Inkjet Printing: A Viable Technology for Biosensor Fabrication
Abstract
1. Introduction
2. Inkjet Printing
3. Inkjet-Printed Biosensors
3.1. Virus Sensors
3.2. Enzymatic Biosensor
3.3. Non-Enzymatic Biosensors
4. Inkjet Printing Technology and the Market Competition
5. Challenges and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring In Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Cammann, K. Bio-sensors based on ion-selective electrodes. Fresenius’ Z. Für Anal. Chem. 1977, 287, 1–9. [Google Scholar] [CrossRef]
- Pandey, C.M.; Malhotra, B.D. Biosensors: Fundamentals and Applications; Sensors and Actuators B, Smithers Rapra Shawbury: Shrewsbury, UK, 2019; Volume 4, pp. 197–206. [Google Scholar]
- Reddy, S.M.; Higson, S.P.J.; Vadgama, P.M. Enzyme and other biosensors: Evolution of a technology. Eng. Sci. Educ. J. 1994, 3, 41–48. [Google Scholar] [CrossRef]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.Z.; Latif, U.; Feroz, S. Gravimetric viral diagnostics: QCM based biosensors for early detection of viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Oplatowska-Stachowiak, M.; Campbell, K. Evaluation of Mass Sensitive Micro-Array biosensors for their feasibility in multiplex detection of low molecular weight toxins using mycotoxins as model compounds. Talanta 2021, 222, 121521. [Google Scholar] [CrossRef]
- Mack, N.H.; Wackerly, J.W.; Malyarchuk, V.; Rogers, J.A.; Moore, J.S.; Nuzzo, R.G. Optical transduction of chemical forces. Nano Lett. 2007, 7, 733–737. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V. Recent advances in synthetic biology–enabled and natural whole-cell optical biosensing of heavy metals. Anal. Bioanal. Chem. 2021, 413, 73–82. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent developments in nanotechnology-based printing electrode systems for electrochemical sensors. Talanta 2021, 255, 121951. [Google Scholar] [CrossRef]
- Elbadawi, M.; Ong, J.J.; Pollard, T.D.; Gaisford, S.; Basit, A.W. Additive Manufacturable Materials for Electrochemical Biosensor Electrodes. Adv. Funct. Mater. 2021, 31, 2006407. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS electrochemical instrumentation for biosensor microsystems: A review. Sensors 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Putnin, T.; Jumpathong, W.; Laocharoensuk, R.; Jakmunee, J.; Ounnunkad, K. A sensitive electrochemical immunosensor based on poly(2-aminobenzylamine) film modified screen-printed carbon electrode for label-free detection of human immunoglobulin G. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1042–1051. [Google Scholar] [CrossRef]
- Hussin, H.; Soin, N.; Wan Muhamad Hatta, S.F.; Md Rezali, F.A.; Abdul Wahab, Y. Review—Recent Progress in the Diversity of Inkjet-Printed Flexible Sensor Structures in Biomedical Engineering Applications. J. Electrochem. Soc. 2021, 168, 077508. [Google Scholar] [CrossRef]
- Krishnan, A.; Hamilton, J.P.; Alqahtani, S.A.; Woreta, T.A. A narrative review of coronavirus disease 2019 (COVID-19): Clinical, epidemiological characteristics, and systemic manifestations. Intern. Emerg. Med. 2021, 16, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Abbas, N.; Shin, S. A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021, 177, 113005. [Google Scholar] [CrossRef]
- Fani, M.; Zandi, M.; Soltani, S.; Abbasi, S. Future developments in biosensors for field-ready SARS-CoV-2 virus diagnostics. Biotechnol. Appl. Biochem. 2021, 68, 695–699. [Google Scholar] [CrossRef]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2021, 22, 117–1189. [Google Scholar] [CrossRef]
- Nuutila, J.; Hohenthal, U.; Oksi, J.; Jalava-Karvinen, P. Rapid detection of bacterial infection using a novel single-tube, four-colour flow cytometric method: Comparison with PCT and CRP. EBioMedicine 2021, 74, 103724. [Google Scholar] [CrossRef]
- Munteanu, F.D.; Titoiu, A.M.; Marty, J.L.; Vasilescu, A. Detection of antibiotics and evaluation of antibacterial activity with screen-printed electrodes. Sensors 2018, 18, 901. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, U.; Ishfaq, U.; Qamar, M.; Azam, M.; Zahra Naqvi, W.; Rafeeq, H.; Noor, Z. Review on Biological Techniques, Microbial Food Testing Approaches, Biosensors Principles and Applications. Sch. Bull. 2021, 7, 82–86. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS ES T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Ross, I.M. The invention of the transistor. Proc. IEEE 1998, 86, 7–28. [Google Scholar] [CrossRef]
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Cotte, S.; Baraket, A.; Bessueille, F.; Gout, S.; Yaakoubi, N.; Leonard, D.; Errachid, A. Fabrication of Microelectrodes Using Original “Soft Lithography” Processes; New Sensors and Processing Chain, Wiley Online Library: Hoboken, NJ, USA, 2014; ISBN 9781119050612. [Google Scholar]
- Kokkinos, C.; Economou, A. Recent advances in voltammetric, amperometric and ion-selective (bio)sensors fabricated by microengineering manufacturing approaches. Curr. Opin. Electrochem. 2020, 23, 21–25. [Google Scholar] [CrossRef]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater. 2020, 32, 1905279. [Google Scholar] [CrossRef]
- Palavesam, N.; Marin, S.; Hemmetzberger, D.; Landesberger, C.; Bock, K.; Kutter, C. Roll-to-roll processing of film substrates for hybrid integrated flexible electronics. Flex. Print. Electron. 2018, 3, 014002. [Google Scholar] [CrossRef]
- Lin, S.; Bai, X.; Wang, H.; Wang, H.; Song, J.; Huang, K.; Wang, C.; Wang, N.; Li, B.; Lei, M.; et al. Roll-to-Roll Production of Transparent Silver-Nanofiber-Network Electrodes for Flexible Electrochromic Smart Windows. Adv. Mater. 2017, 29, 1703238. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Lee, W.; Choi, Y.; Sun, J.; Bak, J.; Noh, J.; Subramanian, V.; Azuma, Y.; Majima, Y.; Cho, G. Scalability of carbon-nanotube-based thin film transistors for flexible electronic devices manufactured using an all roll-To-roll gravure printing system. Sci. Rep. 2015, 5, 14459. [Google Scholar] [CrossRef] [PubMed]
- Bucella, S.G.; Luzio, A.; Gann, E.; Thomsen, L.; McNeill, C.R.; Pace, G.; Perinot, A.; Chen, Z.; Facchetti, A.; Caironi, M. Macroscopic and high-throughput printing of aligned nanostructured polymer semiconductors for MHz large-area electronics. Nat. Commun. 2015, 6, 8394. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Wu, K.Y. Health Risk Assessment of Photoresists Used in an Optoelectronic Semiconductor Factory. Risk Anal. 2019, 39, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Shin, J.A.; Park, H.H.; Yi, G.Y.; Chung, K.J.; Park, H.D.; Kim, K.B.; Lee, S. Exposure to volatile organic compounds and possibility of exposure to by-product volatile organic compounds in photolithography processes in semiconductor manufacturing factories. Saf. Health Work 2011, 2, 210–217. [Google Scholar] [CrossRef]
- Eom, Y.S.; Hong, J.H.; Lee, S.J.; Lee, E.J.; Cha, J.S.; Lee, D.G.; Bang, S.A. Emission factors of air toxics from semiconductor manufacturing in Korea. J. Air Waste Manag. Assoc. 2006, 56, 1518–1524. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H.; Paek, D. The health impacts of semiconductor production: An epidemiologic review. Int. J. Occup. Environ. Health 2014, 20, 95–114. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–689. [Google Scholar] [CrossRef]
- Ursan, I.; Chiu, L.; Pierce, A. Three-dimensional drug printing: A structured review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.N.; Schwartz, D.E.; Lavery, L.L.; Whiting, G.L.; Russo, B.; Krusor, B.; Veres, J.; Bröms, P.; Herlogsson, L.; Alam, N.; et al. Scalable printed electronics: An organic decoder addressing ferroelectric non-volatile memory. Sci. Rep. 2012, 2, 585. [Google Scholar] [CrossRef] [PubMed]
- Sekine, C.; Tsubata, Y.; Yamada, T.; Kitano, M.; Doi, S. Recent progress of high performance polymer OLED and OPV materials for organic printed electronics. Sci. Technol. Adv. Mater. 2014, 15, 034203. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.M.; Khan, Y.; Ostfeld, A.E.; Pandya, S.; Abraham, S.; Arias, A.C. Identifying orthogonal solvents for solution processed organic transistors. Org. Electron. 2016, 30, 18–29. [Google Scholar] [CrossRef]
- Chen, S.-P.; Chiu, H.-L.; Wang, P.-H.; Liao, Y.-C. Inkjet Printed Conductive Tracks for Printed Electronics. ECS J. Solid State Sci. Technol. 2015, 4, P3026–P3033. [Google Scholar] [CrossRef]
- Kimura, J.; Kawana, Y.; Kuriyama, T. An immobilized enzyme membrane fabrication method using an ink jet nozzle. Biosensors 1989, 4, 41–52. [Google Scholar] [CrossRef]
- Sneck, A.; Ailas, H.; Gao, F.; Leppäniemi, J. Reverse-Offset Printing of Polymer Resist Ink for Micrometer-Level Patterning of Metal and Metal-Oxide Layers. ACS Appl. Mater. Interfaces 2021, 13, 41782–41792. [Google Scholar] [CrossRef]
- Goh, G.L.; Zhang, H.; Chong, T.H.; Yeong, W.Y. 3D Printing of Multilayered and Multimaterial Electronics: A Review. Adv. Electron. Mater. 2021, 7, 21004455. [Google Scholar] [CrossRef]
- Goh, G.L.; Agarwala, S.; Yeong, W.Y. Aerosol-Jet-Printed Preferentially Aligned Carbon Nanotube Twin-Lines for Printed Electronics. ACS Appl. Mater. Interfaces 2019, 11, 43719–43730. [Google Scholar] [CrossRef]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning proteins and cells using soft lithography. Biomater. Silver Jubil. Compend. 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Falconnet, D.; Koenig, A.; Assi, F.; Textor, M. A combined photolithographic and molecular-assembly approach to produce functional micropatterns for applications in the biosciences. Adv. Funct. Mater. 2004, 14, 749–756. [Google Scholar] [CrossRef]
- Park, T.H.; Shuler, M.L. Integration of cell culture and microfabrication technology. Biotechnol. Prog. 2003, 19, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Piner, R.D.; Zhu, J.; Xu, F.; Hong, S.; Mirkin, C.A. “Dip-pen” nanolithography. Science 1999, 283, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.L.; Martin, R.; Hong, S.; Cronin-Golomb, M.; Mirkin, C.A.; Kaplan, D.L. Surface organization and nanopatterning of collagen by dip-pen nanolithography. Proc. Natl. Acad. Sci. USA 2001, 98, 13660–13664. [Google Scholar] [CrossRef]
- Rauter, H.; Matyushin, V.; Alguel, Y.; Pittner, F.; Schalkhammer, T. Nanotechnology for smart polymer optical devices. Macromol. Symp. 2004, 217, 109–134. [Google Scholar] [CrossRef]
- Kampfrath, G.; Hintsche, R. Plasma-Polymerized Thin Films for Enzyme Immobilization in Biosensors. Anal. Lett. 1989, 22, 2423–2431. [Google Scholar] [CrossRef]
- Hiratsuka, A.; Kojima, K.I.; Muguruma, H.; Lee, K.H.; Suzuki, H.; Karube, I. Electron transfer mediator micro-biosensor fabrication by organic plasma process. Biosens. Bioelectron. 2005, 21, 957–964. [Google Scholar] [CrossRef]
- Muguruma, H.; Karube, I. Plasma-polymerized films for biosensors. TrAC—Trends Anal. Chem. 1999, 18, 62–68. [Google Scholar] [CrossRef]
- Stempien, Z.; Kozicki, M.; Pawlak, R.; Korzeniewska, E.; Owczarek, G.; Poscik, A.; Sajna, D. Ammonia gas sensors ink-jet printed on textile substrates. In Proceedings of the IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Gonzalez-Macia, L.; Morrin, A.; Smyth, M.R.; Killard, A.J. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analyst 2010, 135, 845–867. [Google Scholar] [CrossRef]
- Schwartz, P.V. Meniscus force nanografting: Nanoscopic patterning of DNA. Langmuir 2001, 17, 5971–5977. [Google Scholar] [CrossRef]
- Wadu-Mesthrige, K.; Amro, N.A.; Garno, J.C.; Xu, S.; Liu, G.Y. Fabrication of nanometer-sized protein patterns using atomic force microscopy and selective immobilization. Biophys. J. 2001, 80, 1891–1899. [Google Scholar] [CrossRef]
- Park, J.U.; Lee, J.H.; Paik, U.; Lu, Y.; Rogers, J.A. Nanoscale patterns of oligonucleotides formed by electrohydrodynamic jet printing with applications in biosensing and nanomaterials assembly. Nano Lett. 2008, 8, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kou, R.; Shang, Z.; Weng, Z.; Zhu, C.; Zhong, Y. Corona-Enabled Electrostatic Printing for Ultra-fast Manufacturing of Binder-Free Multifunctional E-Skins. ACS Appl. Mater. Interfaces 2021, 13, 45966–45976. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Derby, B.; Wong, J. Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 2021, 13, 035006. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Ke, D.; Wang, D.; Zhang, X.-E. In situ graphene-modified carbon microelectrode array biosensor for biofilm impedance analysis. Electrochim. Acta 2021, 403, 139570. [Google Scholar] [CrossRef]
- Baracu, A.M.; Dinu Gugoasa, L.A. Review—Recent Advances in Microfabrication, Design and Applications of Amperometric Sensors and Biosensors. J. Electrochem. Soc. 2021, 168, 037503. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Feng, J.; Chu, Z.; Jin, W. Screen-printing of nanocube-based flexible microchips for the precise biosensing of ethanol during fermentation. AlChE J. 2021, 67, e17142. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, Q.; Xiao, J.; Zheng, M.; Zhang, D.; Yang, J. An inkjet-printed smartphone-supported electrochemical biosensor system for reagentless point-of-care analyte detection. Sens. Actuators B Chem. 2021, 346, 130447. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors—A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Reddy, A.S.G.; Narakathu, B.B.; Atashbar, M.Z.; Rebros, M.; Rebrosova, E.; Joyce, M.K. Gravure printed electrochemical biosensor. Procedia Eng. 2011, 25, 956–959. [Google Scholar] [CrossRef][Green Version]
- Nagamine, K.; Tokito, S. Flexible and printed biosensors based on organic TFT devices. Chem. Gas. Biosens. Internet Things Relat. Appl. 2019, 291–306. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Lloyd, J.S.; Samavat, S.; Deganello, D.; Stanton, R.J.; Teng, K.S. Nanotextured Surface on Flexographic Printed ZnO Thin Films for Low-Cost Non-Faradaic Biosensors. ACS Appl. Mater. Interfaces 2016, 8, 33802–33810. [Google Scholar] [CrossRef] [PubMed]
- Smolyarova, T.E.; Shanidze, L.V.; Lukyanenko, A.V.; Baron, F.A.; Krasitskaya, V.V.; Kichkailo, A.S.; Tarasov, A.S.; Volkov, N. Talanta Protein biosensor based on Schottky barrier nanowire field effect transistor. Talanta 2022, 239, 123092. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jasim, I.; Liu, T.; Huang, J.; Kinzel, E.; Almasri, M. Off-axis microsphere photolithography patterned nanohole array and other structures on an optical fiber tip for glucose sensing. RSC Adv. 2021, 11, 25912–25920. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Sheini, A.; Hashemi, P.; Hajian, A.; Bagheri, H. Disposable paper-based biosensors for the point-of-care detection of hazardous contaminations—A review. Biosensors 2021, 11, 316. [Google Scholar] [CrossRef]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab Chip 2015, 15, 2538. [Google Scholar] [CrossRef]
- Ricci, F.; Amine, A.; Palleschi, G.; Moscone, D. Prussian Blue based screen printed biosensors with improved characteristics of long-term lifetime and pH stability. Biosens. Bioelectron. 2002, 18, 165–174. [Google Scholar] [CrossRef]
- Sánchez, S.; Pumera, M.; Cabruja, E.; Fàbregas, E. Carbon nanotube/polysulfone composite screen-printed electrochemical enzyme biosensors. Analyst 2007, 132, 142–147. [Google Scholar] [CrossRef]
- Crouch, E.; Cowell, D.C.; Hoskins, S.; Pittson, R.W.; Hart, J.P. A novel, disposable, screen-printed amperometric biosensor for glucose in serum fabricated using a water-based carbon ink. Biosens. Bioelectron. 2005, 21, 712–718. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, Z.; Peng, J.; Jin, W. Screen-printed biosensor chips with Prussian blue nanocubes for the detection of physiological analytes. Sens. Actuators B Chem. 2016, 228, 679–687. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Parupudi, T.; Zhao, X.; Yazdi, I.K.; Dokmeci, M.R.; Tamayol, A.; Khademhosseini, A.; Ziaie, B. A low-cost flexible pH sensor array for wound assessment. Sens. Actuators B Chem. 2016, 229, 609–617. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Wu, J.; Wei, Q.; Chen, C.; Bao, N.; Yu, C.; Gu, H. An electrochemical biosensor based on multi-wall carbon nanotube–modified screen-printed electrode immobilized by uricase for the detection of salivary uric acid. Anal. Bioanal. Chem. 2020, 412, 7275–7283. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-based electrochemical potentiostat detection system using pedot: Pss/chitosan/graphene modified screen-printed electrodes for dopamine detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.N.; Lin, Y.L.; Yu, K.J.; Chiou, Y.E.; Leung, W.H.; Weng, W.H. An effective sars-cov-2 electrochemical biosensor with modifiable dual probes using a modified screen-printed carbon electrode. Micromachines 2021, 12, 1171. [Google Scholar] [CrossRef]
- Eissa, S.; Al-Kattan, K.; Zourob, M. Combination of Carbon Nanofiber-Based Electrochemical Biosensor and Cotton Fiber: A Device for the Detection of the Middle-East Respiratory Syndrome Coronavirus. ACS Omega 2021, 6, 32072. [Google Scholar] [CrossRef]
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, T.L.; Le Dang, T.T.; Matteo, T.; Nguyen, T.H.H. A label-free electrochemical biosensor based on screen-printed electrodes modified with gold nanoparticles for quick detection of bacterial pathogens. Mater. Today Commun. 2021, 26, 101726. [Google Scholar] [CrossRef]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef]
- Crowley, K.; O’Malley, E.; Morrin, A.; Smyth, M.R.; Killard, A.J. An aqueous ammonia sensor based on an inkjet-printed polyaniline nanoparticle-modified electrode. Analyst 2008, 133, 391–399. [Google Scholar] [CrossRef]
- Abe, K.; Kotera, K.; Suzuki, K.; Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal. Chem. 2010, 398, 885–893. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Choi, J.S.; Kim, S.; Yoo, B.; Kim, Y.S.; Park, K.; Cho, Y.W. A glucose sensor fabricated by piezoelectric inkjet printing of conducting polymers and bienzymes. Anal. Sci. 2011, 27, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Tomikawa, S.; Suzuki, K.; Citterio, D. Inkjet printing: An integrated and green chemical approach to microfluidic paper-based analytical devices. RSC Adv. 2013, 3, 9258. [Google Scholar] [CrossRef]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Morrin, A.; Shepherd, R.; Crowley, K.; Killard, A.J.; Innis, P.C.; Wallace, G.G. Wholly printed polypyrrole nanoparticle-based biosensors on flexible substrate. J. Mater. Chem. B 2014, 2, 793. [Google Scholar] [CrossRef]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, Z.; Liu, Z.; Weigum, S.E.; Yu, Q.; Chen, M.Y. Inkjet-Printed Flexible Biosensor Based on Graphene Field Effect Transistor. IEEE Sens. J. 2016, 16, 8359–8364. [Google Scholar] [CrossRef]
- Adly, N.; Feng, L.; Krause, K.J.; Mayer, D.; Yakushenko, A.; Offenhäusser, A.; Wolfrum, B. Flexible Microgap Electrodes by Direct Inkjet Printing for Biosensing Application. Adv. Biosyst. 2017, 1, 1600016. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nanostructured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef]
- Martínez-Domingo, C.; Conti, S.; de la Escosura-Muñiz, A.; Terés, L.; Merkoçi, A.; Ramon, E. Organic-based field effect transistors for protein detection fabricated by inkjet-printing. Org. Electron. 2020, 84, 105794. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M.; Calabria, D.; Maddalena Calabretta, M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.M.; Mendonça, D.M.H.; Silva, W.P.; Silva, M.N.T.; Nossol, E.; da Silva, R.A.B.; Richter, E.M.; Muñoz, R.A.A. 3D printing for electroanalysis: From multiuse electrochemical cells to sensors. Anal. Chim. Acta 2018, 1033, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.H.; Chua, C.K.; Pumera, M. DNA biosensing with 3D printing technology. Analyst 2017, 142, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.L.; Katic, V.; Loureiro, H.C.; dos Santos, M.F.; dos Santos, D.P.; Formiga, A.L.B.; Bonacin, J.A. Enhanced performance of 3D printed graphene electrodes after electrochemical pre-treatment: Role of exposed graphene sheets. Sens. Actuators B Chem. 2019, 218, 837–848. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Paschoal, C.W.A.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.; Kim, B.; Ko, S. Fabrication of Microfluidic Structure Based Biosensor Using Roll-to-Roll Gravure Printing. Int. J. Precis. Eng. Manuf. Green Technol. 2018, 5, 369–374. [Google Scholar] [CrossRef]
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250. [Google Scholar] [CrossRef]
- Benson, J.; Fung, C.M.; Lloyd, J.S.; Deganello, D.; Smith, N.A.; Teng, K.S. Direct patterning of gold nanoparticles using flexographic printing for biosensing applications. Nanoscale Res. Lett. 2015, 10, 3596. [Google Scholar] [CrossRef]
- Fung, C.M.; Lloyd, J.S.; Samavat, S.; Deganello, D.; Teng, K.S. Facile fabrication of electrochemical ZnO nanowire glucose biosensor using roll to roll printing technique. Sens. Actuators B Chem. 2017, 247, 807–813. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Samavat, S.; Deganello, D.; Teng, K.S. Vertically aligned graphene prepared by photonic annealing for ultrasensitive biosensors. ACS Appl. Mater. Interfaces 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Pattani, V.P.; Li, C.; Desai, T.A.; Vu, T.Q. Microcontact printing of quantum dot bioconjugate arrays for localized capture and detection of biomolecules. Biomed. Microdevices 2008, 10, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Angeley, D. Fabrication of an optical-quality linear grating of immunoglobulin G proteins by microcontact printing and demonstration of potential biosensing applications. Opt. Eng. 2006, 45, 043402. [Google Scholar] [CrossRef]
- Salomon, S.; Leïchlé, T.; Dezest, D.; Seichepine, F.; Guillon, S.; Thibault, C.; Vieu, C.; Nicu, L. Arrays of nanoelectromechanical biosensors functionalized by microcontact printing. Nanotechnology 2012, 23, 495501. [Google Scholar] [CrossRef]
- Tsai, S.M.; Goshia, T.; Chen, Y.C.; Kagiri, A.; Sibal, A.; Chiu, M.H.; Gadre, A.; Tung, V.; Chin, W.C. High-throughput label-free microcontact printing graphene-based biosensor for valley fever. Colloids Surf. B Biointerfaces 2018, 170, 219–223. [Google Scholar] [CrossRef]
- Touloupakis, E.; Chatzipetrou, M.; Boutopoulos, C.; Gkouzou, A.; Zergioti, I. A polyphenol biosensor realized by laser printing technology. Sens. Actuators B Chem. 2014, 193, 301–305. [Google Scholar] [CrossRef]
- Touloupakis, E.; Boutopoulos, C.; Buonasera, K.; Zergioti, I.; Giardi, M.T. A photosynthetic biosensor with enhanced electron transfer generation realized by laser printing technology. Anal. Bioanal. Chem. 2012, 402, 3237–3244. [Google Scholar] [CrossRef]
- Duocastella, M.; Fernández-Pradas, J.M.; Morenza, J.L.; Zafra, D.; Serra, P. Novel laser printing technique for miniaturized biosensors preparation. Sens. Actuators B Chem. 2010, 145, 596–600. [Google Scholar] [CrossRef]
- Chatzipetrou, M.; Tsekenis, G.; Tsouti, V.; Chatzandroulis, S.; Zergioti, I. Biosensors by means of the laser induced forward transfer technique. Appl. Surf. Sci. 2013, 278, 250–254. [Google Scholar] [CrossRef]
- Tsekenis, G.; Filippidou, M.K.; Chatzipetrou, M.; Tsouti, V.; Zergioti, I.; Chatzandroulis, S. Heavy metal ion detection using a capacitive micromechanical biosensor array for environmental monitoring. Sens. Actuators B Chem. 2015, 208, 628–635. [Google Scholar] [CrossRef]
- Skotadis, E.; Voutyras, K.; Chatzipetrou, M.; Tsekenis, G.; Patsiouras, L.; Madianos, L.; Chatzandroulis, S.; Zergioti, I.; Tsoukalas, D. Label-free DNA biosensor based on resistance change of platinum nanoparticles assemblies. Biosens. Bioelectron. 2016, 81, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Dalman, Y. Fabrication of biosensors by bacterial printing on different carriers using a laser printer. Bioprinting 2020, 20, e00099. [Google Scholar] [CrossRef]
- Rayleigh, Lord On the instability of jets. Proc. Lond. Math. Soc. 1878, s1–10, 4–13. [CrossRef]
- Plog, J.; Jiang, Y.; Pan, Y.; Yarin, A.L. Electrostatically-assisted direct ink writing for additive manufacturing. Addit. Manuf. 2021, 39, 101644. [Google Scholar] [CrossRef]
- Elrod, S.A.; Hadimioglu, B.; Khuri-Yakub, B.T.; Rawson, E.G.; Richley, E.; Quate, C.F.; Mansour, N.N.; Lundgren, T.S. Nozzleless droplet formation with focused acoustic beams. J. Appl. Phys. 1989, 65, 3441. [Google Scholar] [CrossRef]
- Khan, A.; Rahman, K.; Ali, S.; Khan, S.; Wang, B.; Bermak, A. Fabrication of circuits by multi-nozzle electrohydrodynamic inkjet printing for soft wearable electronics. J. Mater. Res. 2021, 36, 3568–3578. [Google Scholar] [CrossRef]
- Mueller, U.; Nyarsik, L.; Horn, M.; Rauth, H.; Przewieslik, T.; Saenger, W.; Lehrach, H.; Eickhoff, H. Development of a technology for automation and miniaturization of protein crystallization. J. Biotechnol. 2001, 85, 7–14. [Google Scholar] [CrossRef]
- Parashkov, R.; Becker, E.; Riedl, T.; Johannes, H.H.; Kowalsky, W. Large area electronics using printing methods. Proc. IEEE 2005, 93, 1321–1329. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P.F.; Marrazza, G. Ink-jet printing for the fabrication of amperometric glucose biosensors. Anal. Chim. Acta 1992, 262, 13–17. [Google Scholar] [CrossRef]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Piana, C.; Bonazzi, S.; Ballarin, B.; Frascaro, D.; Fraleoni-Morgera, A.; Giuliani, S. Thermal inkjet technology for the microdeposition of biological molecules as a viable route for the realization of biosensors. Anal. Lett. 2004, 37, 1559–1570. [Google Scholar] [CrossRef]
- Wang, H.S.; Pan, Q.X.; Wang, G.X. A biosensor based on immobilization of horseradish peroxidase in chitosan matrix cross-linked with glyoxal for amperometric determination of hydrogen peroxide. Sensors 2005, 5, 266–276. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, G.M.; Markey, A.A.; Holloway, C.K. Protein damage in drop-on-demand printers. J. Am. Chem. Soc. 2004, 126, 16320–16321. [Google Scholar] [CrossRef]
- De Gans, B.J.; Schubert, U.S. Inkjet printing of polymer micro-arrays and libraries: Instrumentation, requirements, and perspectives. Macromol. Rapid Commun. 2003, 24, 659–666. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.Y.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar] [CrossRef]
- Beedasy, V.; Smith, P.J. Printed Electronics as Prepared by Inkjet Printing. Materials 2020, 13, 704. [Google Scholar] [CrossRef]
- Andresen, P.; Faubel, M.; Haeusler, D.; Kraft, G.; Luelf, H.W.; Skofronick, J.G. Characteristics of a piezoelectric pulsed nozzle beam. Rev. Sci. Instrum. 1985, 56, 2038. [Google Scholar] [CrossRef]
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet printed (bio)chemical sensing devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, M.; Liu, X.; Xu, S.; Du, T.; Xu, J.; Yang, Q.; Xu, Y.; Han, Y.; Li, T.; et al. Significance of serology testing to assist timely diagnosis of SARS-CoV-2 infections: Implication from a family cluster. Emerg. Microbes Infect. 2020, 9, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19)—StatPearls—NCBI Bookshelf; NCBI: Bethesda, MD, USA, 2020.
- Fathi-Hafshejani, P.; Azam, N.; Wang, L.; Kuroda, M.A.; Hamilton, M.C.; Hasim, S.; Mahjouri-Samani, M. Two-Dimensional-Material-Based Field-Effect Transistor Biosensor for Detecting COVID-19 Virus (SARS-CoV-2). ACS Nano 2021, 15, 11461–11469. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Singh, R.; Hong, S.; Jang, J. Label-free Detection of Influenza Viruses using a Reduced Graphene Oxide-based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 42771. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Z.; Xie, Z.; Luo, S.; Xie, L.; Huang, L.; Fan, Q.; Zhang, Y.; Wang, S.; Zeng, T. Silver nanoparticles coated graphene electrochemical sensor for the ultrasensitive analysis of avian influenza virus H7. Anal. Chim. Acta 2016, 913, 121–127. [Google Scholar] [CrossRef]
- Gowri, A.; Ashwin Kumar, N.; Suresh Anand, B.S. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19—A minireview. TrAC–Trends Anal. Chem. 2021, 137, 116205. [Google Scholar] [CrossRef]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed electrochemical biosensors: Opportunities and metrological challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. Npj Flex. Electron. 2018, 2, 30. [Google Scholar] [CrossRef]
- Sanguino, P.; Monteiro, T.; Marques, F.; Dias, C.J.; Igreja, R.; Franco, R. Interdigitated capacitive immunosensors with PVDF immobilization layers. IEEE Sens. J. 2014, 14, 1260–1265. [Google Scholar] [CrossRef]
- Tursunniyaz, M.; Andrews, J. Printed Capacitive Immunoassay for Detecting SARS-CoV-2 Viral Particles. In Proceedings of the IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 20–23 June 2021. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S. wen Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid Detection of SARS-CoV-2 Antigens Using High-Purity Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 2019, 186, 224. [Google Scholar] [CrossRef]
- Sayhi, M.; Ouerghi, O.; Belgacem, K.; Arbi, M.; Tepeli, Y.; Ghram, A.; Anik, Ü.; Österlund, L.; Laouini, D.; Diouani, M.F. Electrochemical detection of influenza virus H9N2 based on both immunomagnetic extraction and gold catalysis using an immobilization-free screen printed carbon microelectrode. Biosens. Bioelectron. 2018, 107, 170–177. [Google Scholar] [CrossRef]
- Koo, H.L.; Neill, F.H.; Estes, M.K.; Munoz, F.M.; Cameron, A.; DuPont, H.L.; Atmar, R.L. Noroviruses: The most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J. Pediatric Infect. Dis. Soc. 2013, 2, 57–60. [Google Scholar] [CrossRef]
- Hall, A.J.; Lopman, B.A.; Payne, D.C.; Patel, M.M.; Gastañaduy, P.A.; Vinjé, J.; Parashar, U.D. Norovirus disease in the united states. Emerg. Infect. Dis. 2013, 19, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Niwa, O.; Xu, Y.; Halsall, H.B.; Heineman, W.R. Small-Volume Voltammetric Detection of 4-Aminophenol with Interdigitated Array Electrodes and Its Application to Electrochemical Enzyme Immunoassay. Anal. Chem. 1993, 65, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.B.; Reilley, C.N. Thin-layer electrochemistry: Steady-state methods of studying rate processes. J. Electroanal. Chem. 1965, 10, 295–305. [Google Scholar] [CrossRef]
- Kanno, Y.; Ino, K.; Shiku, H.; Matsue, T. A local redox cycling-based electrochemical chip device with nanocavities for multi-electrochemical evaluation of embryoid bodies. Lab Chip 2015, 23, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Zheng, Z.H.; Li, H.W.; Huck, W.T.S.; Sirringhaus, H. Dewetting of conducting polymer inkjet droplets on patterned surfaces. Nat. Mater. 2004, 3, 171–176. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. Reagentless substrate analysis with immobilized enzymes. Science 1967, 158, 270–272. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Meas. J. Int. Meas. Confed. 2021, 167, 108293. [Google Scholar] [CrossRef]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Review: Recent Developments in Enzyme-Based Biosensors for Biomedical Analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme-surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Mass, M.; Veiga, L.S.; Garate, O.; Longinotti, G.; Moya, A.; Ramón, E.; Villa, R.; Ybarra, G.; Gabriel, G. Fully inkjet-printed biosensors fabricated with a highly stable ink based on carbon nanotubes and enzyme-functionalized nanoparticles. Nanomaterials 2021, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A. Glucose biosensors for clinical and personal use. Electrochem. Commun. 2021, 125, 106973. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J. Technological leap for sweat sensing. Nature 2016, 529, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible Electrochemical Bioelectronics: The Rise of In Situ Bioanalysis. Adv. Mater. 2020, 32, 1902083. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Tai, L.C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sensors 2018, 3, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.R.; Zhou, Y.; Dey, A.A.; Arellano, D.L.G.; Okoroanyanwu, U.; Secor, E.B.; Hersam, M.C.; Morse, J.; Rothstein, J.P.; Carter, K.R.; et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab Chip 2022, 22, 156–169. [Google Scholar] [CrossRef]
- Ahmed, J.; Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Novel SWCNTs-mesoporous silicon nanocomposite as efficient non-enzymatic glucose biosensor. Appl. Surf. Sci. 2021, 552, 149477. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Kim, D.U.; Cui, J.Z.; Kang, S.W. Enzyme-based glucose biosensor using a dye couple system. Dye. Pigment. 2001, 49, 103–108. [Google Scholar] [CrossRef]
- Hrapovic, S.; Liu, Y.; Male, K.B.; Luong, J.H.T. Electrochemical Biosensing Platforms Using Platinum Nanoparticles and Carbon Nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef]
- Shi, W.; Ma, Z. Amperometric glucose biosensor based on a triangular silver nanoprisms/chitosan composite film as immobilization matrix. Biosens. Bioelectron. 2010, 26, 1098–1103. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, W.; Guo, Q.; Li, Y. Metal oxide-based composite for non-enzymatic glucose sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 16111–16136. [Google Scholar] [CrossRef]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Rakesh Kumar, R.K.; Shaikh, M.O.; Chuang, C.H. A review of recent advances in non-enzymatic electrochemical creatinine biosensing. Anal. Chim. Acta 2021, 1183, 338748. [Google Scholar] [CrossRef] [PubMed]
- Archana, V.; Xia, Y.; Fang, R.; Gnana Kumar, G. Hierarchical CuO/NiO-Carbon Nanocomposite Derived from Metal Organic Framework on Cello Tape for the Flexible and High Performance Nonenzymatic Electrochemical Glucose Sensors. ACS Sustain. Chem. Eng. 2019, 7, 6707–6719. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Tominaga, M.; Shimazoe, T.; Nagashima, M.; Taniguchi, I. Electrocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem. Commun. 2005, 7, 189–193. [Google Scholar] [CrossRef]
- Chen, X.M.; Lin, Z.J.; Chen, D.J.; Jia, T.T.; Cai, Z.M.; Wang, X.R.; Chen, X.; Chen, G.N.; Oyama, M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2010, 25, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Kim, K.B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.S.; Shim, Y.B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Deng, J. A glucose biosensor based on immobilization of glucose oxidase in electropolymerized o-aminophenol film on platinized glassy carbon electrode. Anal. Chem. 1996, 68, 1632–1638. [Google Scholar] [CrossRef]
- Hu, C.; Yang, D.P.; Zhu, F.; Jiang, F.; Shen, S.; Zhang, J. Enzyme-labeled Pt@BSA nanocomposite as a facile electrochemical biosensing interface for sensitive glucose determination. ACS Appl. Mater. Interfaces 2014, 6, 4170–4178. [Google Scholar] [CrossRef]
- Shrivas, K.; Monisha; Kant, T.; Karbhal, I.; Kurrey, R.; Sahu, B.; Sinha, D.; Patra, G.K.; Deb, M.K.; Pervez, S. Smartphone coupled with paper-based chemical sensor for on-site determination of iron(III) in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 1573–1583. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, S.; Sinha, D.; Thakur, S.S.; Patle, T.K.; Kant, T.; Dewangan, K.; Satnami, M.L.; Nirmalkar, J.; Kumar, S. Colorimetric and smartphone-integrated paper device for on-site determination of arsenic (III) using sucrose modified gold nanoparticles as a nanoprobe. Microchim. Acta 2020, 187, 173. [Google Scholar] [CrossRef]
- Ghosale, A.; Shankar, R.; Ganesan, V.; Shrivas, K. Direct-Writing of Paper Based Conductive Track using Silver Nano-ink for Electroanalytical Application. Electrochim. Acta 2016, 209, 511–520. [Google Scholar] [CrossRef]
- Ghosale, A.; Shrivas, K.; Shankar, R.; Ganesan, V. Low-Cost Paper Electrode Fabricated by Direct Writing with Silver Nanoparticle-Based Ink for Detection of Hydrogen Peroxide in Wastewater. Anal. Chem. 2017, 89, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Gu, S.X.; Li, W.W.; Chen, T.T.; Xu, Q.; Wang, W. A paper disk equipped with graphene/polyaniline/Au nanoparticles/glucose oxidase biocomposite modified screen-printed electrode: Toward whole blood glucose determination. Biosens. Bioelectron. 2014, 56, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qian, D.; Wang, Q.; Li, Y.; Bao, N.; Gu, H.; Yu, C. Fully-drawn origami paper analytical device for electrochemical detection of glucose. Sens. Actuators B Chem. 2016, 231, 230–238. [Google Scholar] [CrossRef]
- Kant, T.; Shrivas, K.; Tapadia, K.; Devi, R.; Ganesan, V.; Deb, M.K. Inkjet-printed paper-based electrochemical sensor with gold nano-ink for detection of glucose in blood serum. New J. Chem. 2021, 45, 8297–8305. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. Electrochemistry of glucose at gold nanoparticles modified graphite/SrPdO3 electrode–Towards a novel non-enzymatic glucose sensor. J. Electroanal. Chem. 2015, 749, 42–52. [Google Scholar] [CrossRef]
- Rafatmah, E.; Hemmateenejad, B. Dendrite gold nanostructures electrodeposited on paper fibers: Application to electrochemical non-enzymatic determination of glucose. Sens. Actuators B Chem. 2020, 304, 127335. [Google Scholar] [CrossRef]
- Yang, M.; Liu, M.; Cheng, J.; Wang, H. A movable type bioelectronics printing technology for modular fabrication of biosensors. Sci. Rep. 2021, 11, 22323. [Google Scholar] [CrossRef]
- Ghorbani, L.; Rabbani, M. Fabrication of a time-temperature indicator by inkjet printing of a spore-based bio-ink. Bioprinting 2021, 21, e00109. [Google Scholar] [CrossRef]
- Kosmala, A.; Wright, R.; Zhang, Q.; Kirby, P. Synthesis of silver nano particles and fabrication of aqueous Ag inks for inkjet printing. Mater. Chem. Phys. 2011, 129, 1075–1080. [Google Scholar] [CrossRef]
- Hiraoka, M.; Hasegawa, T.; Abe, Y.; Yamada, T.; Tokura, Y.; Yamochi, H.; Saito, G.; Akutagawa, T.; Nakamura, T. Ink-jet printing of organic metal electrodes using charge-transfer compounds. Appl. Phys. Lett. 2006, 89, 173504. [Google Scholar] [CrossRef]
- Sun, T.X.; Jabbour, G.E. Combinatorial screening and optimization of luminescent materials and organic light-emitting devices. MRS Bull. 2002, 113, 231101. [Google Scholar] [CrossRef]
- Trotter, M.; Juric, D.; Bagherian, Z.; Borst, N.; Gläser, K.; Meissner, T.; von Stetten, F.; Zimmermann, A. Inkjet-printing of nanoparticle gold and silver ink on cyclic olefin copolymer for DNA-sensing applications. Sensors 2020, 20, 1333. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef]
- Castrejón-Pita, J.R.; Baxter, W.R.S.; Morgan, J.; Temple, S.; Martin, G.D.; Hutchings, I.M. Future, opportunities and challenges of inkjet technologies. At. Sprays 2013, 23, 541–565. [Google Scholar] [CrossRef]
- Colella, R.; Rivadeneyra, A.; Palma, A.J.; Tarricone, L.; Capitan-Vallvey, L.F.; Catarinucci, L.; Salmeron, J.F. Comparison of Fabrication Techniques for Flexible UHF RFID Tag Antennas [Wireless Corner]. IEEE Antennas Propag. Mag. 2017, 59, 159–168. [Google Scholar] [CrossRef]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet printing of mammalian cells–Theory and applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Sumaiya, S.; Kardel, K.; El-Shahat, A. Organic Solar Cell by Inkjet Printing—An Overview. Technologies 2017, 5, 53. [Google Scholar] [CrossRef]
- Martin, G.D.; Hoath, S.D.; Hutchings, I.M. Inkjet printing–The physics of manipulating liquid jets and drops. J. Phys. Conf. Ser. 2008, 105, 012001. [Google Scholar] [CrossRef]
- Noh, Y.Y.; Zhao, N.; Caironi, M.; Sirringhaus, H. Downscaling of self-aligned, all-printed polymer thin-film transistors. Nat. Nanotechnol. 2007, 2, 784–789. [Google Scholar] [CrossRef]
- Ko, H.Y.; Park, J.; Shin, H.; Moon, J. Rapid self-assembly of monodisperse colloidal spheres in an ink-jet printed droplet. Chem. Mater. 2004, 16, 4212–4215. [Google Scholar] [CrossRef]
- Bartolo, D.; Boudaoud, A.; Narcy, G.; Bonn, D. Dynamics of non-newtonian droplets. Phys. Rev. Lett. 2007, 99, 174502. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Hardy, M.; Kang, S.J.; Barton, K.; Adair, K.; Mukhopadhyay, D.K.; Lee, C.Y.; Strano, M.S.; Alleyne, A.G.; Georgiadis, J.G.; et al. High-resolution electrohydrodynamic jet printing. Nat. Mater. 2007, 6, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Pan, H.; Grigoropoulos, C.P.; Luscombe, C.K.; Fréchet, J.M.J.; Poulikakos, D. All-inkjet-printed flexible electronics fabrication on a polymer substrate by low-temperature high-resolution selective laser sintering of metal nanoparticles. Nanotechnology 2007, 18, 345202. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, J.; Moon, H.; Lim, T.W.; Hong, S.; Nam, K.H.; Yoo, S.; Grigoropoulos, C.P.; Yang, D.Y.; Ko, S.H. Nanoscale electronics: Digital fabrication by direct femtosecond laser processing of metal nanoparticles. Adv. Mater. 2011, 23, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Soltman, D.; Subramanian, V. Inkjet-printed line morphologies and temperature control of the coffee ring effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef]
- Yu, X.; Xing, R.; Peng, Z.; Lin, Y.; Du, Z.; Ding, J.; Wang, L.; Han, Y. To inhibit coffee ring effect in inkjet printing of light-emitting polymer films by decreasing capillary force. Chin. Chem. Lett. 2019, 30, 135–138. [Google Scholar] [CrossRef]
- Sliz, R.; Czajkowski, J.; Fabritius, T. Taming the Coffee Ring Effect: Enhanced Thermal Control as a Method for Thin-Film Nanopatterning. Langmuir 2020, 36, 9562–9570. [Google Scholar] [CrossRef]
- Rodrigues, J.; Desai, S. The nanoscale Leidenfrost effect. Nanoscale 2019, 11, 12139–12151. [Google Scholar] [CrossRef]
- Graddage, N.; Chu, T.Y.; Ding, H.; Py, C.; Dadvand, A.; Tao, Y. Inkjet printed thin and uniform dielectrics for capacitors and organic thin film transistors enabled by the coffee ring effect. Org. Electron. 2016, 29, 114–119. [Google Scholar] [CrossRef]
- Lee, A.; Sudau, K.; Ahn, K.H.; Lee, S.J.; Willenbacher, N. Optimization of experimental parameters to suppress nozzle clogging in inkjet printing. Ind. Eng. Chem. Res. 2012, 51, 13195–13204. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
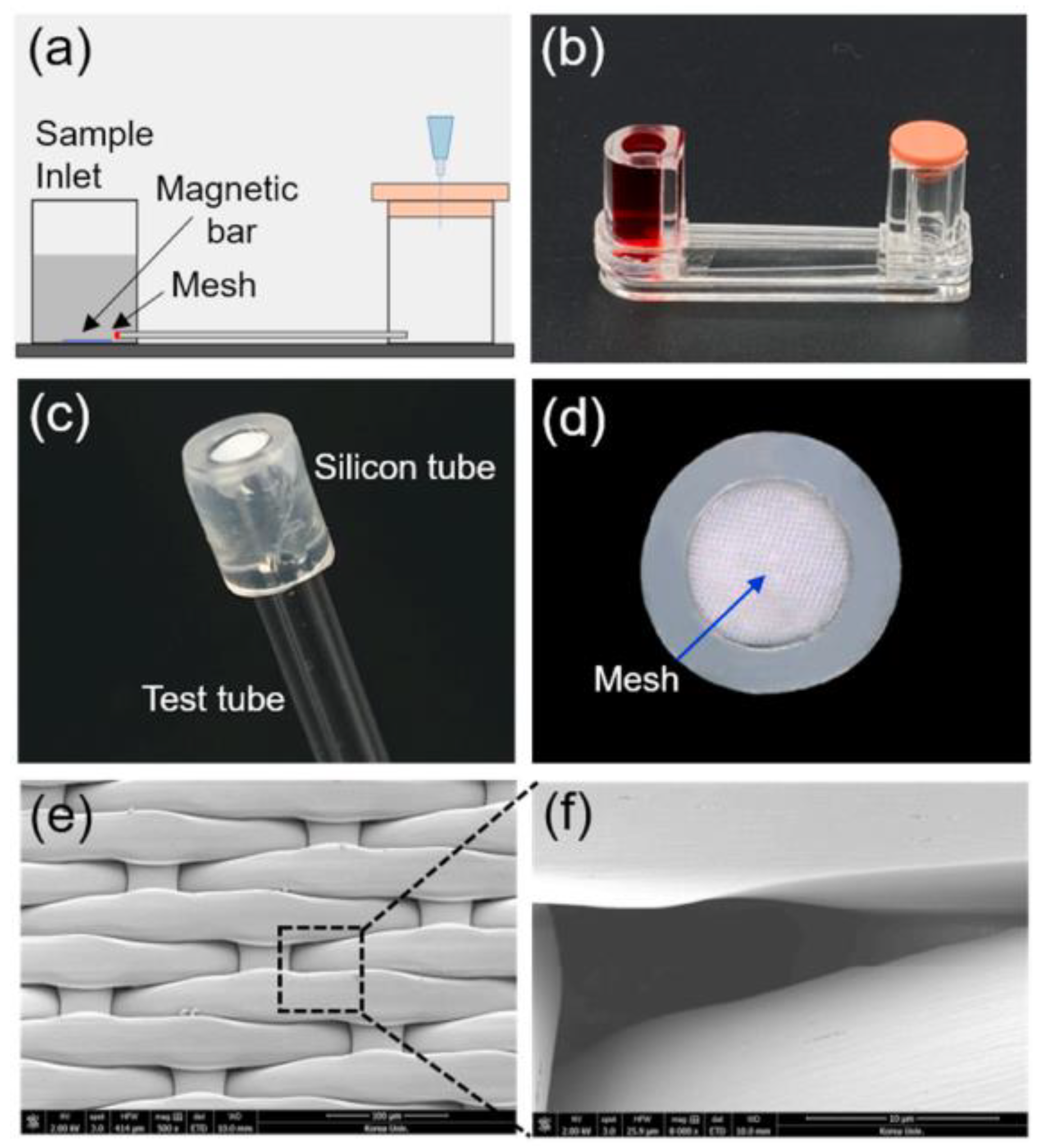
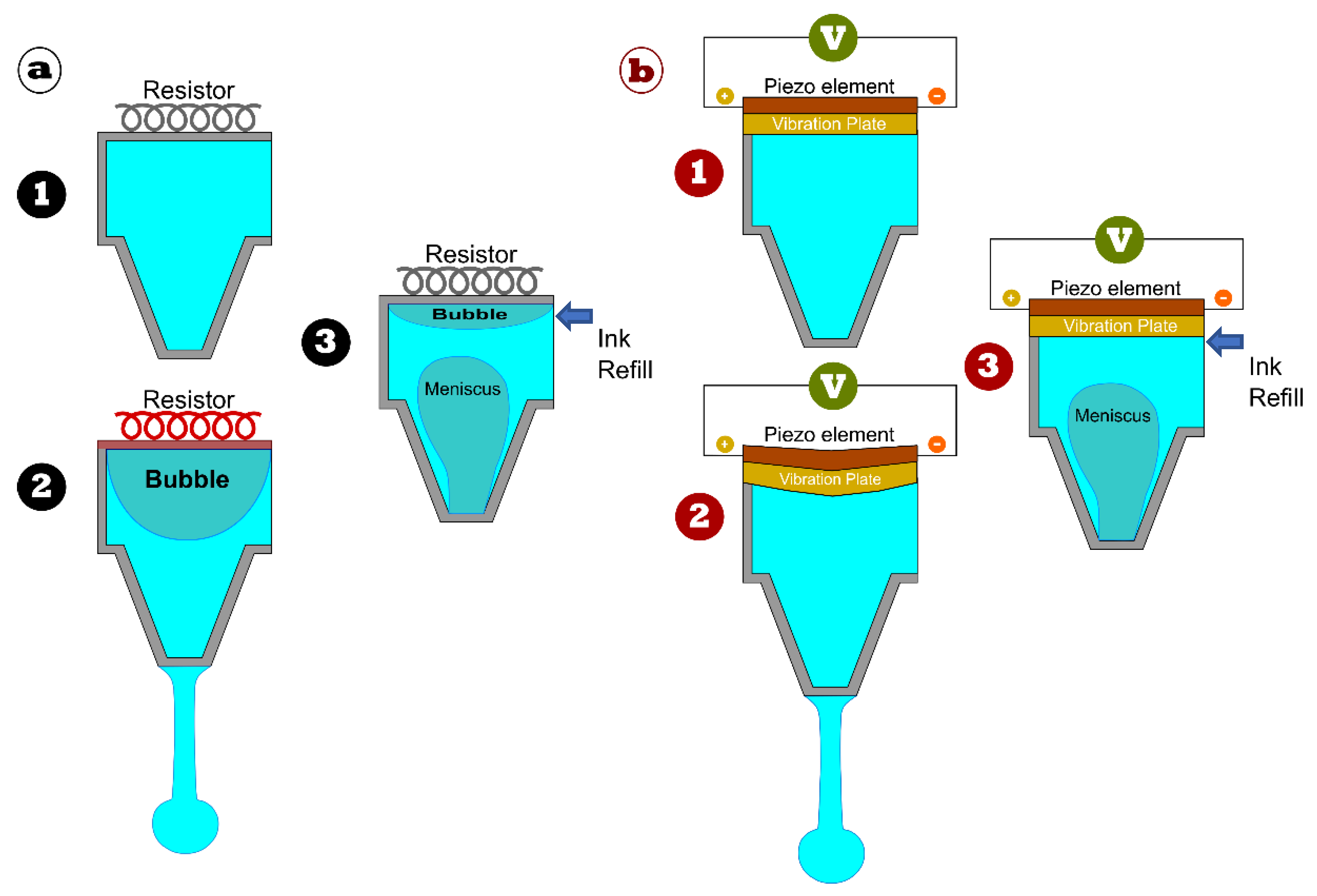

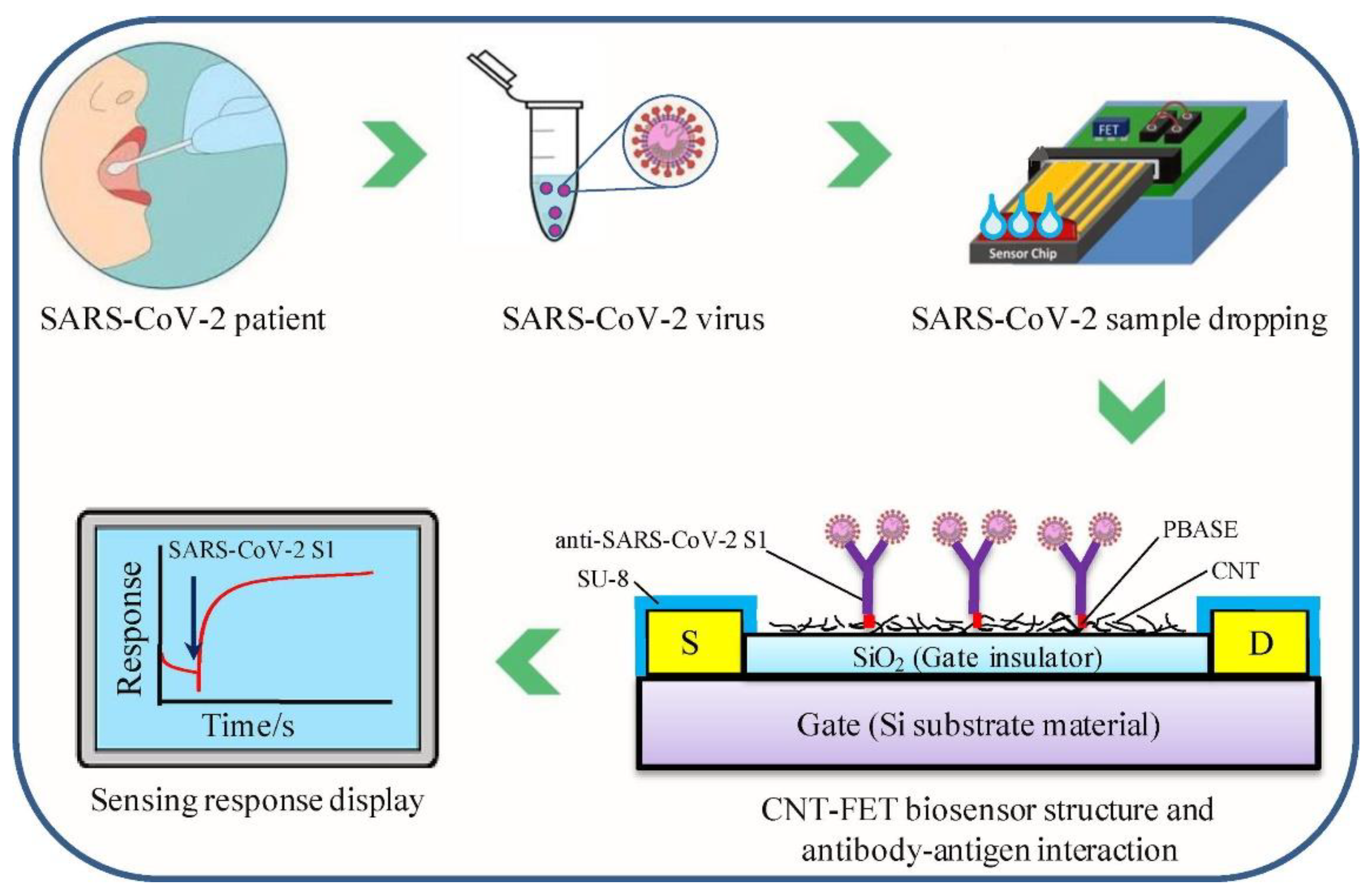
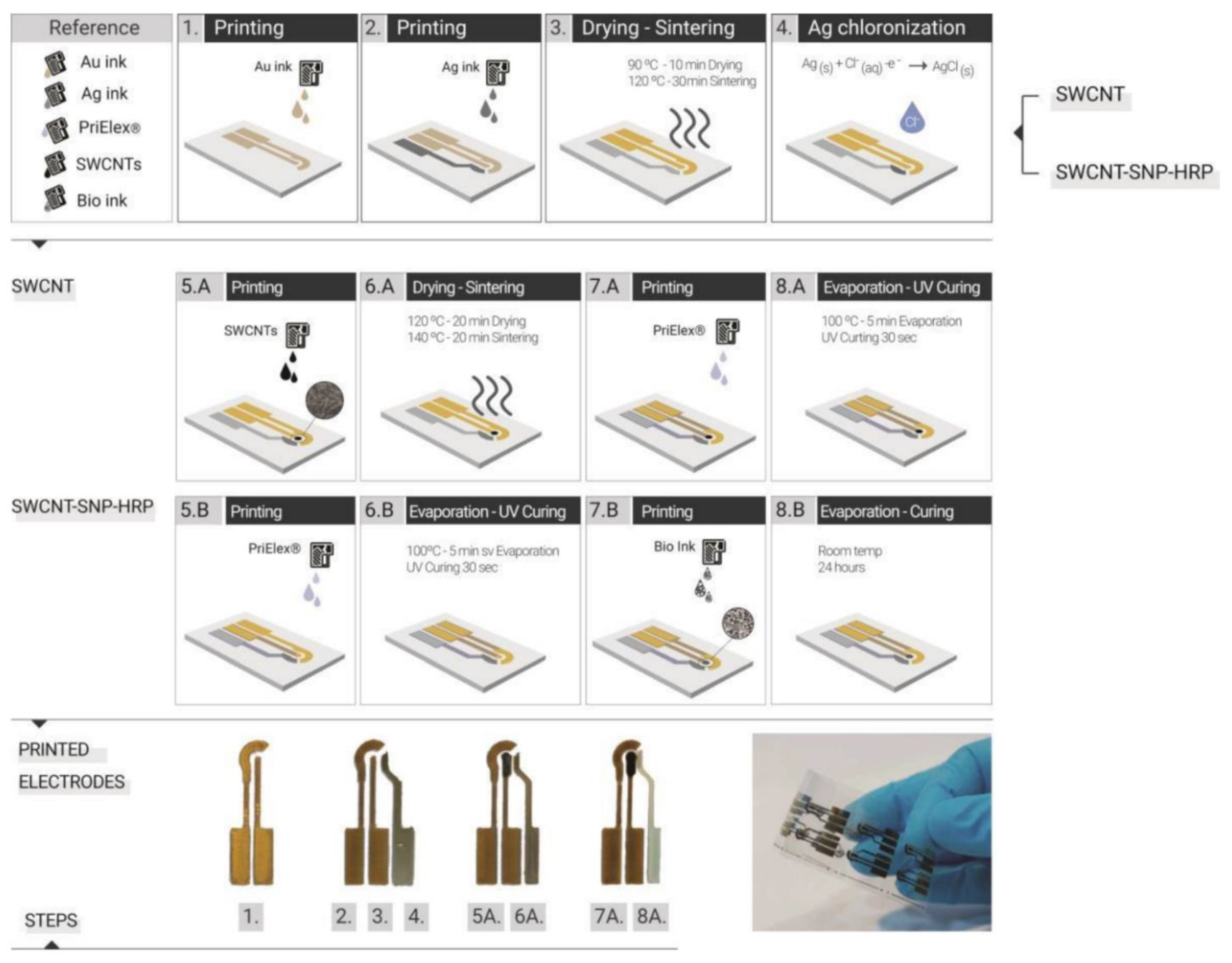
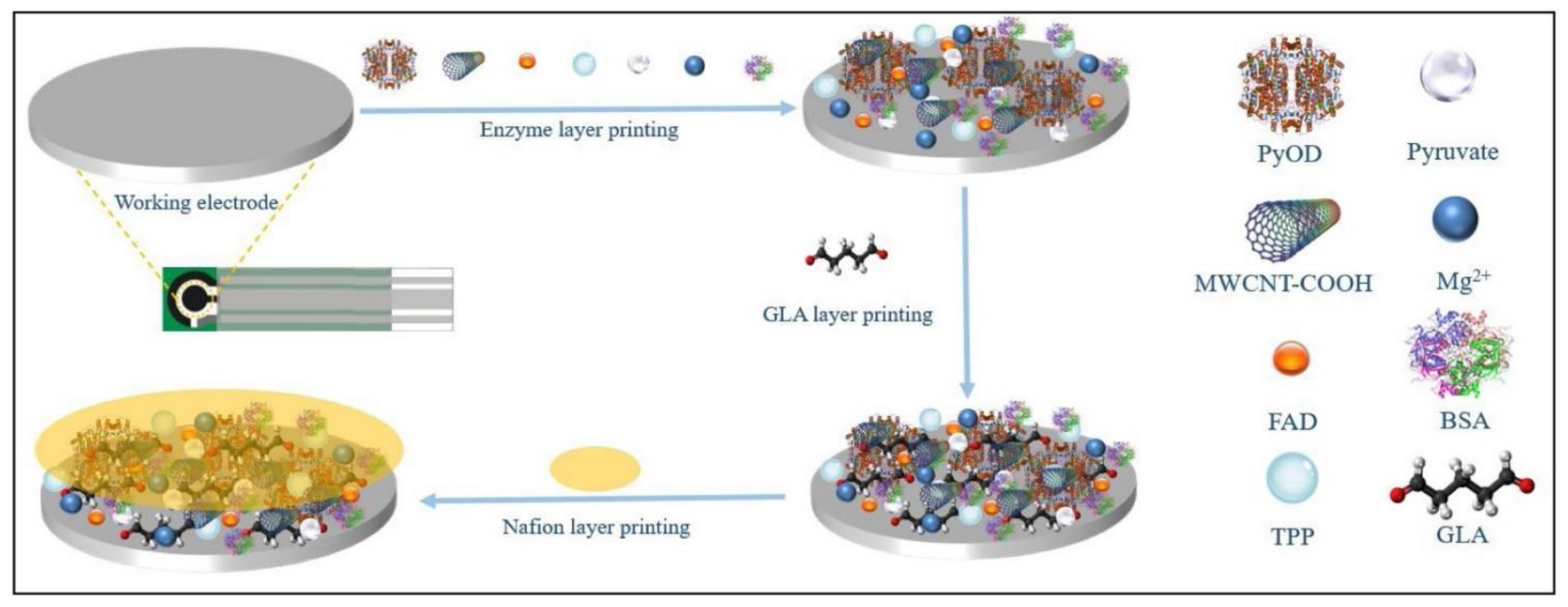
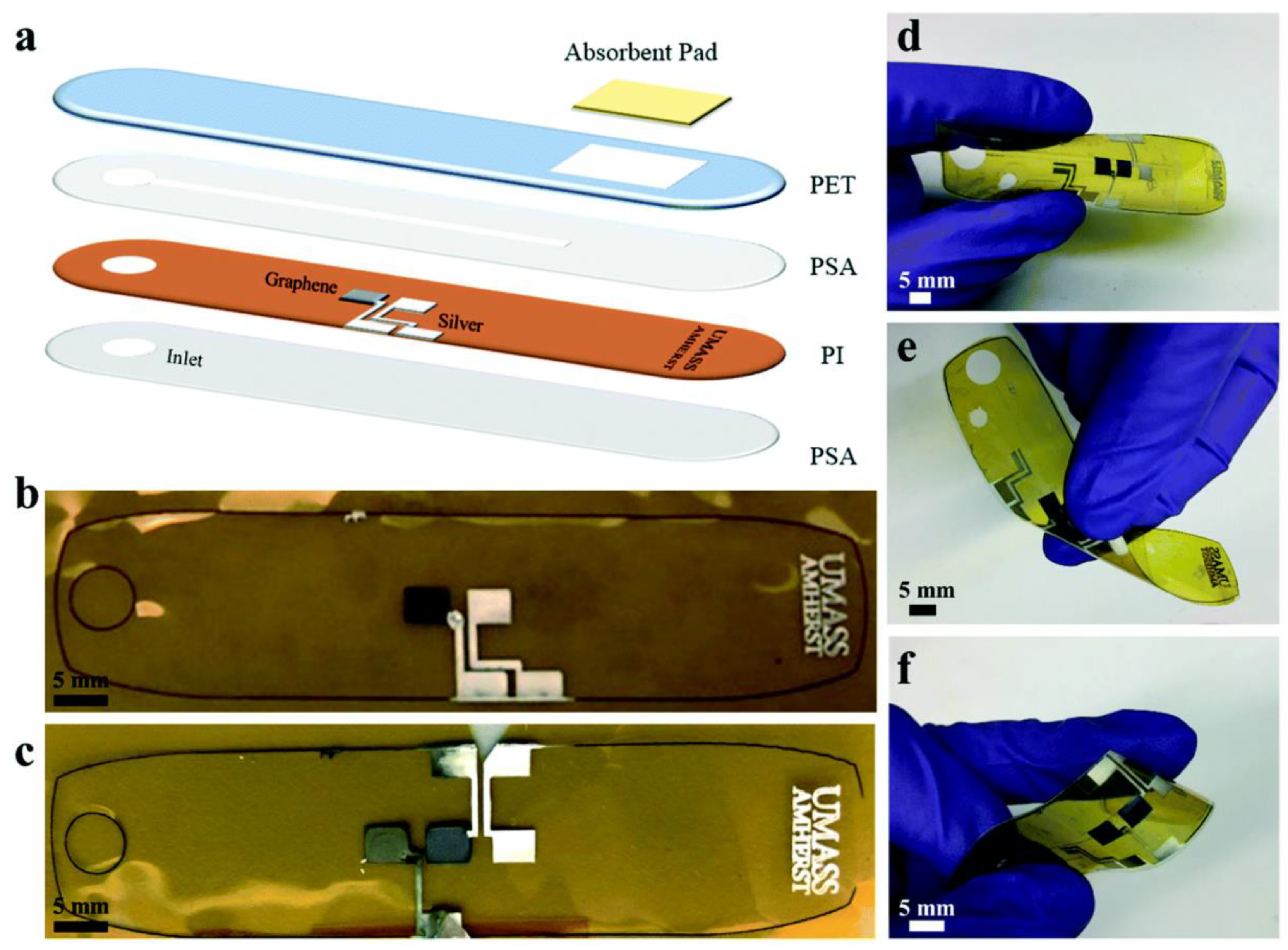
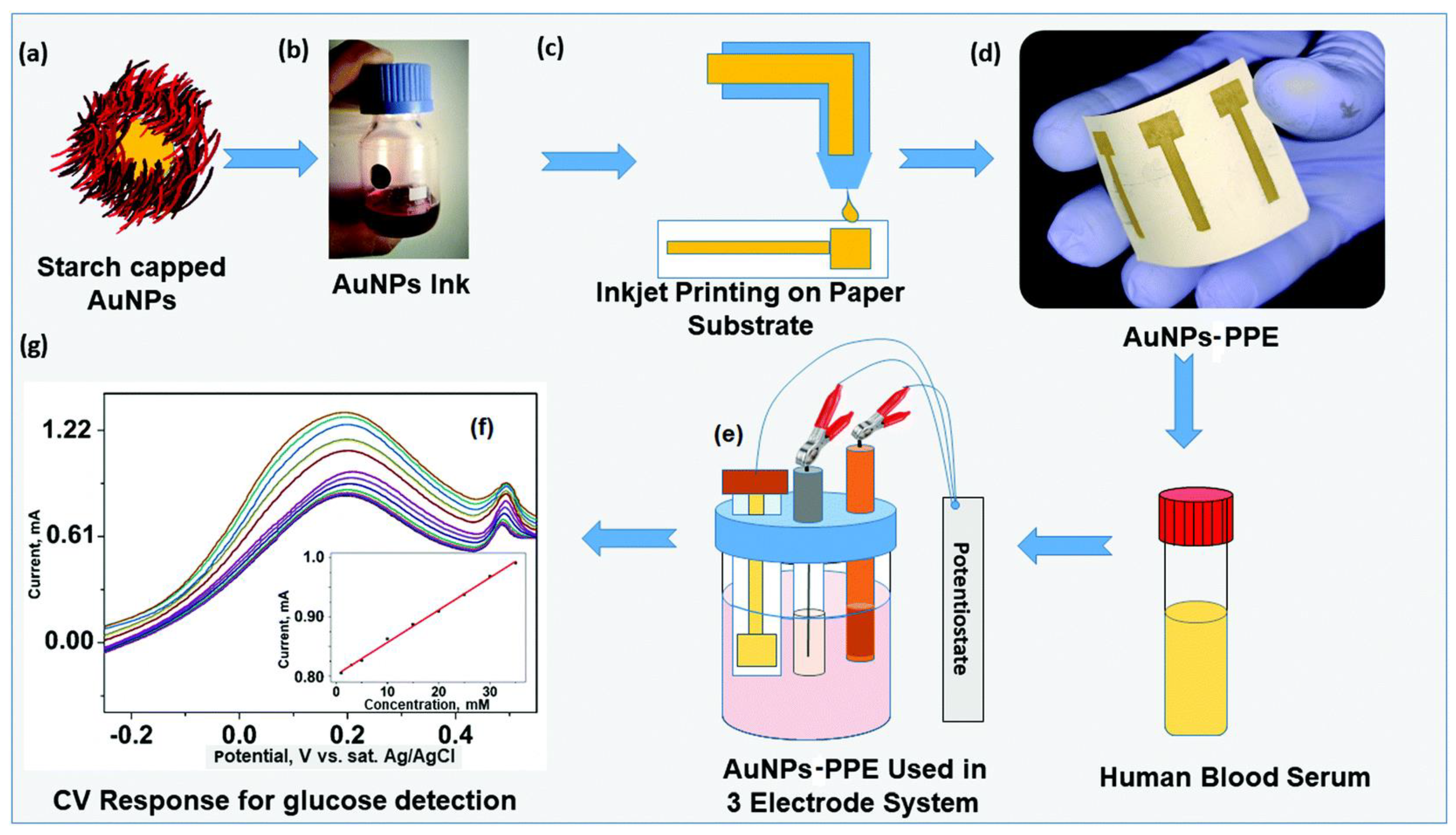
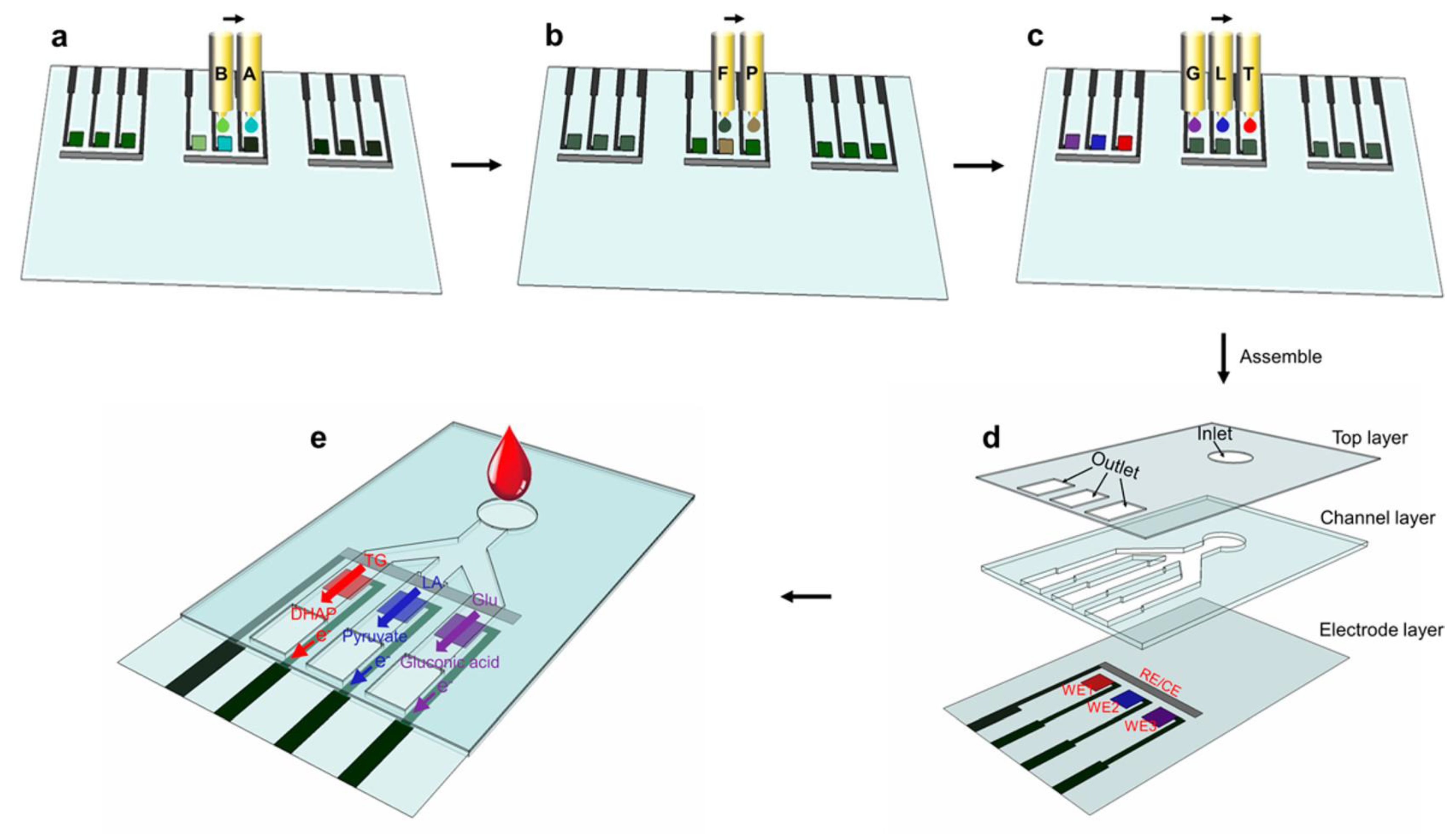
| Printing Technology | Biosensors | Merits | References | Limitations |
|---|---|---|---|---|
| Screen Printing | H2O2 detection | Simple, high throughput, economical, robust, rapid, scalable, miniaturization, reliable | [80,81] | Mask-based, contact-based, low production rate compared to roll-to-roll printing, material waste, high material consumption, limited for large scale production |
| Glucose sensor | [82] | |||
| Glucose, glutamate, lactate sensor | [83] | |||
| pH sensor | [84] | |||
| Uric Acid sensor | [85] | |||
| Dopamine detection | [86] | |||
| SARS-CoV-2 | [87,88] | |||
| MERS-CoV | [89] | |||
| Escherichia coli (E. coli) | [90] | |||
| Inkjet Printing | Multianalyte (pH, Protein, Glucose) sensor | Drop-on-demand (DOD), maskless, can create patterns on non-planar surfaces, well-defined patterning, simple, capacity for mass production | [91] | Nozzle clogging, highly specified ink formulation, limited resolution |
| Ammonia sensor | [92] | |||
| Pathogen detection | [93] | |||
| Bienzymatic Glucose biosensor | [94] | |||
| Calorimetric sensor/ H2O2 detection | [95,96] | |||
| H2O2 and glucose detection | [97] | |||
| Pressure ulcer detection | [98] | |||
| Wearable biosensor detection system/norovirus detection | [99] | |||
| HIV-related ssDNA detection | [100] | |||
| Multiplexed biosensor | [101] | |||
| Protein detection | [102] | |||
| SARS-CoV-2 detection | [103] | |||
| 3-D Printing | Lactate detection | Ease of design geometries using CAD software and 3D scanner, ability to print complex 3D features | [104] | Limited printing resolution, limited by printable materials |
| Multiuse (dopamine, tert-butyl hydroquinone, dipyrone, and diclofenac) sensors | [105] | |||
| DNA sensing | [106] | |||
| Dopamine detection | [107] | |||
| Enzyme biosensor/H2O2 detection | [108] | |||
| Gravure Printing | Cadmium sulphide, lead sulphide, D-proline, mouse IgG detection | Roll-to-roll printing, rapid printing, large-area patterning | [73] | High pressure required, low resolution, used for long runs |
| Antioxidant Biosensor | [109] | |||
| Ions, metabolites, heavy metals detection, perspiration monitoring | [110] | |||
| Glucose sensor | [111] | |||
| Flexographic Printing | Glucose sensor | Roll-to-roll printing, rapid printing, comparatively low pressure required than gravure printing with better resolution, large-area patterning | [112,113,114] | High pressure required, used for short and medium runs |
| Algal toxin detection in water | [115] | |||
| Human cytomegalovirus (HCMV) detection | [75] | |||
| Microcontact Printing | Protein detection | High resolution down to nm scale, more flexible in terms of ink rheology as compared to inkjet printing, can create patterns on non-planar surfaces | [116] | Transfer printing, requires mask created via photolithography |
| Antibody detection | [117,118] | |||
| Coccidioidomycosis (Valley Fever) detection | [119] | |||
| Laser Printing | Catechol detection/polyphenol biosensor | High spatial resolution, contactless direct-writing technique, no requirement for masks or nozzles, ability to transfer materials both in liquid and solid phase | [120] | Cannot transfer complex multi-component materials, weak bonding between material and substrate, suitable for a few materials only depending on the optical and mechanical properties of the material |
| Herbicides detection | [121] | |||
| Proteins and DNA sensor | [122,123] | |||
| Heavy metal ions detection | [124] | |||
| DNA hybridization event detection | [125] | |||
| Bacteria sensor | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. https://doi.org/10.3390/chemosensors10030103
Hussain A, Abbas N, Ali A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors. 2022; 10(3):103. https://doi.org/10.3390/chemosensors10030103
Chicago/Turabian StyleHussain, Arif, Naseem Abbas, and Ahsan Ali. 2022. "Inkjet Printing: A Viable Technology for Biosensor Fabrication" Chemosensors 10, no. 3: 103. https://doi.org/10.3390/chemosensors10030103
APA StyleHussain, A., Abbas, N., & Ali, A. (2022). Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors, 10(3), 103. https://doi.org/10.3390/chemosensors10030103







