection of Olive Oil Rancidity Levels and Aroma Profiles Using Near-Infrared Spectroscopy, a Low-Cost Electronic Nose and Machine Learning Modelling. Chemosensors, 10(5), 159. https://doi.org/10.3390/chemosensors10050159







