Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View
Abstract
1. Introduction
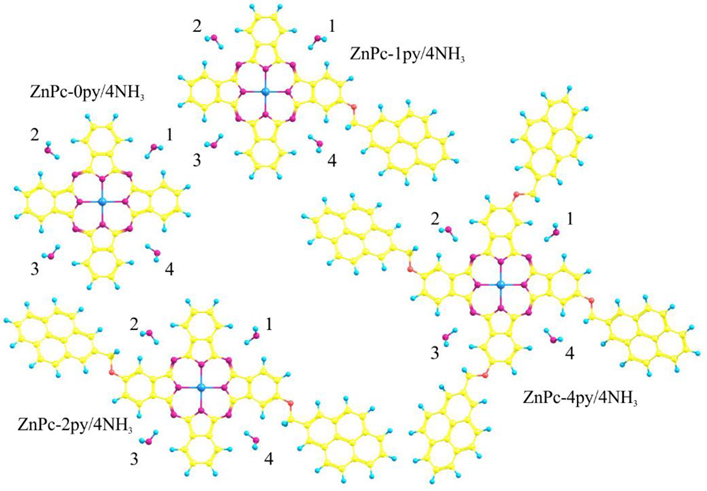
2. Objects and Methods of Investigation
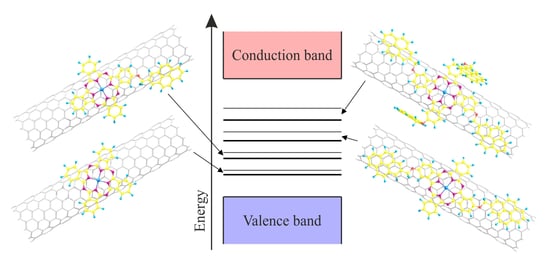
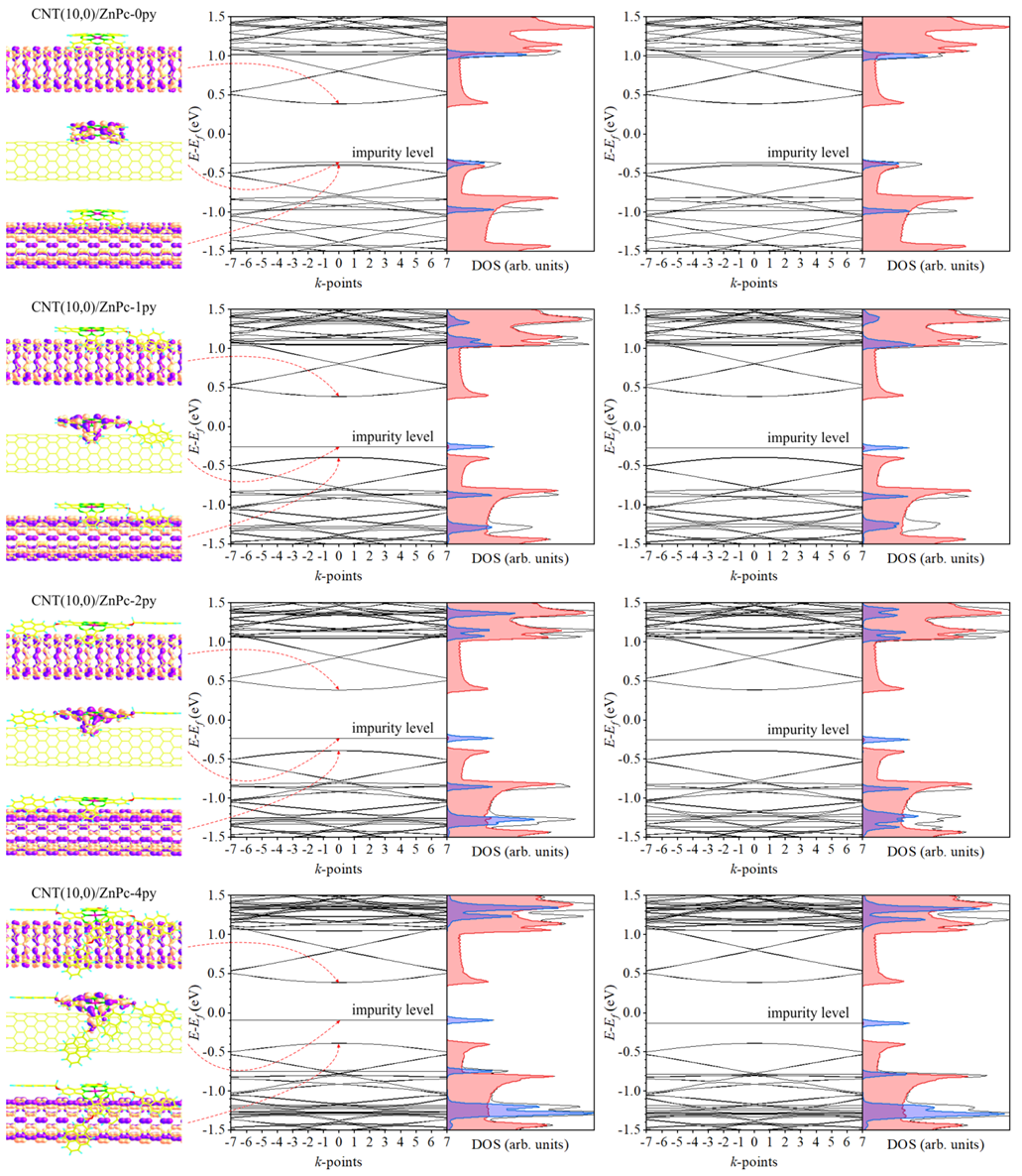
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Norizan, M.N.; Siti Zulaikha, N.D.; Norhana, A.B.; Syakir, M.I.; Norli, A. Carbon nanotubes-based sensor for ammonia gas detection—An overview. Polimery 2021, 66, 175–186. [Google Scholar] [CrossRef]
- Giordano, C.; Filatrella, G.; Sarno, M.; Di Bartolomeo, A. Multi-walled carbon nanotube films for the measurement of the alcoholic concentration. Micro Nano Lett. 2019, 14, 304–308. [Google Scholar] [CrossRef]
- Le, X.V.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Synergistic enhancement of ammonia gas-sensing properties at low temperature by compositing carbon nanotubes with tungsten oxide nanobricks. Vacuum 2019, 168, 108861. [Google Scholar] [CrossRef]
- Vu, T.D.; Cong, T.N.; Huu, B.L.; Duc, C.N.; Huu, L.N. Surface-modified carbon nanotubes for enhanced ammonia gas sensitivity at room temperature. J. Nanosci. Nanotechnol. 2019, 19, 7447–7451. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Saxena, S.; Srivastava, A.K.; Srivastava, R.; Kheraj, V. Metal-phthalocyanine functionalized CNTs sensor for chloroform series. Eur. J. Eng. Sci. Technol. 2019, 2, 71–80. [Google Scholar] [CrossRef]
- Ansari, N.; Lone, M.Y.; Shumaila; Ali, J.; Zulfequar, M.; Husain, M.; Islam, S.S.; Husain, S. Trace level toxic ammonia gas sensing of single-walled carbon nanotubes wrapped polyaniline nanofibers. J. Appl. Phys. 2020, 127, 044902. [Google Scholar] [CrossRef]
- Bouanis, F.Z.; Bensifia, M.; Florea, I.; Mahouche-chergui, S.; Carbonnier, B.; Grande, D.; Léonard, C.; Yassar, A.; Pribat, D. Non-covalent functionalization of single walled carbon nanotubes with Fe-/Co-porphyrin and Co-phthalocyanine for field-effect transistor applications. Org. Electron. 2021, 96, 106212. [Google Scholar] [CrossRef]
- Polyakov, M.S.; Basova, T.V. Hybrid materials of zinc (II) tetra-tert-butylphthalocyanine and zinc (II) tetra-tert-butylnaphthalocyanine with single walled carbon nanotubes: Structure and sensing propertie. Macroheterocycles 2017, 10, 31–36. [Google Scholar] [CrossRef]
- Kaya, E.N.; Tuncel, S.; Basova, T.; Banimuslem, H.; Hassan, A.; Gürek, A.G.; Ahsen, V.; Durmuş, M. Effect of pyrene substitution on the formation and sensor properties of phthalocyanine-single walled carbon nanotube hybrids. Sens. Actuators B. Chem. 2014, 199, 277–283. [Google Scholar] [CrossRef]
- Krasnov, P.O.; Ivanova, V.N.; Basova, T.V. Carbon nanotubes functionalized with Zinc (II) phthalocyanines: Effect of the expanded aromatic system and aromatic substituents on the binding energy. Appl. Surf. Sci. 2021, 547, 149172. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc p hthalocyanine as ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Saini, R.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Debnath, A.K. Room temperature ppb level Cl2 detection and sensing mechanism of highly selective and sensitive phthalocyanine nanowires. Sens. Actuators B. Chem. 2014, 203, 17–24. [Google Scholar] [CrossRef]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of copper phthalocyanine with nitrogen dioxide and ammonia investigation using X-ray absorption spectroscopy and chemiresistive gas measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Li, X.; Guo, Z.; Zhou, X.; Wu, Y. The effects of amino substituents on the enhanced ammonia sensing performance of PcCo/rGO hybrids. RSC Adv. 2018, 8, 41280–41287. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B. Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Kang, D.; Wang, B.; Wang, X.; Li, Y.; Chen, Z.; He, C.; Wu, Y. Stably dispersed metallophthalocyanine noncovalently bonded to multiwalled carbon nanotubes for ammonia sensing at room temperature. Sens. Actuators B. Chem. 2017, 246, 262–270. [Google Scholar] [CrossRef]
- Prasongkit, J.; Tangsukworakhun, S.; Jaisutti, R.; Osotchan, T. Highly sensitive and selective sensing of acetone and hydrogen sulfide using metal phthalocyanine—carbon nanotube hybrids. Appl. Surf. Sci. 2020, 532, 147314. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Huerta, L.; Basiuk, V.A. Noncovalent bonding of 3d metal (II) phthalocyanines with single-walled carbon nanotubes: A combined DFT and XPS study. Appl. Surf. Sci. 2019, 470, 622–630. [Google Scholar] [CrossRef]
- Mendoza-Domínguez, C.U.; Basiuk, V.A. Adsorption of yttrium bisphthalocyanine on pristine and defect-contaning graphene models: A DFT study. Diam. Relat. Mater. 2022, 126, 109051. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Yu, Z.; Zhou, X.; Kang, D.; Wu, Y.; Chen, Z.; He, C.; Zhou, X. The effects of central metals on ammonia sensing of metallophthalocyanines covalently bonded to graphene oxide hybrids. RSC Adv. 2017, 7, 34215–34225. [Google Scholar] [CrossRef]
- Ridhi, R.; Neeru; Gautam, S.; Saini, G.S.S.; Tripathi, S.K.; Rawat, J.S.; Jha, P. Study of the effect of orbital on interaction behaviour of SWCNT-metal phthalocyanines composites with ammonia gas. Sens. Actuators B. Chem. 2021, 337, 129767. [Google Scholar] [CrossRef]
- Dasari, B.S.; Taube, W.R.; Agarwal, P.B.; Rajput, M.; Kumar, A.; Akhtar, J. Room temperature single walled carbon nanotubes (SWCNT) chemiresistive ammonia gas sensor. Sens. Transducers 2015, 190, 24–30. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Kaya, E.N.; Basova, T.; Polyakov, M.; Durmuş, M.; Hassan, A. Hybrid materials of pyrene substituted phthalocyanines with single-walled carbon nanotubes: Structure and sensing properties. RSC Adv. 2015, 5, 91855–91862. [Google Scholar] [CrossRef]
- Polyakov, M.S.; Basova, T.V.; Göksel, M.; Şenocak, A.; Demirbaş, E.; Durmuş, M.; Kadem, B.; Hassan, A. Effect of covalent and non-covalent linking of zinc(II) phthalocyanine functionalised carbon nanomaterials on the sensor response to ammonia. Synth. Met. 2017, 227, 78–86. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, B.; Wang, X.; Li, Y.; Gai, S.; Wu, Y.; Cheng, X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9, 37518–37525. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.M.; Zhang, J.L.; Wu, F.; He, C.H.; Ren, Z.Y.; Wu, Y.Q. Manipulating polyaniline fibrous networks by doping tetra-β-carboxyphthalocyanine cobalt(II) for remarkably enhanced ammonia sensing. Chem. Mater. 2017, 29, 9509–9517. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Xian, L.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B. Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y. Layer-by-Layer Self-assembly of Co3O4 Nanorod-Decorated MoS2 Nanosheet-Based Nanocomposite toward High-Performance Ammonia Detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Ridhi, R.; Singh, S.; Saini, G.S.S.; Tripathi, S.K. Comparison of interaction mechanisms of copper phthalocyanine and nickel phthalocyanine thin films with chemical vapours. J. Phys. Chem. Solids 2018, 115, 119–126. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Z.; Wu, H.; Guo, L.; He, C.; Wang, B.; Wu, Y. Enhanced NH3-sensing behavior of 2,9,16,23-tetrakis(2,2,3,3-tetrafluoropropoxy) metal(II) phthalocyanine/multi-walled carbon nanotube hybrids: An investigation of the effects of central metals. Carbon 2014, 80, 268–278. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, Q.; Lu, Y.; Liu, H.; Hu, Y. Density functional theory study on the interaction between metalloporphyrins and NH3. Int. J. Quantum Chem. 2012, 113, 1137–1146. [Google Scholar] [CrossRef]
- Baggio, A.R.; Machado, D.F.S.; Carvalho-Silva, V.H.; Paterno, L.G.; de Oliveira, H.C.B. Rovibrational spectroscopic constants of the interaction between ammonia and metallo-phthalocyanines: A theoretical protocol for ammonia sensor design. Phys. Chem. Chem. Phys. 2017, 19, 10843–10853. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–24. [Google Scholar] [CrossRef]
- Kaya, E.N.; Şenocak, A.; Klyamer, D.D.; Demirbaş, E.; Basova, T.V.; Durmuş, M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci. Mater. Electron. 2019, 30, 7543–7551. [Google Scholar] [CrossRef]
- Ivanova, V.; Klyamer, D.; Krasnov, P.; Kaya, E.N.; Kulu, İ.; Kostakoglu, S.T.; Durmuş, M.; Basova, T. Hybrid materials based on pyrene-substituted metallo phthalocyanines as sensing layers for ammonia detection: Effect of the number of pyrene substituents. Sens. Actuators B. Chem. 2022, 375, 132843. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitric, T.; Dominguez, A.; et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef]
- Lu, X.; Gaus, M.; Elstner, M.; Cui, Q. Parametrization of DFTB3/3OB for magnesium and zinc for chemical and biological applications. J. Phys. Chem. B 2015, 119, 1062–1082. [Google Scholar] [CrossRef] [PubMed]
- Gaus, M.; Goez, A.; Elstner, M. Parametrization and benchmark of DFTB3 for organic molecules. J. Chem. Theory Comput. 2013, 9, 338–354. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, P.O.; Basova, T.V.; Hassan, A. Interaction of metal phthalocyanines with carbon zigzag and armchair nanotubes with different diameters. Appl. Surf. Sci. 2018, 457, 235–240. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. J. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of Xα theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Van Alsenoy, C. Ab initio calculations on large molecules: The multiplicative integral approximation. J. Comp. Chem. 1988, 9, 620–626. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Todd, A.; Keith, T.K. AIMAll, version 19.10.12; Gristmill Software: Overland Park, KS, USA, 2019; Available online: aim.tkgristmill.com (accessed on 24 October 2022).
- Bushmarinov, I.S.; Lyssenko, K.A.; Antipin, M.Y. Atomic energy in the ’Atoms in Molecules’ theory and its use for solving chemical problems. Russ. Chem. Rev. 2009, 78, 283–302. [Google Scholar] [CrossRef]

| x | Eg1, eV | Eg2, eV | ΔE, eV | n1/n2 |
|---|---|---|---|---|
| 0 | 0.774 | 0.774 | 0.005 | 1.10 |
| 1 | 0.774 | 0.773 | 0.013 | 1.29 |
| 2 | 0.775 | 0.774 | 0.019 | 1.45 |
| 4 | 0.772 | 0.772 | 0.038 | 2.10 |
| x | Position Number of the NH3 Molecule (in Accordance with Figure 2) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 0 | 0.141 | 0.141 | 0.141 | 0.141 |
| 1 | 0.133 | 0.127 | 0.141 | 0.215 |
| 2 | 0.139 | 0.149 | 0.145 | 0.204 |
| 4 | 0.156 | 0.156 | 0.156 | 0.156 |
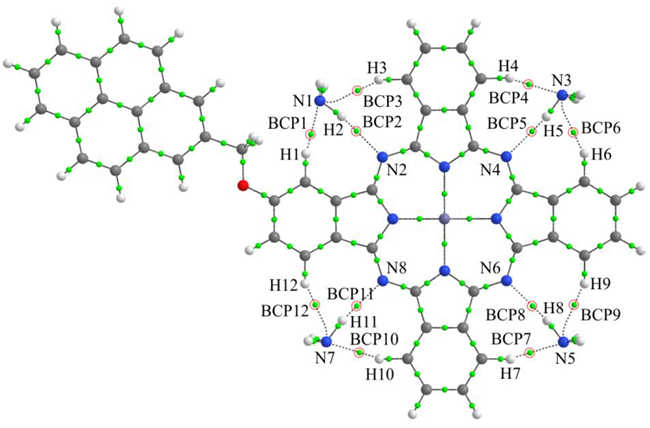
| BCP | Atoms | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 | q, e |
|---|---|---|---|---|
| 1 | N1-H1 | 0.146 | 1.602 | 0.148 |
| 2 | N2-H2 | 0.117 | 1.162 | - |
| 3 | N1-H3 | 0.096 | 1.050 | 0.051 |
| 4 | N3-H4 | 0.140 | 1.543 | 0.106 |
| 5 | N4-H5 | 0.129 | 1.270 | - |
| 6 | N3-H6 | 0.098 | 1.069 | 0.046 |
| 7 | N5-H7 | 0.137 | 1.527 | 0.107 |
| 8 | N6-H8 | 0.125 | 1.238 | - |
| 9 | N5-H9 | 0.100 | 1.092 | 0.047 |
| 10 | N7-H10 | 0.138 | 1.530 | 0.107 |
| 11 | N8-H11 | 0.126 | 1.250 | - |
| 12 | N7-H12 | 0.099 | 1.082 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnov, P.; Ivanova, V.; Klyamer, D.; Fedorov, A.; Basova, T. Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View. Chemosensors 2022, 10, 479. https://doi.org/10.3390/chemosensors10110479
Krasnov P, Ivanova V, Klyamer D, Fedorov A, Basova T. Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View. Chemosensors. 2022; 10(11):479. https://doi.org/10.3390/chemosensors10110479
Chicago/Turabian StyleKrasnov, Pavel, Victoria Ivanova, Darya Klyamer, Aleksandr Fedorov, and Tamara Basova. 2022. "Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View" Chemosensors 10, no. 11: 479. https://doi.org/10.3390/chemosensors10110479
APA StyleKrasnov, P., Ivanova, V., Klyamer, D., Fedorov, A., & Basova, T. (2022). Phthalocyanine-Carbon Nanotube Hybrid Materials: Mechanism of Sensor Response to Ammonia from Quantum-Chemical Point of View. Chemosensors, 10(11), 479. https://doi.org/10.3390/chemosensors10110479