Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques
Abstract
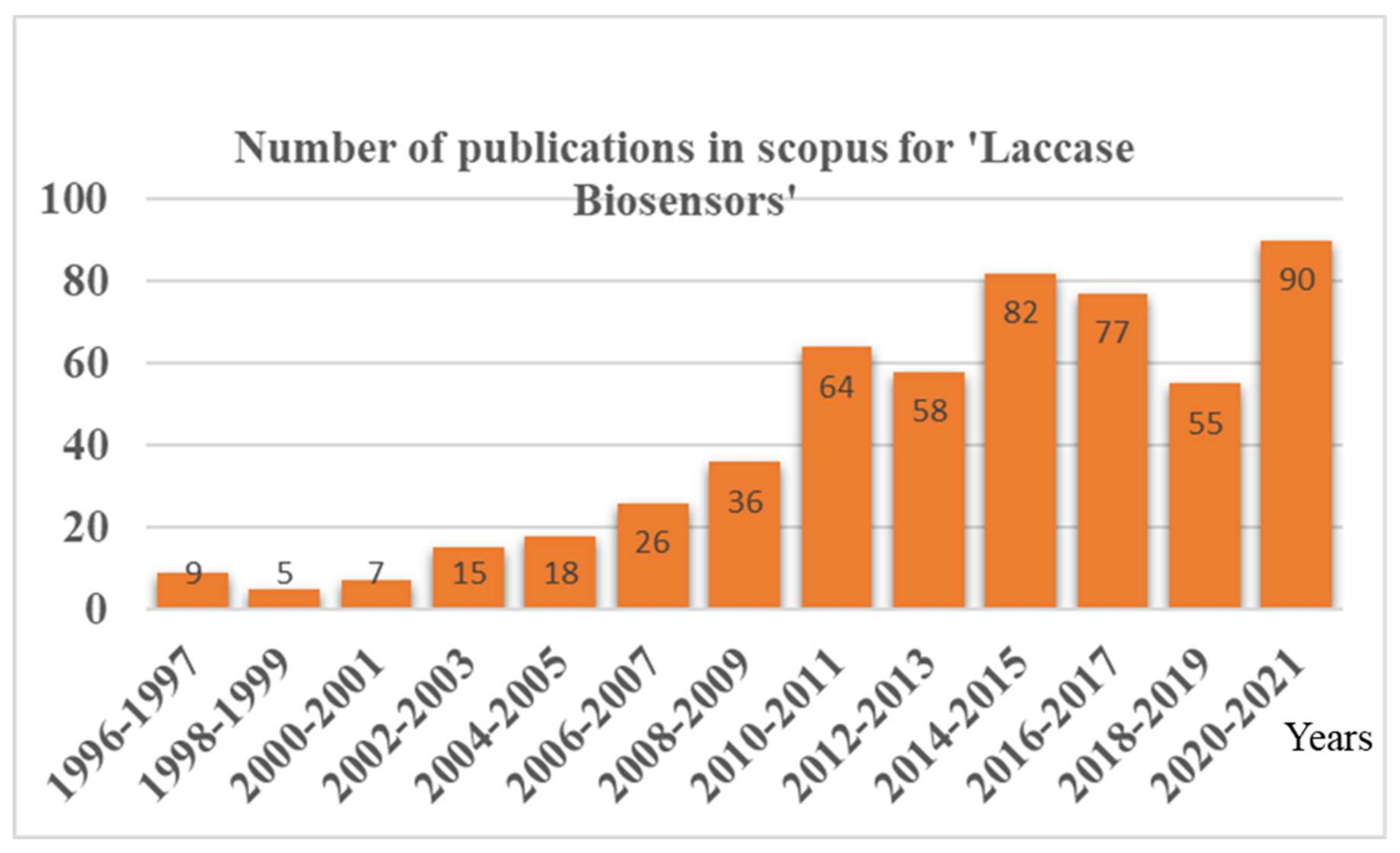
:1. Introduction
2. Laccase Information and Reaction
3. Structure of Typical Laccase-Based Biosensors
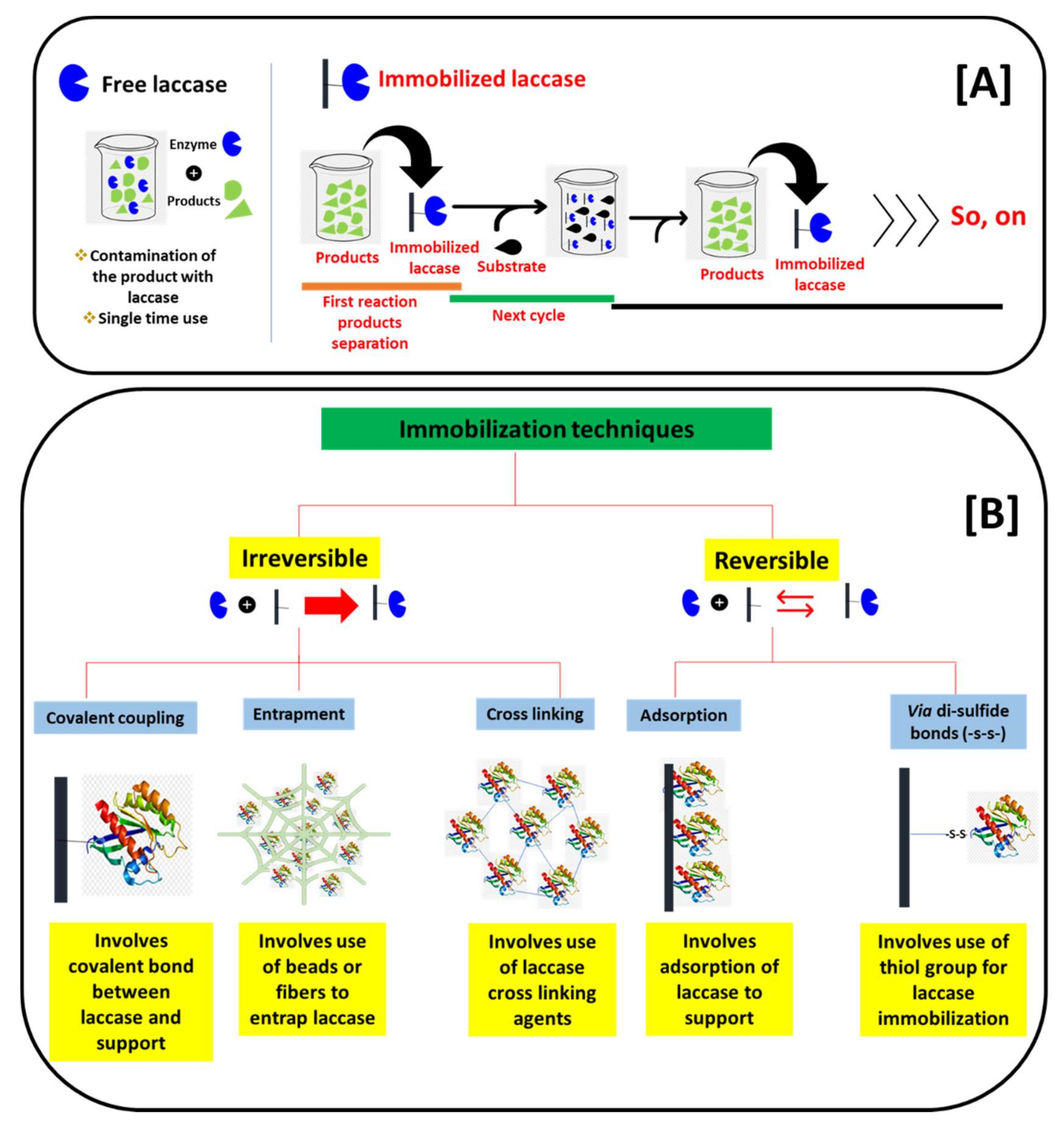
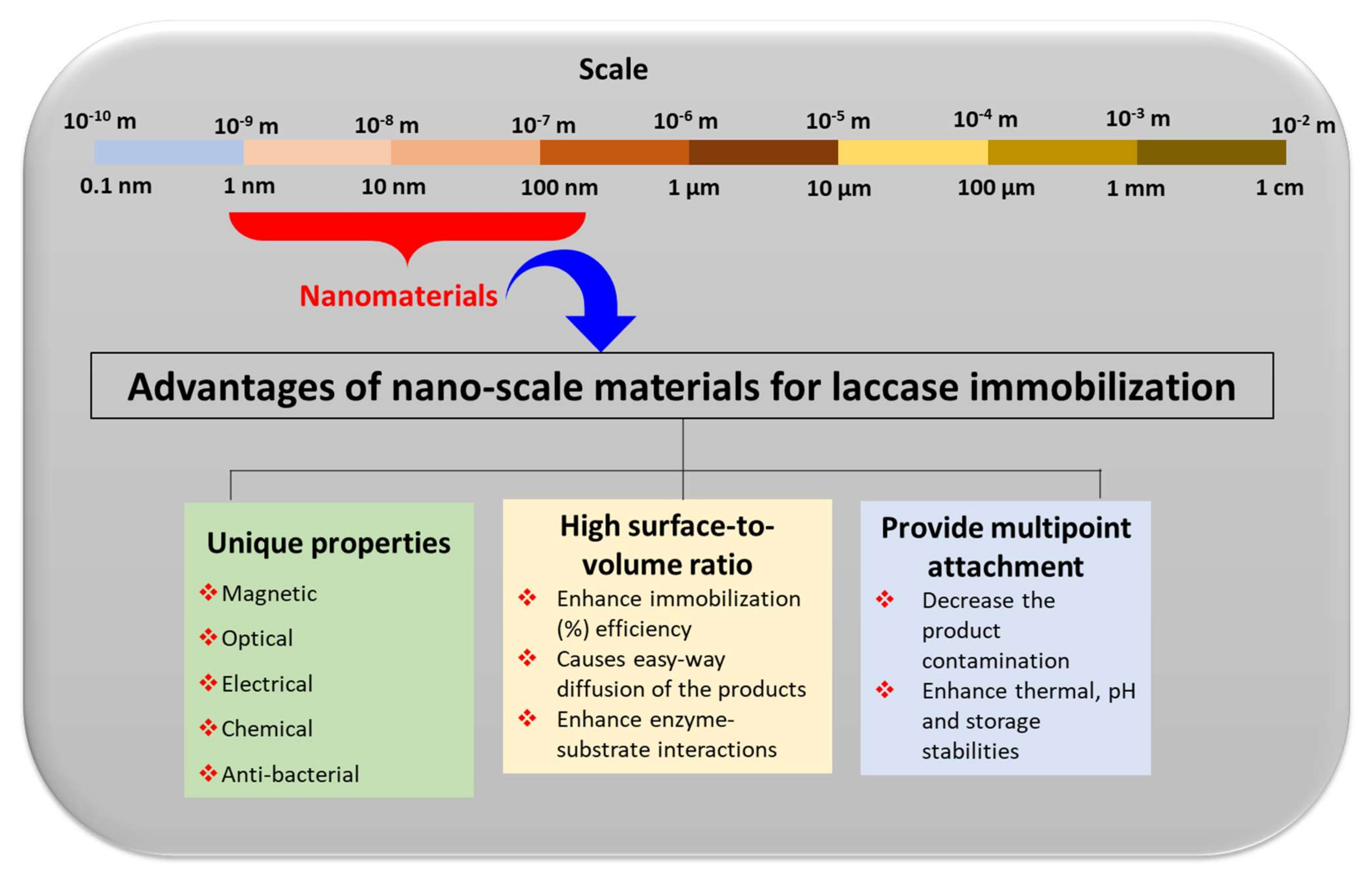
4. Nano-Immobilization of Laccase in Laccase-Based Biosensors
4.1. Adsorption-Based Laccase Immobilization
4.2. Entrapment-Based Laccase Immobilization
4.3. Cross-Linking-Based Laccase Immobilization
4.4. Covalent-Based Laccase Immobilization
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Mamo, M.A.; Penny, P.W.; Govender, P.P. Phenolic Compounds Compounds in Water: Sources, Phenolic Toxicity and Treatment Methods. In Phenolic Compounds: Natural Sources, Importance and Applications; Intech Open: London, UK, 2017; pp. 419–443. [Google Scholar]
- Barrico, M.L.; Nabais, C.; Martins, M.J.; Freitas, H. Sources of phenolic compounds in two catchments of southern Portugal-Effect of season, land use and soil type. Chemosphere 2006, 65, 482–488. [Google Scholar] [CrossRef]
- Stadlmair, L.F.; Letzel, T.; Graßmann, J. Monitoring enzymatic degradation of emerging contaminants using a chip-based robotic nano-ESI-MS tool. Anal. Bioanal. Chem. 2018, 410, 27–32. [Google Scholar] [CrossRef]
- Yuan, T.; Tazaki, A.; Hashimoto, K.; Al Hossain, M.M.A.; Kurniasari, F.; Ohgami, N.; Aoki, M.; Ahsan, N.; Akhand, A.A.; Kato, M. Development of an efficient remediation system with a low cost after identification of water pollutants including phenolic compounds in a tannery built-up area in Bangladesh. Chemosphere 2021, 280, 130959. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.A.; Pasquali, C.E.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero, Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Parada, H.; Gammon, M.D.; Ettore, H.L.; Chen, J.; Calafat, A.M.; Neugut, A.I.; Santella, R.M.; Wolff, M.S.; Teitelbaum, S.L. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Phenol; U.S. Department of Health and Human Services, Public Health Services: Atlanta, GA, USA, 2008.
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC-Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Delfino, I.; Diano, N.; Lepore, M. Advanced optical sensing of phenolic compounds for environmental applications. Sensors 2021, 21, 7563. [Google Scholar] [CrossRef]
- Karim, F.; Fakhruddin, A.N.M. Recent advances in the development of biosensor for phenol: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 261–274. [Google Scholar] [CrossRef]
- Pasternak, G.; Hanczyc, M.M. Novel method for detecting and quantifying phenol with transient response of glycolytic oscillations of synchronised yeast cells. Sens. Bio-Sens. Res. 2019, 22, 100259. [Google Scholar] [CrossRef]
- Flachbart, L.K.; Gerhard, C.; Gertzen, W.; Gohlke, H.; Marienhagen, J. Development of a Biosensor Platform for Phenolic Compounds Using a Transition Ligand Strategy. ACS Synth. Biol. 2021, 10, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Hemmati, A.; Majidi, M.R.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Adlpour-Azar, R.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. TrAC-Trends Anal. Chem. 2021, 143, 116344. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Perna, V.; Meyer, A.S.; Holck, J.; Eltis, L.D.; Eijsink, V.G.H.; Wittrup Agger, J. Laccase-Catalyzed Oxidation of Lignin Induces Production of H2O2. ACS Sustain. Chem. Eng. 2020, 8, 831–841. [Google Scholar] [CrossRef]
- Mehra, R.; Muschiol, J.; Meyer, A.S.; Kepp, K.P. A structural-chemical explanation of fungal laccase activity. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Kulys, J.J.; Duke, K.; Li, K.; Krikstopaitis, K.; Deussen, H.J.W.; Abbate, E.; Galinyte, V.; Schneider, P. Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds. Appl. Environ. Microbiol. 2000, 66, 2052–2056. [Google Scholar] [CrossRef] [Green Version]
- Mazlan, S.Z.; Lee, Y.H.; Hanifah, S.A. A new Laccase based biosensor for tartrazine. Sensors 2017, 17, 2859. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.P.; Feng, J.J.; Wu, L.; Zhou, J.Y.; Chen, J.R.; Wang, A.J. Novel phenol biosensor based on laccase immobilized on reduced graphene oxide supported palladium-copper alloyed nanocages. Biosens. Bioelectron. 2015, 74, 347–352. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G.L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Qu, R.; Wang, Z.; Huang, Q. The laccase-like reactivity of manganese oxide nanomaterials for pollutant conversion: Rate analysis and cyclic voltammetry. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Parra Guardado, A.L.; Belleville, M.P.; Rostro Alanis, M.D.J.; Parra Saldivar, R.; Sanchez-Marcano, J. Effect of redox mediators in pharmaceuticals degradation by laccase: A comparative study. Process Biochem. 2019, 78, 123–131. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Witayakran, S.; Ragauskas, A.J. Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Yang, J.; Shinde, S.S.; Mistry, B.M.; Kim, D.Y.; Sung, J.S.; Kadam, A.A. Paper waste extracted A-cellulose fibers super-magnetized and chitosan-functionalized for covalent laccase immobilization. Bioresour. Technol. 2018, 261, 420–427. [Google Scholar] [CrossRef]
- Martinez-Ortiz, J.; Flores, R.; Vazquez-Duhalt, R. Molecular design of laccase cathode for direct electron transfer in a biofuel cell. Biosens. Bioelectron. 2011, 26, 2626–2631. [Google Scholar] [CrossRef]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 1–2. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. A low-cost wheat bran medium for biodegradation of the benzidine-based carcinogenic dye trypan blue using a microbial consortium. Int. J. Environ. Res. Public Health 2015, 12, 3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Fact. 2019, 18, 1–33. [Google Scholar] [CrossRef]
- Kumar Mishra, S.; Kumar Srivastava, S.; Ash, K. Laccase sources and their applications in environmental pollution. Int. J. Life-Sci. Sci. Res. 2015, 1, 71–73. [Google Scholar]
- Leontievsky, A.A.; Vares, T.; Lankinen, P.; Shergill, J.K.; Pozdnyakova, N.N.; Myasoedova, N.M.; Kalkkinen, N.; Golovleva, L.A.; Cammack, R.; Thurston, C.F.; et al. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol. Lett. 1997, 156, 9–14. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, E.Y.; Lin, P.Y.; Wang, P.F.; Kuo, T.R.; Chen, C.H.; Manga, Y.B.; Hsiao, Y.C. Label-free, smartphone-based, and sensitive nano-structural liquid crystal aligned by ceramic silicon compound–constructed dmoap-based biosensor for the detection of urine albumin. Int. J. Nanomed. 2021, 16, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-L.; Fan, Y.-J.; Lin, J.-D.; Hsiao, Y.-C. Label-free, color-indicating, and sensitive biosensors of cholesteric liquid crystals on a single vertically aligned substrate. Biomed. Opt. Express 2019, 10, 4636. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Chen, F.L.; Liou, J.C.; Huang, Y.W.; Chen, C.H.; Hong, Z.Y.; De Lin, J.; Hsiao, Y.C. Label-free multi-microfluidic immunoassays with liquid crystals on polydimethylsiloxane biosensing chips. Polymers 2020, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Ardhaoui, M.; Bhatt, S.; Zheng, M.; Dowling, D.; Jolivalt, C.; Khonsari, F.A. Biosensor based on laccase immobilized on plasma polymerized allylamine/carbon electrode. Mater. Sci. Eng. C 2013, 33, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Gkantzou, E.; Chatzikonstantinou, A.V.; Fotiadou, R.; Giannakopoulou, A.; Patila, M.; Stamatis, H. Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol. Adv. 2021, 51, 107738. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gu, H.; Chen, W.; Shi, H.; Yang, B.; Huang, X.; Zhang, Q. Immobilized laccase on activated poly(vinyl alcohol) microspheres for enzyme thermistor application. Appl. Biochem. Biotechnol. 2014, 173, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Calandra, P.; Antonacci, A.; Scognamiglio, V. The convergence of forefront technologies in the design of laccase-based biosensors—An update. TrAC-Trends Anal. Chem. 2019, 119, 115615. [Google Scholar] [CrossRef]
- Datta, S.; Veena, R.; Samuel, M.S.; Selvarajan, E. Immobilization of laccases and applications for the detection and remediation of pollutants: A review. Environ. Chem. Lett. 2021, 19, 521–538. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef]
- Kadam, A.A.; Sharma, B.; Shinde, S.K.; Ghodake, G.S.; Saratale, G.D.; Saratale, R.G.; Kim, D.Y.; Sung, J.S. Thiolation of chitosan loaded over super-magnetic halloysite nanotubes for enhanced laccase immobilization. Nanomaterials 2020, 10, 2560. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Jee, S.C.; Sung, J.S.; Lee, D.S. Chitosan-functionalized supermagnetic halloysite nanotubes for covalent laccase immobilization. Carbohydr. Polym. 2018, 194, 208–216. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Lee, D.S. Supermagnetically Tuned Halloysite Nanotubes Functionalized with Aminosilane for Covalent Laccase Immobilization. ACS Appl. Mater. Interfaces 2017, 9, 15492–15501. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Sung, J.S.; Kadam, A.A. Supermagnetic sugarcane bagasse hydrochar for enhanced osteoconduction in human adipose tissue-derived mesenchymal stem cells. Nanomaterials 2020, 10, 1793. [Google Scholar] [CrossRef]
- Min, K.; Jee, S.C.; Sung, J.S.; Kadam, A.A. Anti-proliferative applications of laccase immobilized on super-magnetic chitosan-functionalized halloysite nanotubes. Int. J. Biol. Macromol. 2018, 118, 228–237. [Google Scholar]
- Ghodake, G.S.; Shinde, S.K.; Saratale, G.D.; Saratale, R.G.; Kim, M.; Jee, S.C.; Kim, D.Y.; Sung, J.S.; Kadam, A.A. α-cellulose fibers of paper-waste origin surface-modified with Fe3O4 and thiolated-chitosan for efficacious immobilization of laccase. Polymers 2021, 13, 581. [Google Scholar] [CrossRef]
- Othman, A.M.; Wollenberger, U. Amperometric biosensor based on coupling aminated laccase to functionalized carbon nanotubes for phenolics detection. Int. J. Biol. Macromol. 2020, 153, 855–864. [Google Scholar] [CrossRef]
- Wang, K.; Liu, P.; Ye, Y.; Li, J.; Zhao, W.; Huang, X. Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sens. Actuators B Chem. 2014, 197, 292–299. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.M.; Yang, T.C.K.; Pan, Y.F.; Chen, T.W.; Velusamy, V.; Selvam, S. A novel Laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Zhang, Y.; Tang, J. Synthesis of three-dimensional laccase-Cu3(PO4)2⋅3H2O microflowers via biomineralization for UV–vis epinephrine biosensing. Microchem. J. 2022, 172, 106911. [Google Scholar] [CrossRef]
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase bioconjugate and multi-walled carbon nanotubes-based biosensor for bisphenol A analysis. Bioelectrochemistry 2021, 144, 108033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. A smartphone-assisted portable biosensor using laccase-mineral hybrid microflowers for colorimetric determination of epinephrine. Talanta 2021, 224, 121840. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, L.G.; Aranda, P.; Messina, G.A.; Nazareno, M.A.; Pereira, S.V.; Raba, J.; Bertolino, F.A. Amperometric biosensor based on laccase immobilized onto a nanostructured screen-printed electrode for determination of polyphenols in propolis. Microchem. J. 2019, 144, 13–18. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Fu, J.; Huang, F.; Wei, Q. TiO2-CuCNFs based laccase biosensor for enhanced electrocatalysis in hydroquinone detection. J. Electroanal. Chem. 2016, 766, 16–23. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Vasilescu, I.; Radoi, A.; Litescu, S.C.; Radu, G.L. Disposable biosensor based on platinum nanoparticles-reduced graphene oxide-laccase biocomposite for the determination of total polyphenolic content. Talanta 2013, 110, 164–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, D.; Wei, Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf. B Biointerfaces 2020, 186, 110683. [Google Scholar] [CrossRef]
- Rubio-Govea, R.; Hickey, D.P.; García-Morales, R.; Rodriguez-Delgado, M.; Domínguez-Rovira, M.A.; Minteer, S.D.; Ornelas-Soto, N.; García-García, A. MoS2 nanostructured materials for electrode modification in the development of a laccase based amperometric biosensor for non-invasive dopamine detection. Microchem. J. 2020, 155, 104792. [Google Scholar] [CrossRef]
- Kavetskyy, T.; Smutok, O.; Demkiv, O.; Maťko, I.; Švajdlenková, H.; Šauša, O.; Novák, I.; Berek, D.; Čechová, K.; Pecz, M.; et al. Microporous carbon fibers as electroconductive immobilization matrixes: Effect of their structure on operational parameters of laccase-based amperometric biosensor. Mater. Sci. Eng. C 2020, 109, 110570. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Peng, Z.; Li, A.; Liu, J. Simultaneous Enhancement of Bioactivity and Stability of Laccase by Cu2+/PAA/PPEGA Matrix for Efficient Biosensing and Recyclable Decontamination of Pyrocatechol. Anal. Chem. 2017, 89, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.H.; Eisele, A.P.P.; Valezi, C.F.; Mattos, G.J.; Schirmann, J.G.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Sartori, E.R. Exploring the exocellular fungal biopolymer botryosphaeran for laccase-biosensor architecture and application to determine dopamine and spironolactone. Talanta 2019, 204, 475–483. [Google Scholar] [CrossRef]
- Wardak, C.; Paczosa-Bator, B.; Malinowski, S. Application of cold plasma corona discharge in preparation of laccase-based biosensors for dopamine determination. Mater. Sci. Eng. C 2020, 116, 111199. [Google Scholar] [CrossRef] [PubMed]
- Cevher, Ş.C.; Bekmezci, S.A.; SaniyeSoylemez; Udum, Y.A.; Toppare, L.; Çırpan, A. Indenoquinoxalinone based conjugated polymer substrate for laccase biosensor. Mater. Chem. Phys. 2021, 257, 123788. [Google Scholar] [CrossRef]
- Gomes, A.; Mattos, G.J.; Coldibeli, B.; Dekker, R.F.H.; Barbosa Dekker, A.M.; Sartori, E.R. Covalent attachment of laccase to carboxymethyl-botryosphaeran in aqueous solution for the construction of a voltammetric biosensor to quantify quercetin. Bioelectrochemistry 2020, 135, 107543. [Google Scholar] [CrossRef]
- Zhao, K.; Veksha, A.; Ge, L.; Lisak, G. Near real-time analysis of para-cresol in wastewater with a laccase-carbon nanotube-based biosensor. Chemosphere 2021, 269, 128699. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef]
- Karami, C.; Taher, M.A. A catechol biosensor based on immobilizing laccase to Fe3O4 @Au core-shell nanoparticles. Int. J. Biol. Macromol. 2019, 129, 84–90. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Anwar, M.Z.; Kumar, A.; Otari, S.V.; Pagolu, R.T.; Kim, S.Y.; Kim, I.W.; Lee, J.K. Fe2O3 yolk-shell particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem. Eng. J. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Romero-Arcos, M.; Garnica-Romo, M.G.; Martínez-Flores, H.E. Characterization of Amperometric Laccase Biosensor Based on Carbon Nanotube. Procedia Technol. 2017, 27, 279–281. [Google Scholar] [CrossRef]
- Maleki, N.; Kashanian, S.; Nazari, M.; Shahabadi, N. A novel sensitive laccase biosensor using gold nanoparticles and poly L-arginine to detect catechol in natural water. Biotechnol. Appl. Biochem. 2019, 66, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yasa, M.; Deniz, A.; Forough, M.; Yildirim, E.; Persil Cetinkol, O.; Udum, Y.A.; Toppare, L. Construction of amperometric biosensor modified with conducting polymer/carbon dots for the analysis of catechol. J. Polym. Sci. 2020, 58, 3336–3348. [Google Scholar] [CrossRef]
- Chakroun Galai, H.; Rassas, I.; Namour, P.; Bonhomme, A.; Raimondi, G.; Besbes Hentati, S.; Jaffrezic-Renault, N. A Laccase/Chitosan-Lambda-Carrageenan Based Voltammetric Biosensor for Phenolic Compound Detection. Electroanalysis 2020, 32, 732–740. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Development of a novel electrochemical biosensor based on carbon nanofibers–cobalt phthalocyanine–laccase for the detection of p-coumaric acid in phytoproducts. Int. J. Mol. Sci. 2021, 22, 9302. [Google Scholar] [CrossRef] [PubMed]
- Bonet-San-emeterio, M.; Montiel, N.F.; Del Valle, M. Graphene for the building of electroanalytical enzyme-based biosensors. Application to the inhibitory detection of emerging pollutants. Nanomaterials 2021, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Salvo-Comino, C.; González-Gil, A.; Rodriguez-Valentin, J.; Garcia-Hernandez, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Biosensors platform based on chitosan/AuNPs/phthalocyanine composite films for the electrochemical detection of catechol. the role of the surface structure. Sensors 2020, 20, 2152. [Google Scholar] [CrossRef] [Green Version]
- Mercante, L.A.; Pavinatto, A.; Pereira, T.S.; Migliorini, F.L.; dos Santos, D.M.; Correa, D.S. Nanofibers interfaces for biosensing: Design and applications. Sens. Actuators Rep. 2021, 3, 100048. [Google Scholar] [CrossRef]
- Litescu, S.C.; Eremia, S.A.V.; Bertoli, A.; Pistelli, L.; Radu, G.L. Laccase-nafion based biosensor for the determination of polyphenolic secondary metabolites. Anal. Lett. 2010, 43, 1089–1099. [Google Scholar] [CrossRef]
- Sigawi, S.; Smutok, O.; Demkiv, O.; Zakalska, O.; Gayda, G.; Nitzan, Y.; Nisnevitch, M.; Gonchar, M. Immobilized formaldehyde-metabolizing enzymes from Hansenula polymorpha for removal and control of airborne formaldehyde. J. Biotechnol. 2011, 153, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, S.; Wardak, C.; Jaroszyńska-Wolińska, J.; Herbert, P.A.F.; Panek, R. Cold Plasma as an Innovative Construction Method of Voltammetric Biosensor Based on Laccase. Sensors 2018, 18, 4086. [Google Scholar] [CrossRef] [Green Version]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020, 163, 112299. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-Linked Enzyme Aggregates as Industrial Biocatalysts. Pharm. Process Chem. 2010, 15, 159–181. [Google Scholar]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021, 169, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.; Khan, F.; Micklefield, J. Selective covalent protein immobilization: Strategies and applications. Chem. Rev. 2009, 109, 4025–4053. [Google Scholar] [CrossRef]
- Jabbari, S.; Dabirmanesh, B.; Khajeh, K. Specificity enhancement towards phenolic substrate by immobilization of laccase on surface plasmon resonance sensor chip. J. Mol. Catal. B Enzym. 2015, 121, 32–36. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, P.T.; Le, T.N.; Kim, M.I. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens. Bioelectron. 2021, 182, 113187. [Google Scholar] [CrossRef] [PubMed]




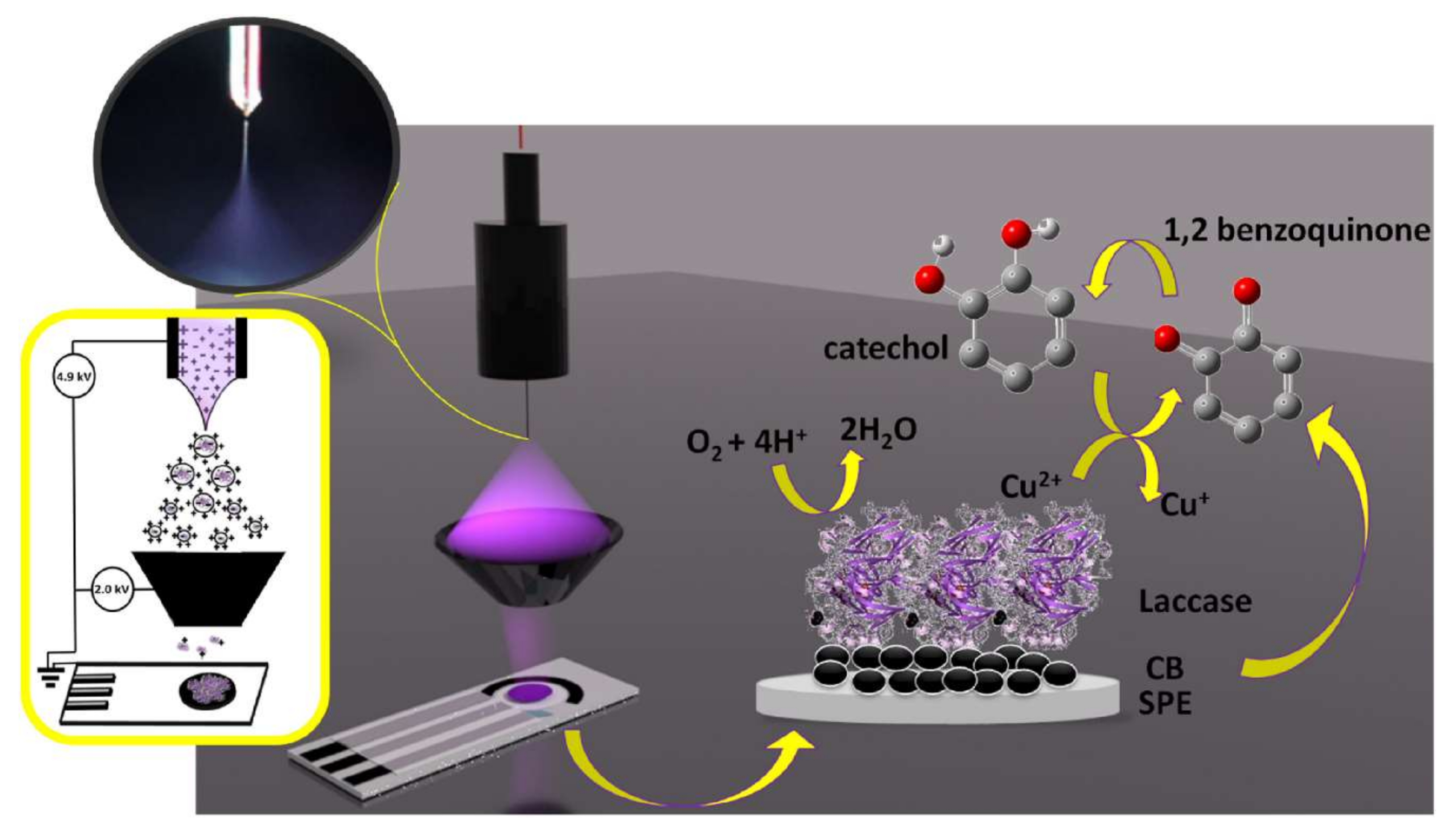
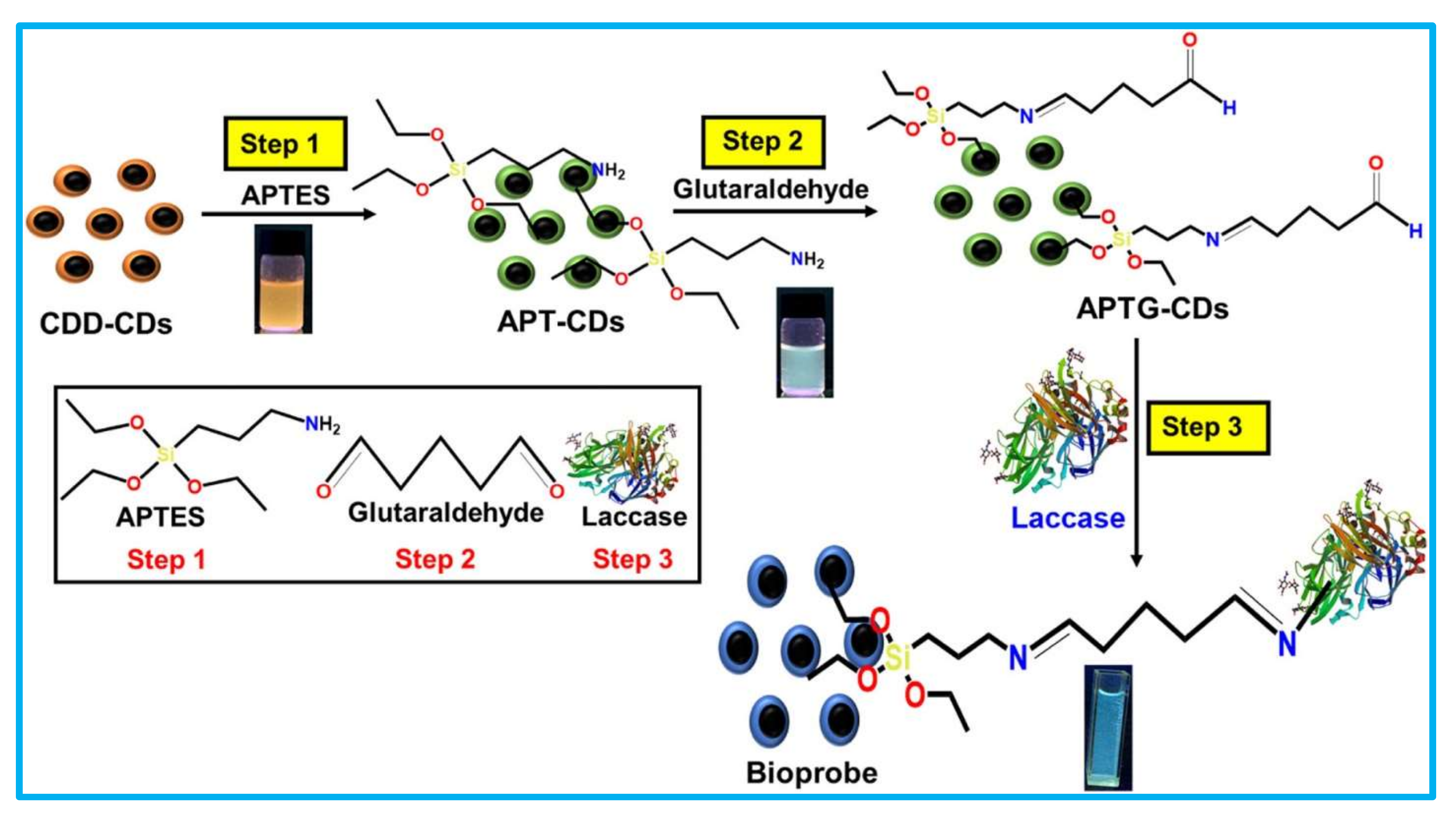
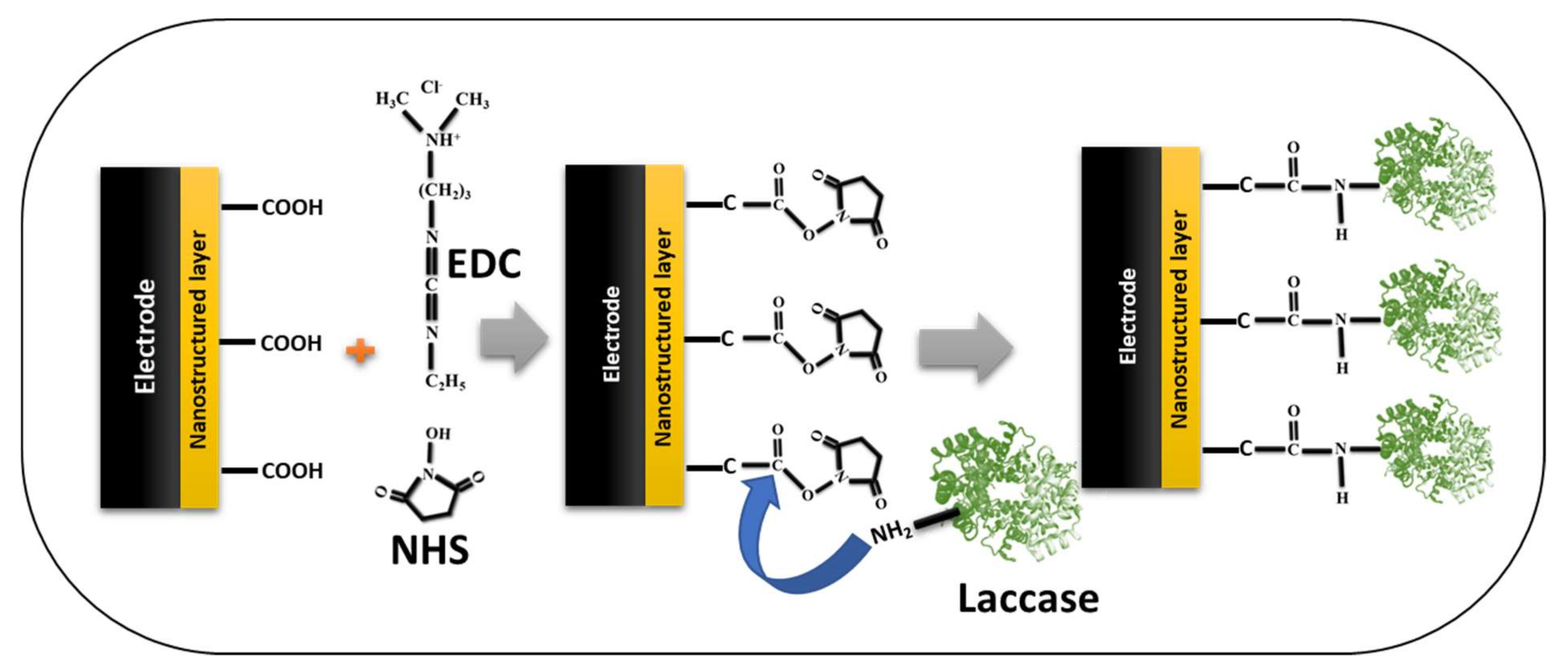
| Nano-Immobilization Parameters | Biosensor Development | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nano-Supports | Modification Made with Nano-Support | Laccase Source | Immobilization Conditions (pH: P, Temperature: T °C) | Type of Immobilization | Analyte (Analyte Detection Range) | Biosensor Type/Transduction | Stability of Laccase in Biosensor | Selectivity/Real Samples | Application | |
| Carboxyl functionalized multi-walled carbon nanotubes (COOH–MWCNT) | PEI coated AuNP | Coriolus hirsuta (Trametes hirsuta) | P: 4.2, T: room temperature | Adsorption | Catechol/ (0–1 mM) | Electrochemical/Amperometric | 77% catalytic response in 10 cycles. Laccase retained 86.0% after 30 days | - | Environment | [51] |
| Silica nanoparticles | Phytic acid | Trametes versicolor | - | Adsorption | Dopamine (0.99–103.10 μM) | Electrochemical/Amperometric | After 20 days of storage at 4 °C (>90%) | Determination of the recovery of DA in pharmaceutical injection | Pharmacological research | [52] |
| Graphene-cellulose Microfiber | - | Trametes versicolor | - | Adsorption | Catechol/(0.2 to 209.7 μM) | Electrochemical/Voltammetric | After 132 h 96.8 % initial response retained | Detection of catechol in different water samples | Environment | [53] |
| Cu3(PO4)2°3H2O microflowers | - | Trametes versicolor | P: 7.4, T: 4 | Entrapment | Epinephrine (0.4–400 μg/mL) | Optical/Colorimetric | Laccase reserved 96.6% and 64.4% of initial activity after stored for 30 days and 5 recycles | Favorable applicability for EP detection in human blood serum and urine samples | Clinical diagnosis and pharmacological research. | [54] |
| Multiwalled carboxylic-functionalized carbon nanotubes | Chitosan solution, BMIMBF4 ionic liquid | Trametes versicolor | T: 4 | Entrapment | Bisphenol A/(0.5–12 μM) | Electrochemical/Potentiostat–galvanostat | Laccase kept 87% of the initial response after one month | Bisphenol A detection from river water | Environment | [55] |
| Cu3(PO4)2·3H2O microflowers | - | Trametes versicolor | P: 7.4, T: 4 | Entrapment | Epinephrine/(0.4–400 μM) | Optical/Colorimetric | Laccase decreased the sensing capacity of epinephrine only 2% after being stored for 30 days | Ascorbic acid, citric acid, vitamin C, glucose, glycine, L-lysine, and urea had no interference | Clinical diagnosis and pharmacological research | [56] |
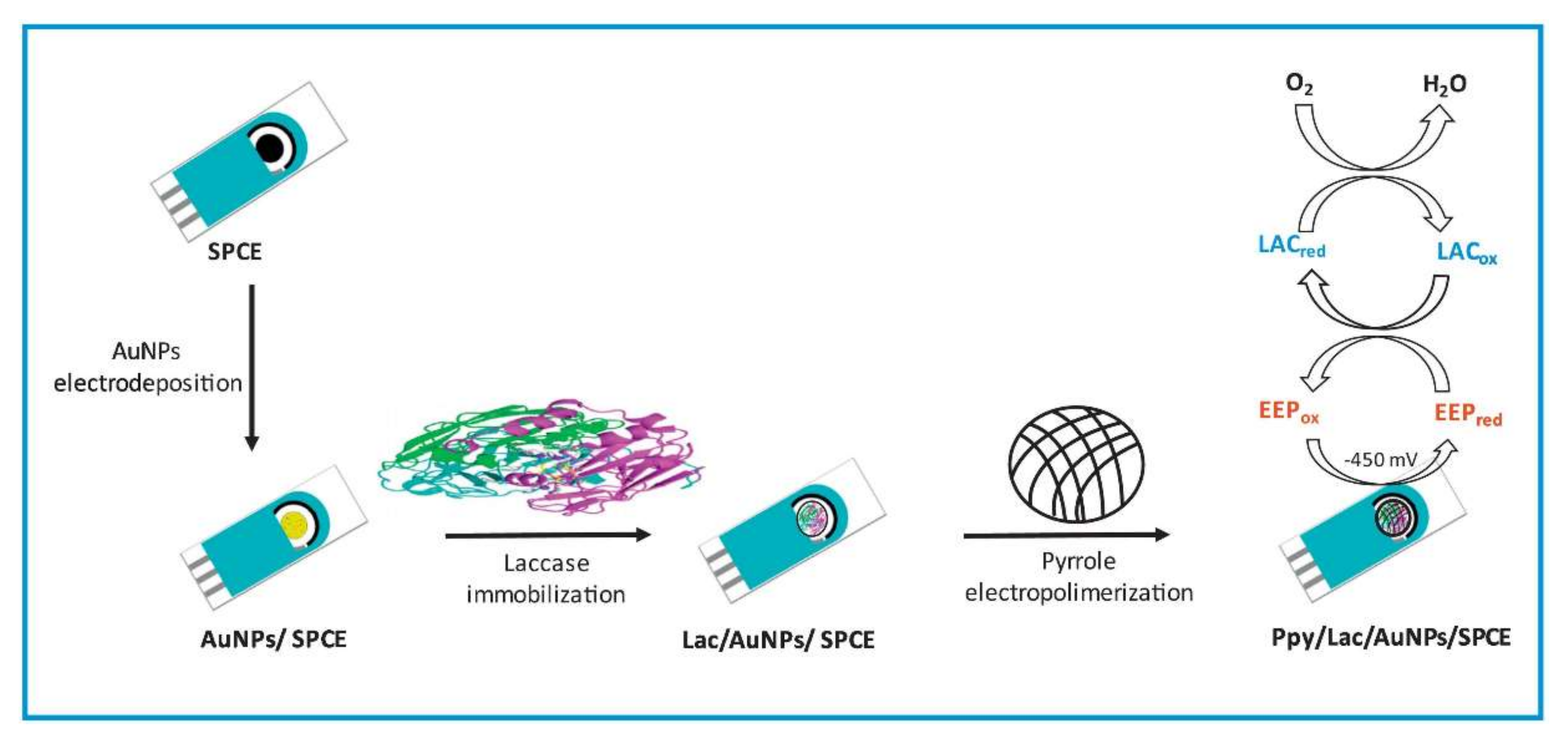
| AuNPs/SPCE | Polypyrrole | - | P: 7, T: 4 | Entrapment | Polyphenols/ (1–250 μM) | Electrochemical/Amperometric | Laccase sensor showed 85% activity retention after one-month storage | Polyphenols in propolis samples | Food | [57] |
| Poly (glycidyl methacrylate-co-n butyl acrylate) microspheres | Colloidal AuNPs, Glutaraldehyde | - | - | Entrapment | Tartrazine/ (0.2 to 14 µM) | Electrochemical/Amperometric | Laccase biosensor response was stable up to 30 days | Foods and beverages used as real samples | Food | [19] |
| Carbon nanofiber | Magnetic, Polydopamine (PDA), nickel nanoparticle | Trametes versicolor | P: 5.5 | Entrapment | Catechol/ (1 μM to 9.1 mM) | Electrochemical/Amperometric | Laccase-biosensor response to catechol was within 2.0% for 10 successive measurements indicated good stability. | Catechin, epicatechin, gallic acid, guaiacol, phenol, and aminophenol had no interference/catechol successfully detected in real tap water, and lake water | Environment | [44] |
| TiO2/CuCNFs | Nafion polymer | Trametes versicolor | P: 6.8 | Entrapment | Hydroquinon/(1–89.8 μM) | Electrochemical/Chronoamperometry | Even after a month, the biosensor still retained 93.45% of the initial response | No interference from guaiacol, 3,5-dinitro salicylic acid, vanillin, phenol, and catechol | Environment | [58] |
| Platinum nanoparticles and reduced graphene oxide | Nafion polymer | Trametes versicolor | - | Entrapment | Caffeic acid/(0.2–2 μM) | Electrochemical/Amperometric | After six weeks, a remnant response of 87.4% from its initial value | Total polyphenolic content from tea infusions | Food | [59] |
| AuNPs-MoS2 | Nafion polymer | - | P: 5, T: 4 | Entrapment | Catechol (2–2000 μM) | Electrochemical/Amperometric | Ten repeated cycles gave a 3 % decreased response | Resorcinol, salicylic acid, phenol, and p-nitrophenol do not affect the response | Environment | [60] |
| MoS2 | Nafion polymer/TBAB | Trametes versicolor laccase from P. sanguineus CS43 (LacI and LacII) | - | Entrapment | Dopamine/(0.1 to 0.5 µM) | Electrochemical/Amperometric | - | Determination of dopamine in synthetic urine samples Table | Pharmacological research | [61] |
| Microporous carbon fibers | Cathodic polymer GY 83-0270 0005 | Trametes versicolor | P: 4.5 | Entrapment | Catechol, ABTS | Electrochemical/Amperometric | - | Wastewater sample | Environment | [62] |
| Pyrene-terminated block copolymer | Pyrolytic graphite (HOPG) | Trametes Versicolor | T: 4 | Entrapment | Pyrocatechol/(50 nM to 1 Mm) | Electrochemical/Voltammetric | After 30 days at 4 °C, response decreased by 4.23% | Highly sensitive (lowest detection limit of 50 nM) | Environment | [63] |
| MWCNTs | Botryosphaeran | B. rhodinaMAMB-05 | T: 4 | Entrapment | Dopamine (2.99–38.5 μmol/L) and Spironolactone (2.97–28.9 μmol/L) | Electrochemical/Voltammetric | In 60 days, 86.2% of the initial response was retained | Acetaminophen, epinephrine, guaiacol, catechol, and hydroquinone were detected. The biosensor was not specific but selective. | Pharmacological research | [64] |
| Multi-walled carbon nanotubes (MWCNT) | Soft Plasma Polymerization (SPP) technique | Cerrena unicolor C-139 | - | Cross-linking | Dopamine/(0.1–10 μM) | Electrochemical/Voltammetric | - | Dopamine determination in pharmaceutical preparation | Pharmacological research | [65] |
| PM1 electrolyzer | Glutaraldehyde | Trametes Versicolor | T: room temperature | Covalent | Catechol/(0.005–0.175 mM) | Electrochemical/amperometric | Ten repeated cycles gave the stable response | Catechol detection in tap water samples | Environment | [66] |
| Carboxymethyl-botryosphaeran | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide (EDC/NHS) | Botryosphaeria rhodian MAMB-0 | P: 6, T: 16 | Covalent | Quercetin/ (4. 98–50.0 × 10−8 mol L−1) | Electrochemical/Voltammetric | Ten repeated cycles gave stable response | Quercetin detection in red wine, green tea, apple juice lemon juice | Food | [67] |
| Carbon nanotubes (CNTs) | 1-ethyl-3-(3-dimethylaminopropycarbodiimide and N-hydroxysuccinimide (EDC/NHS), Glutaraldehyde | Rhus vernicifera | - | Covalent | p-cresol/ (0.2–25 ppm) | Electrochemical/Voltammetric | Twenty repeated cycles gave the stable response | Detects p-cresol from environmental lab wastewater under the interference of metal ions and other organics | Environment | [68] |
| Carbon dots (CDs) | Curcumin, dimethylformamide, 3-(aminopropyl)-triethoxysilane, Glutaraldehyde | Trametes versicolor | P: 7.4, T: 25 | Covalent | Dopamine/(0–30 μM) | Optical/fluorescence | Good stability to the continuous illumination under Xe lamp for 1 h (5-min intervals) | Detection in human serum and cerebrospinal fluid | Pharmacological research | [69] |
| Fe3O4@Au core-shell nanoparticles | NHS and EDC | Trametes versicolor | P: 6.8, T: 4 | Covalent | Catechol/(5.0–70.0 μM) | Optical/colorimetric | - | - | Environment | [70] |
| Fe2O3 yolk-shell particle | Glutaraldehyde, APTES, carbodiimide, cyano, and PEI | Trametes Versicolor | - | Covalent | 2,6-dimethoxyphenol (0.25–250 μM) | Electrochemical/Voltammetric | - | Gave a response to other phenolic compounds, as well as guaiacol, pyrogallol, and L-dopa/synthetic wastewater | Environment | [71] |
| Carbon nanotube | H2SO4 (98%) and HNO3 (70%), Glutaraldehyde | - | - | Covalent | Phenolic content | Electrochemical/Amperometric | - | - | Environment | [72] |
| Plasma-polymerized allylamine (PPAA) | EDC/NHS activation | Trametes versicolor (ATCC 32745) | P: 7 | Covalent | 2,6-dimethoxyphenol (DMP) | Electrochemical/Amperometric | - | Biosensor retained laccase activity for more than 6 months | Environment | [38] |
| Gold nanoparticles (AuNPs) | Poly-L-arginine, Glutaraldehyde | Trametes Versicolor | P: 6 | Covalent | Catechol/ (24–274 nM) | Electrochemical/Voltammetric | - | Catechin, phenol, and aminophenol did not affect sensitivity/tap and river water | Environment | [73] |
| Carbon dots (CDs) | PFTBD, Glutaraldehyde | Trametes versicolor | P: 7 | Covalent | Catechol/ (1.25–175 μM) | Electrochemical/Amperometric | Ten repeated cycles gave a stable response | Detection of catechol in tap water | Environment | [74] |
| Chitosan and λ-carrageenan polyelectrolyte complex | Glutaraldehyde | Trametes Versicolor | - | Covalent | Catechol/ (10−20 to 10−14 M) | Electrochemical/Voltammetric | The laccase biosensor re-trained more than 95% of its original response for 4 weeks | Detection of Polyphenols in Natural Oils | Environment | [75] |
| Carbon Nanofibers | Cobalt phthalocyanine, Glutaraldehyde | Trametes Versicolor | - | Covalent | p-Coumaric Acid/(0.4–6.4 µM) | Electrochemical/Voltamperometric | Biosensor laccase decreases 10.56 and 10.20 % after 8 days | Gallic acid, ascorbic acid, vanillic acid, and ferrulic acid have not shown much interference | Environment | [76] |
| Graphene oxide | Carbodiimide (EDAC), Sulfo-NHS solution | Agaricus bisporus | - | Covalent | Catechol/ (0–1.6 mmol/L) | Electrochemical/Voltammetric | - | EDTA and benzoic acid inhibited laccase reaction | Environment | [77] |
| Chitosan/AuNPs | Phthalocyanine, Glutaraldehyde | Agaricus bisporus | P: 7 | Covalent | Catechol/ (2.4–20.0 mmol/L) | Electrochemical/Amperometric | Good repeatability in 3 cycles | - | Environment | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadam, A.A.; Saratale, G.D.; Ghodake, G.S.; Saratale, R.G.; Shahzad, A.; Magotra, V.K.; Kumar, M.; Palem, R.R.; Sung, J.-S. Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors 2022, 10, 58. https://doi.org/10.3390/chemosensors10020058
Kadam AA, Saratale GD, Ghodake GS, Saratale RG, Shahzad A, Magotra VK, Kumar M, Palem RR, Sung J-S. Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors. 2022; 10(2):58. https://doi.org/10.3390/chemosensors10020058
Chicago/Turabian StyleKadam, Avinash A., Ganesh D. Saratale, Gajanan S. Ghodake, Rijuta G. Saratale, Asif Shahzad, Verjesh Kumar Magotra, Manu Kumar, Ramasubba Reddy Palem, and Jung-Suk Sung. 2022. "Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques" Chemosensors 10, no. 2: 58. https://doi.org/10.3390/chemosensors10020058
APA StyleKadam, A. A., Saratale, G. D., Ghodake, G. S., Saratale, R. G., Shahzad, A., Magotra, V. K., Kumar, M., Palem, R. R., & Sung, J.-S. (2022). Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors, 10(2), 58. https://doi.org/10.3390/chemosensors10020058










