Deferoxamine-Based Materials and Sensors for Fe(III) Detection
Abstract
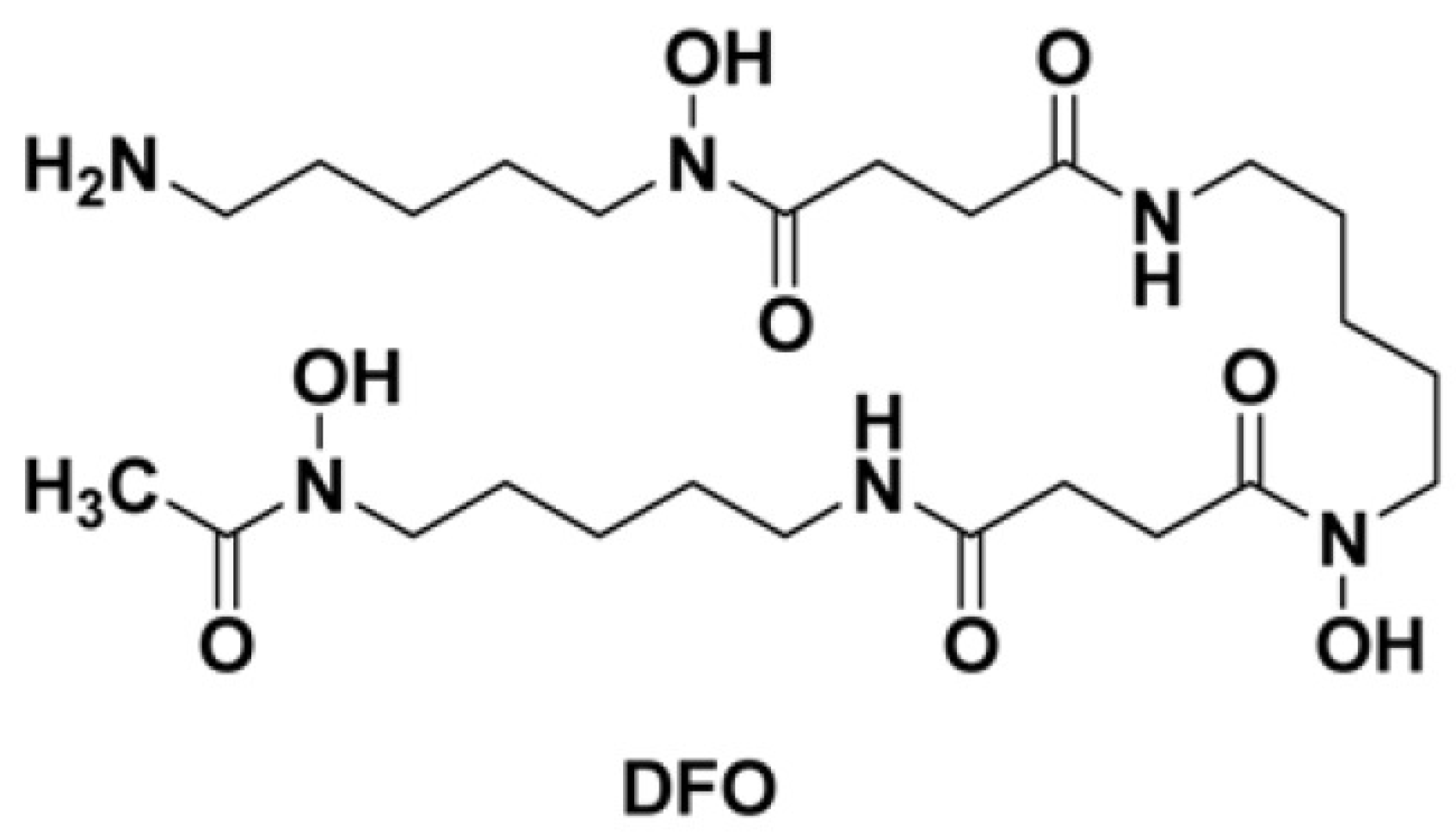
1. Introduction
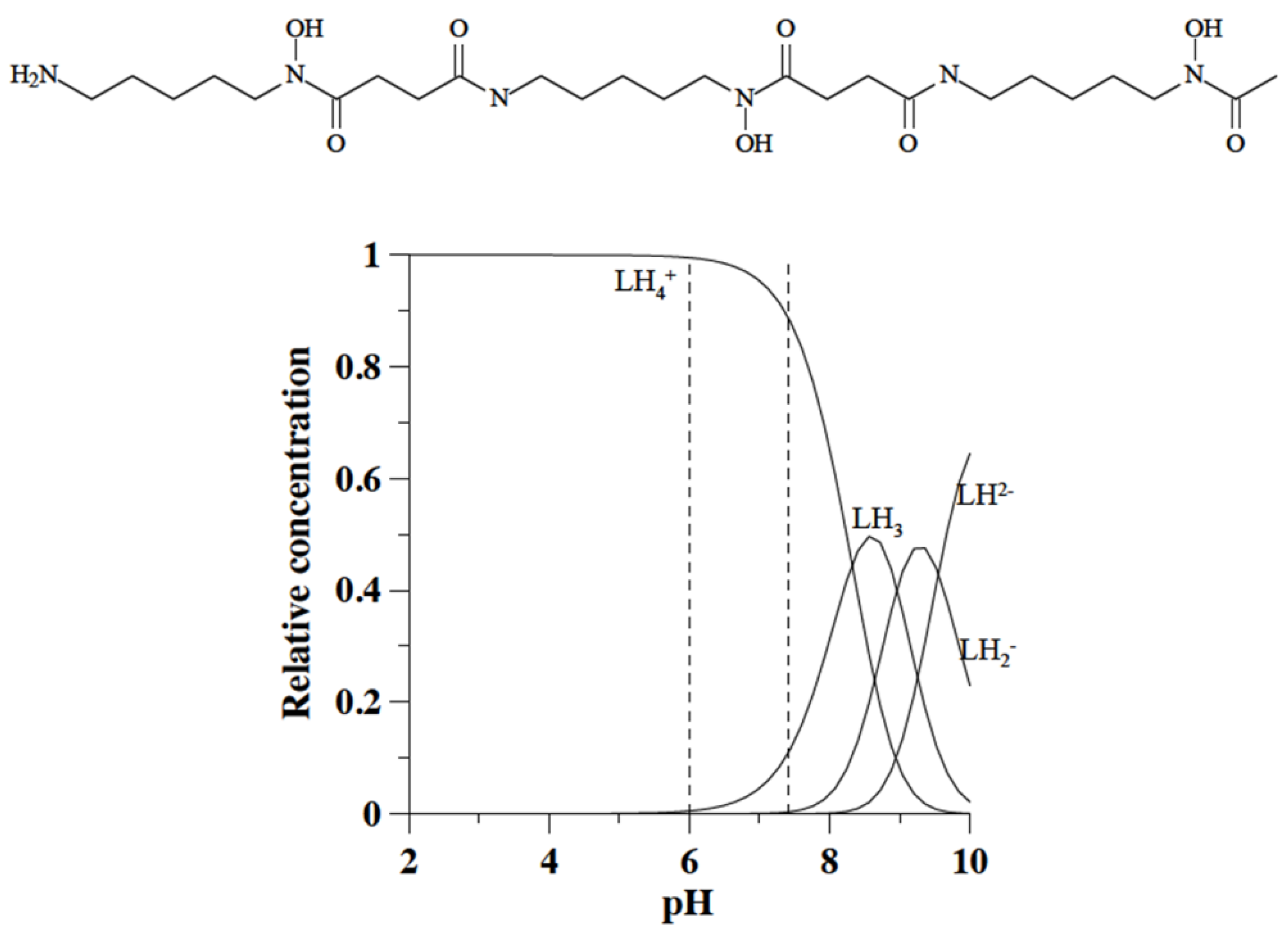
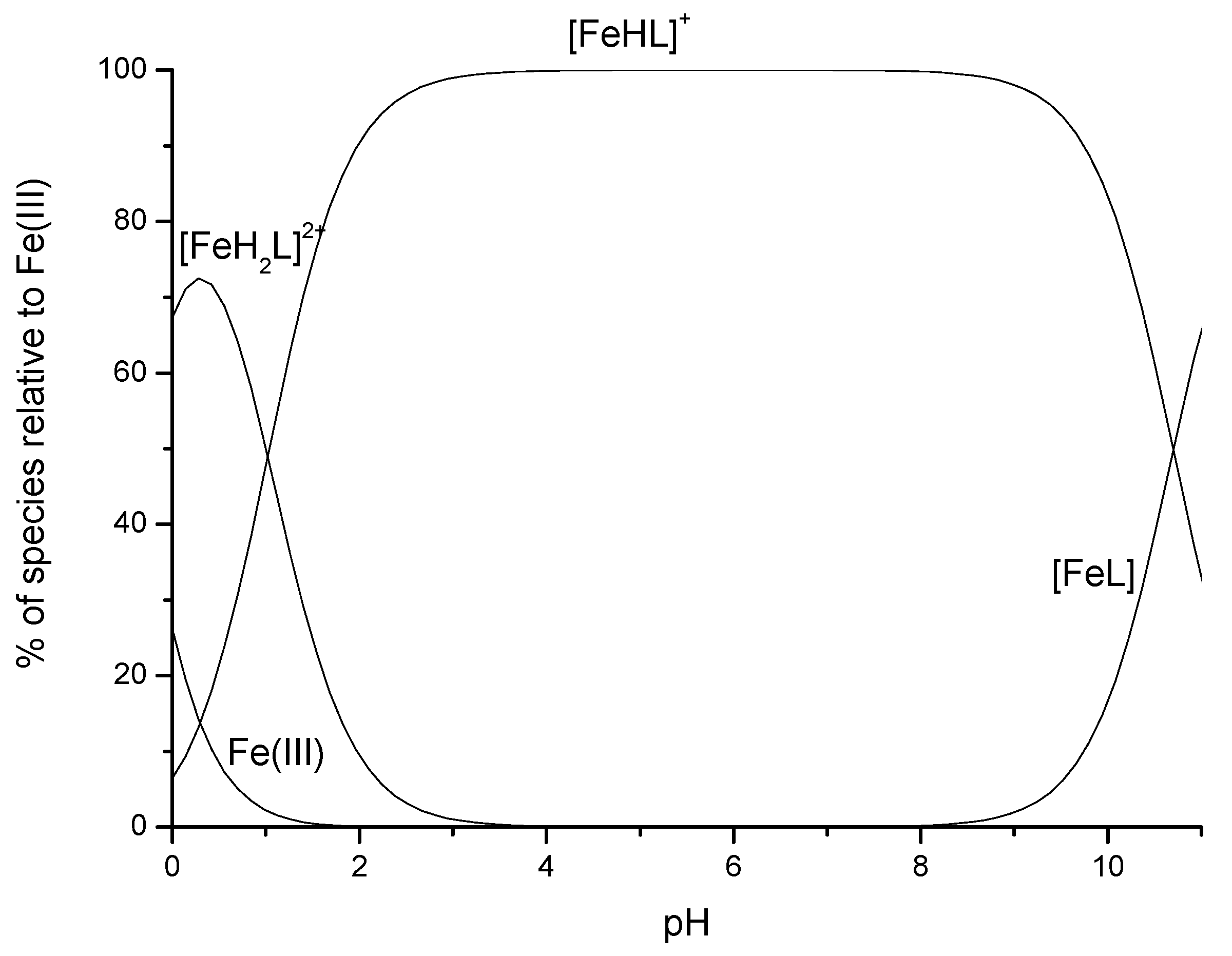
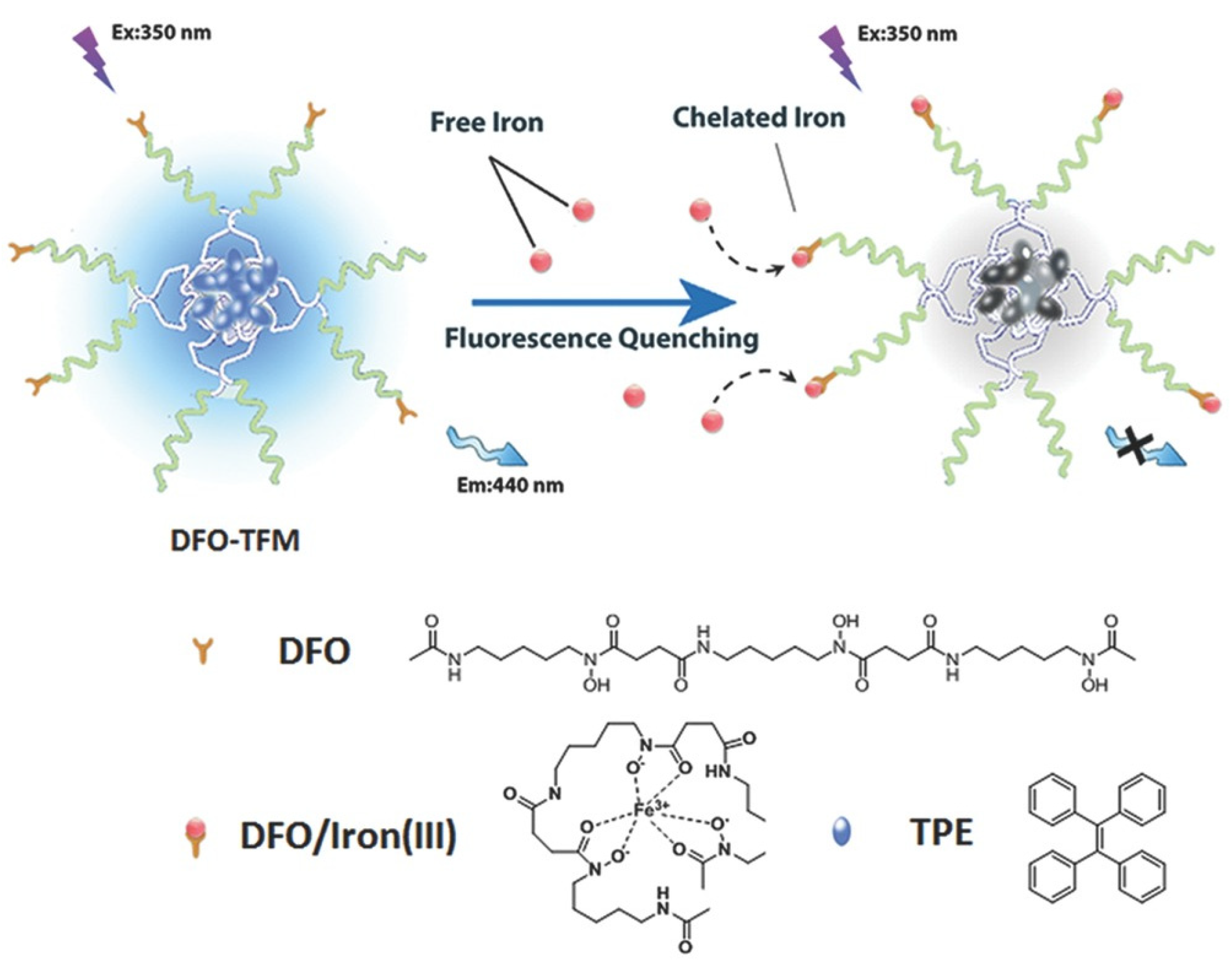
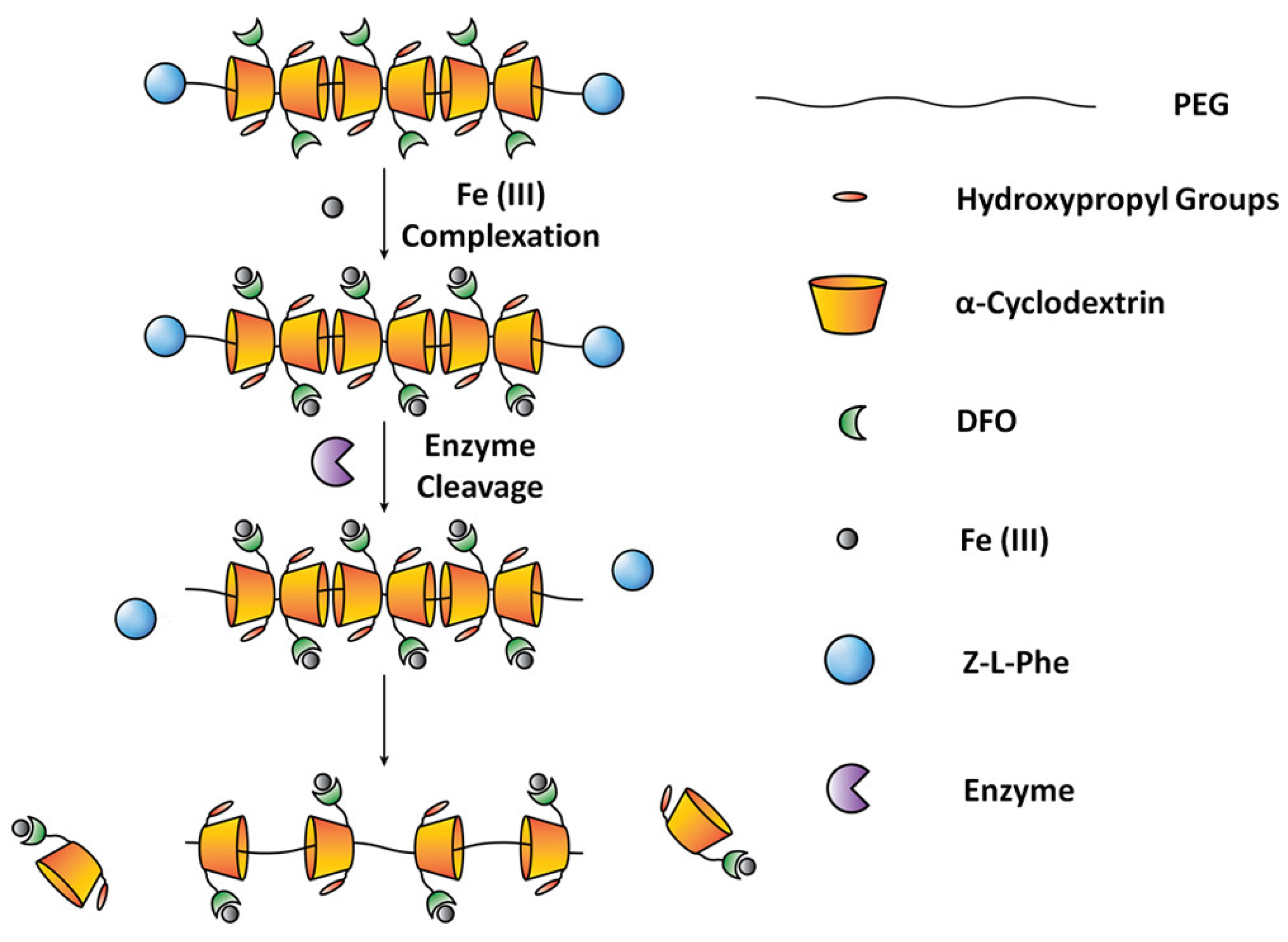
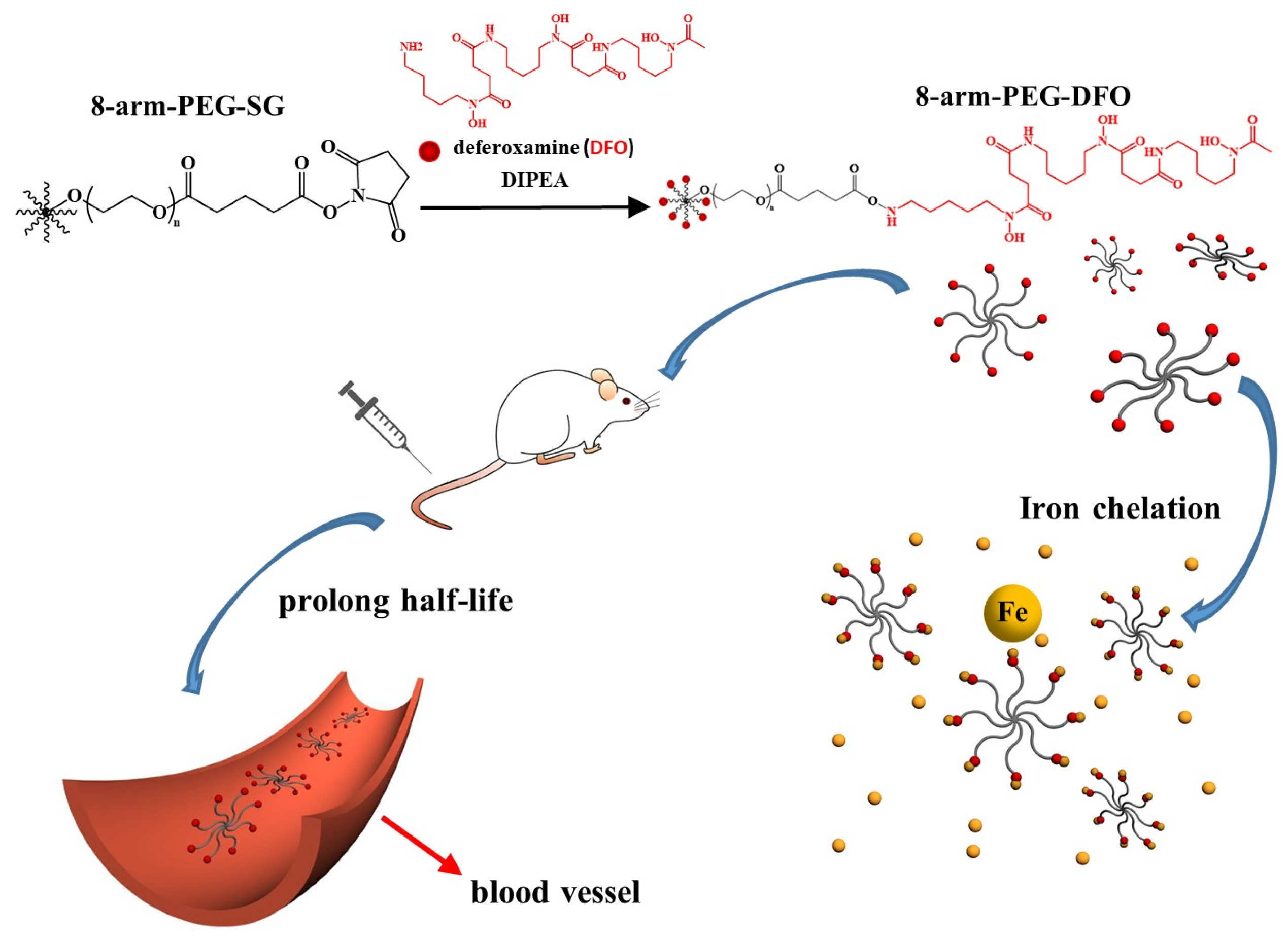
2. DFO-Based Biopolymers for Chelation and Detection of Fe(III)
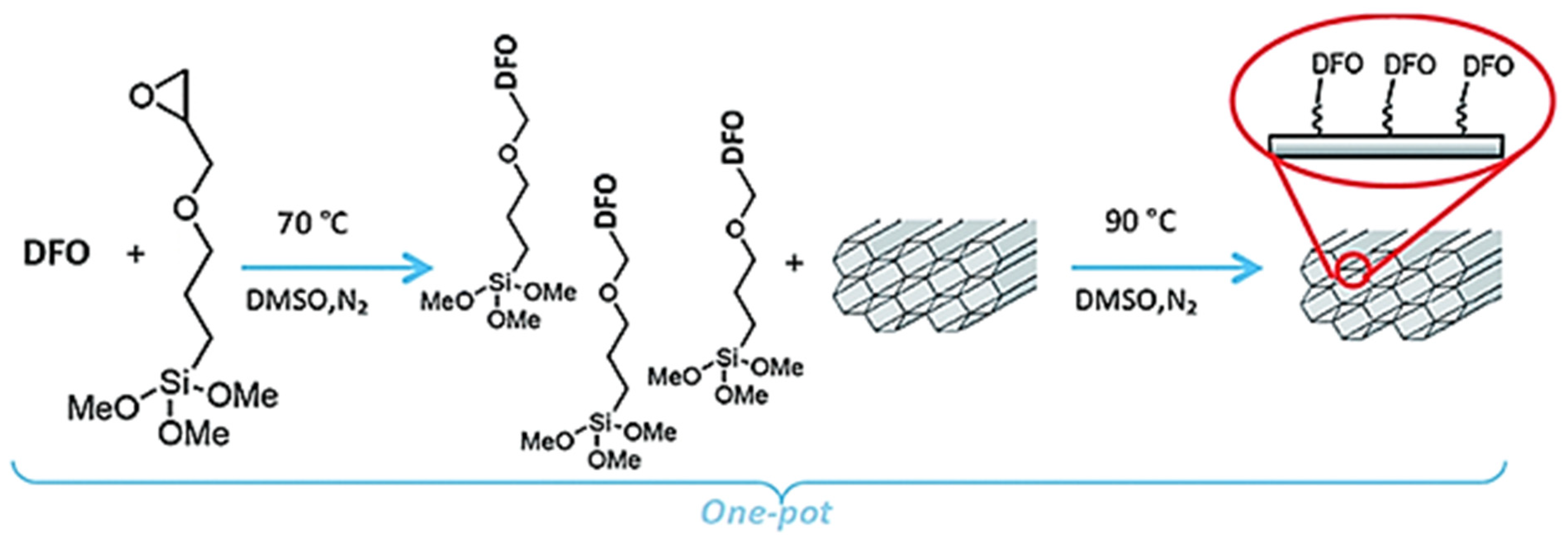
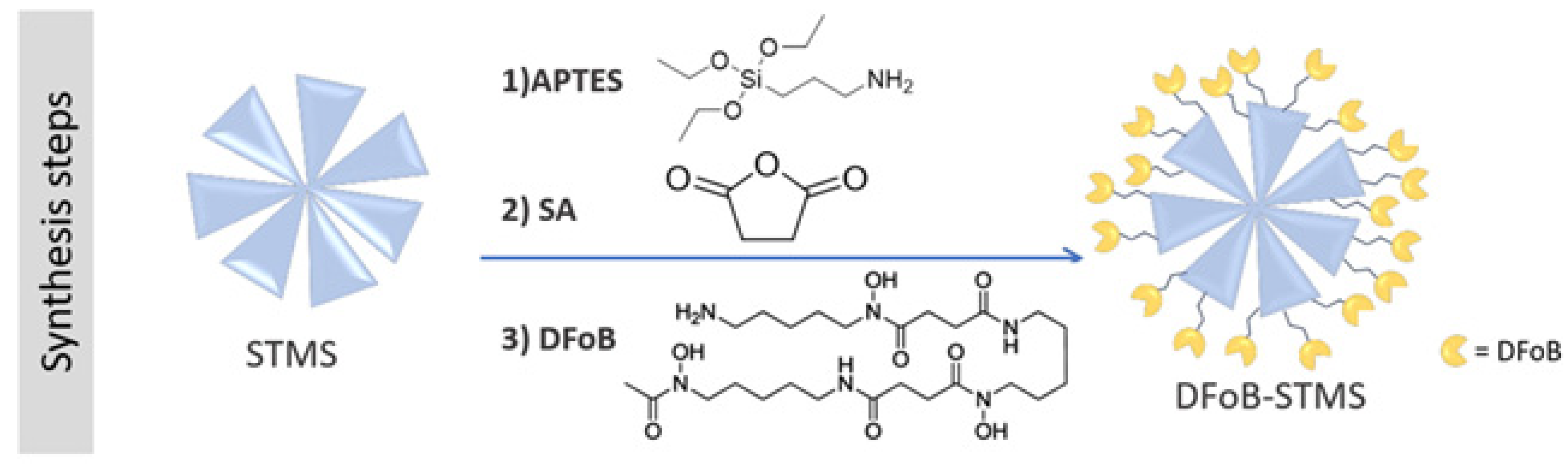
3. Solid Phase Chelating Materials for Fe(III) Sensing
4. DFO-Based Sensors for Fe(III) Detection
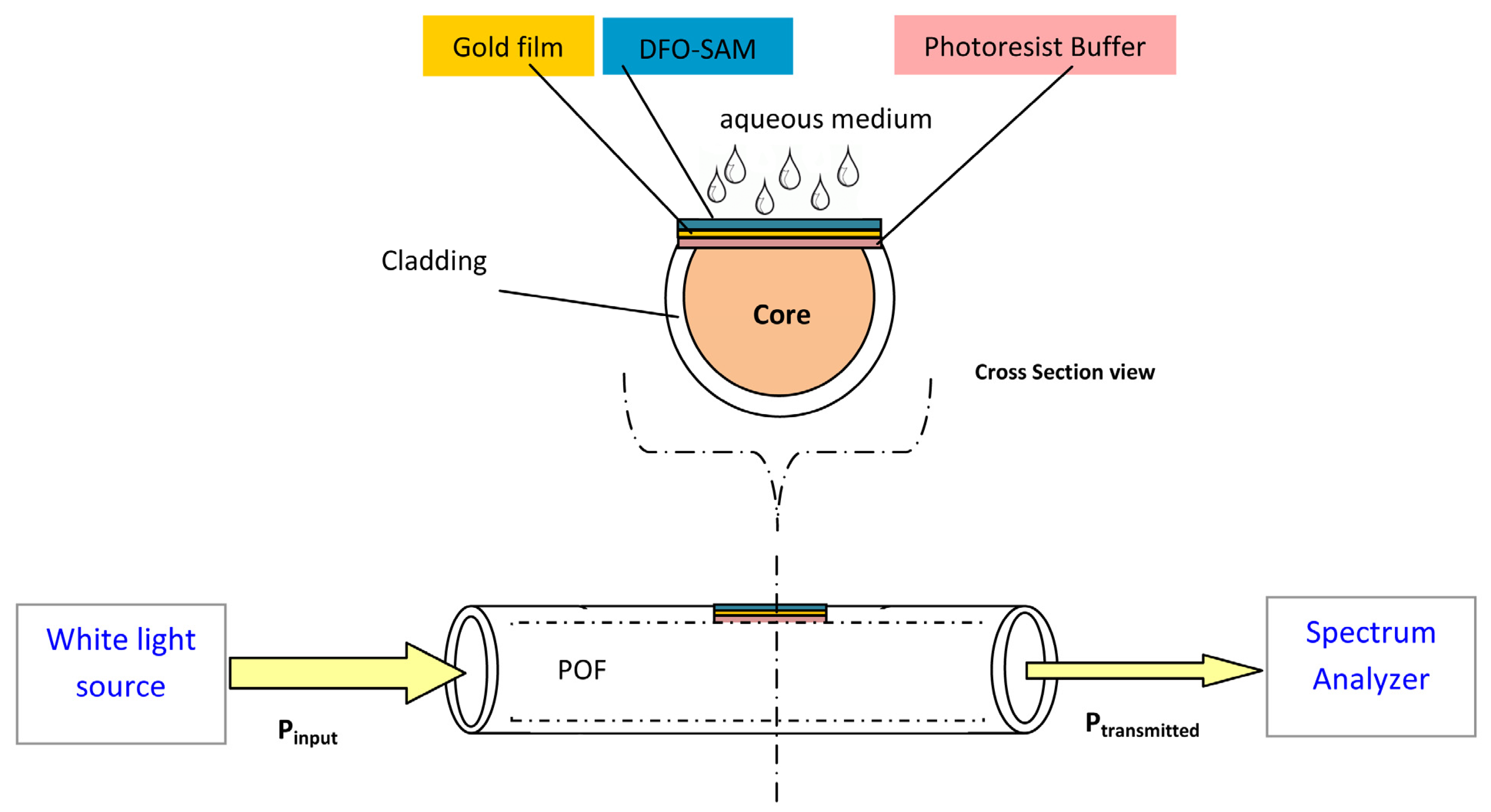
4.1. Optical Sensors
4.2. Electrochemical Sensors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Codd, R.; Richardson-Sanchez, T.; Telfer, T.J.; Gotsbacher, M.P. Advances in the Chemical Biology of Desferrioxamine B. ACS Chem. Biol. 2018, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Fang, Q. Siderophores for medical applications: Imaging, sensors, and therapeutics. Int. J. Pharm. 2021, 597, 120306. [Google Scholar] [CrossRef] [PubMed]
- Raymond, K.N.; Müller, G.; Matzanke, B.F. Complexation of iron by siderophores a review of their solution and structural chemistry and biological function. In Structural Chemistry: Topics in Current Chemistry, 1st ed.; Boschke, F.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 123. [Google Scholar]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, J.; Xiu, J.; Bai, L.; Guan, F.; Zhang, L.; Liu, J.; Zhang, L. Deferoxamine Compensates for Decreases in B Cell Counts and Reduces Mortality in Enterovirus 71-Infected Mice. Mar. Drugs 2014, 12, 4086–4095. [Google Scholar] [CrossRef]
- Anderegg, G.; L’Eplattenier, F.; Schwarzenbach, G. Hydroxamatkomplexe II. Die Anwendung der pH-Methode. Helv. Chim. Acta 1963, 46, 1400–1408. [Google Scholar] [CrossRef]
- Borgias, B.; Hugi, A.D.; Raymond, K.N. Isomerization and solution structures of desferrioxamine B complexes of aluminum(3+) and gallium(3+). Inorg. Chem. 1989, 28, 3538–3545. [Google Scholar] [CrossRef]
- Evers, A.; Hancock, R.D.; Martell, A.E.; Motekaitis, R.J. Metal ion recognition in ligands with negatively charged oxygen donor groups. Complexation of iron(III), gallium(III), indium(III), aluminum(III), and other highly charged metal ions. Inorg. Chem. 1989, 28, 2189–2195. [Google Scholar] [CrossRef]
- Hou, Z.; Whisenhunt, D.W., Jr.; Xu, J.; Raymond, K.N. Potentiometric, spectrophotometric, and 1H NMR study of four desferrioxamine B derivatives and their ferric complexes. J. Am. Chem. Soc. 1994, 116, 840–846. [Google Scholar] [CrossRef]
- Buglyó, P.; Culeddu, N.; Kiss, T.; Micera, G.; Sanna, D. Vanadium (IV) and vanadium (V) complexes of deferoxamine B in aqueous solution. J. Inorg. Biochem. 1995, 60, 45–59. [Google Scholar] [CrossRef]
- Hernlem, B.J.; Vane, L.M.; Sayles, G.D. Stability constants for complexes of the siderophore desferrioxamine B with selected heavy metal cations. Inorg. Chim. Acta 1996, 244, 179–184. [Google Scholar] [CrossRef]
- Farkas, E.; Enyedy, É.A.; Csóka, H. A comparison between the chelating properties of some dihydroxamic acids, desferrioxamine B and acetohydroxamic acid. Polyhedron 1999, 18, 2391–2398. [Google Scholar] [CrossRef]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Crespo-Alonso, M.; Sanna, G.; Zoroddu, M.A.; Alberti, G.; Biesuz, R. A Speciation Study on the Perturbing Effects of Iron Chelators on the Homeostasis of Essential Metal Ions. PLoS ONE 2015, 10, e0133050. [Google Scholar] [CrossRef]
- Toporivska, Y.; Gumienna-Kontecka, E. The solution thermodynamic stability of desferrioxamine B (DFO) with Zr(IV). J. Inorg. Biochem. 2019, 198, 110753. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M.; Rogers, J.T.; Leedman, P.J. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int. J. Biochem. Cell Biol. 1999, 31, 1139–1152. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Pattichis, K.; Neocleous, K.; Kolnagou, A. The design and development of deferiprone (L1) and other iron chelators for clinical use: Targeting methods and application prospects. Curr. Med. Chem. 2004, 11, 2161–2183. [Google Scholar] [CrossRef] [PubMed]
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. World J. Gastroenterol. 2007, 13, 4755–4760. [Google Scholar] [CrossRef]
- Porter, J.B. Concepts and goals in the management of transfusional iron overload. Am. J. Hematol. 2007, 82, 1136–1139. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and Deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef]
- Zhu, F.; Zhong, J.; Hu, J.; Yang, P.; Zhang, J.; Zhang, M.; Li, Y.; Gu, Z. Carrier-Free Deferoxamine Nanoparticles against Iron Overload in Brain. CCS Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Jones, G.; Goswami, S.K.; Kang, H.; Choi, H.S.; Kim, J. Combating iron overload: A case for deferoxamine-based nanochelators. Nanomedicine 2020, 15, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, R.A.; Cerny, E.A.; Rahman, Y.E. Pharmacokinetics of the iron chelator desperrioxamine as affected by liposome encapsulation: Potential in treatment of chronic hemosiderosis. Life Sci. 1978, 22, 313–320. [Google Scholar] [CrossRef]
- Lau, E.H.; Cerny, E.A.; Rahman, Y.E. Liposome-encapsulated desferrioxamine in experimental iron overload. Br. J. Haematol. 1981, 47, 505–518. [Google Scholar] [CrossRef]
- Young, S.P.; Baker, E.; Huehns, E.R. Liposome entrapped desferrioxamine and iron transporting ionophores: A new approach to iron chelation therapy. Br. J. Haematol. 1979, 41, 357–363. [Google Scholar] [CrossRef]
- Salimi, A.; Zadeh, B.S.M.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293–307. [Google Scholar] [PubMed]
- Liu, Z.; Purro, M.; Qiao, J.; Xiong, M.P. Multifunctional polymeric micelles for combining chelation and detection of iron in living cells. Adv. Healthc. Mater. 2017, 6, 1700162. [Google Scholar] [CrossRef]
- Harmatz, P.; Grady, R.W.; Dragsten, P.; Vichinsky, E.; Giardina, P.; Madden, J.; Jeng, M.; Miller, B.; Hanson, G.; Hedlund, B. Phase Ib clinical trial of starch-conjugated Deferoxamine (40SD02): A novel long-acting iron chelator. Br. J. Haematol. 2007, 138, 374–381. [Google Scholar] [CrossRef]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and In-Vitro Characterization of Chitosan-Nanoparticles Loaded with the Iron Chelator Deferoxamine Mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef]
- Ashrafi, H.; Azadi, A.; Mohammadi-Samani, S.; Hamidi, M. New candidate delivery system for Alzheimer’s disease: Deferoxamine nanogels. Biointerface Res. Appl. Chem. 2020, 10, 7106–7119. [Google Scholar]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Salis, A.; Porcu, E.P.; Giunchedi, P.; Roldo, M.; Gavini, E. Composite chitosan/alginate hydrogel for controlled release of Deferoxamine: A system to potentially treat iron dysregulation diseases. Carbohydr. Polym. 2016, 136, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Loh, X.J. Cyclodextrin-based Supramolecular Architectures: Syntheses, Structures, and Applications for Drug and Gene Delivery. Adv. Drug Delivery Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Mondjinou, Y.; Hyun, S.H.; Kulkarni, A.; Lu, Z.R.; Thompson, D.H. Gd3+-1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic-2-hydroxypropyl-β-cyclodextrin/Pluronic Polyrotaxane as a Long Circulating High Relaxivity MRI Contrast Agent. ACS Appl. Mater. Interfaces 2015, 7, 22272–22276. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, T.M.; Purro, M.; Xiong, M.P. Enzymatically Biodegradable Polyrotaxane–Deferoxamine Conjugates for Iron Chelation. ACS Appl. Mater. Interfaces 2016, 8, 25788–25797. [Google Scholar] [CrossRef]
- Liu, Z.; Simchick, G.A.; Qiao, J.; Ashcraft, M.M.; Cui, S.; Nagy, T.; Zhao, Q.; Xiong, M.P. Reactive oxygen species-triggered dissociation of a polyrotaxane-based nanochelator for enhanced clearance of systemic and hepatic iron. ACS Nano 2021, 15, 419–433. [Google Scholar] [CrossRef]
- Rossi, N.A.A.; Zou, Y.; Scott, M.D.; Kizhakkedathu, J.N. RAFT synthesis of acrylic copolymers containing poly(ethylene glycol) and dioxolane functional groups: Towards well-defined aldehyde containing copolymers for bio-conjugation. Macromolecules 2008, 41, 5272–5282. [Google Scholar] [CrossRef]
- Rossi, N.A.; Mustafa, I.; Jackson, J.K.; Burt, H.M.; Horte, S.A.; Scott, M.D.; Kizhakkedathu, J.N. In vitro chelating, cytotoxicity, and blood compatibility of degradable poly(ethylene glycol)-based macromolecular iron chelators. Biomaterials 2009, 30, 638–648. [Google Scholar] [CrossRef]
- Gu, Z.; Gao, D.; Al-Zubaydi, F.; Li, S.; Singh, Y.; Rivera, K.; Holloway, J.; Szekely, Z.; Love, S.; Sinko, P.J. The effect of size and polymer architecture of doxorubicin-poly(ethylene) glycol conjugate nanocarriers on breast duct retention, potency and toxicity. Eur. J. Pharm. Sci. 2018, 121, 118–125. [Google Scholar] [CrossRef]
- Pasut, G.; Canal, F.; Via, L.D.; Arpicco, S.; Veronese, F.M.; Schiavon, O. Antitumoral activity of PEG–gemcitabine prodrugs targeted by folic acid. J. Control. Release 2008, 127, 239–248. [Google Scholar] [CrossRef]
- Schiavon, O.; Pasut, G.; Moro, S.; Orsolini, P.; Guiotto, A.; Veronese, F.M. PEG–Ara-C conjugates for controlled release. Eur. J. Med. Chem. 2004, 39, 123–133. [Google Scholar] [CrossRef]
- Dai, L.; Wang, L.; Deng, L.; Liu, J.; Lei, J.; Li, D.; He, J. Novel Multiarm Polyethylene glycol-Dihydroartemisinin Conjugates Enhancing Therapeutic Efficacy in Non-Small-Cell Lung Cancer. Sci. Rep. 2014, 4, 5871. [Google Scholar] [CrossRef]
- Zhao, H.; Rubio, B.; Sapra, P.; Wu, D.; Reddy, P.; Sai, P.; Martinez, A.; Gao, Y.; Lozanguiez, Y.; Longley, C.; et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjugate Chem. 2008, 19, 849–859. [Google Scholar] [CrossRef]
- Yu, B.; Yang, Y.; Liu, Q.; Zhan, A.; Yang, Y.; Liu, H. A Novel Star Like Eight-Arm Polyethylene Glycol-Deferoxamine Conjugate for Iron Overload Therapy. Pharmaceutics 2020, 12, 329. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Biesuz, R.; Emma, G.; Milanese, C.; Dacarro, G.; Taglietti, A.; Nurchi, V.M.; Alberti, G. Novel DFO-SAM on mesoporous silica for iron sensing. Part I. Synthesis optimization and characterization of the material. Analyst 2014, 139, 3932–3939. [Google Scholar] [CrossRef]
- Alberti, G.; Emma, G.; Colleoni, R.; Pesavento, M.; Nurchi, V.M.; Biesuz, R. Novel DFO-functionalized mesoporous silica for iron sensing. Part 2. Experimental detection of free iron concentration (pFe) in urine samples. Analyst 2014, 139, 3940–3948. [Google Scholar] [CrossRef] [PubMed]
- Duenas-Ramirez, P.; Bertagnolli, C.; Müller, R.; Sartori, K.; Boos, A.; Elhabiri, M.; Bégin-Colin, S.; Mertz, D. Highly chelating stellate mesoporous silica nanoparticles for specific iron removal from biological media. J. Colloid Interface Sci. 2020, 579, 140–151. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Deferoxamine-Modified Hybrid Materials for Direct Chelation of Fe(III) Ions from Aqueous Solutions and Indication of the Competitiveness of In Vitro Complexing toward a Biological System. ACS Omega 2021, 6, 15168–15181. [Google Scholar] [CrossRef]
- Yehuda, Z.; Hadar, Y.; Chen, Y. Immobilization of Fe Chelators on Sepharose Gel and Its Effect on Their Chemical Properties. J. Agric. Food Chem. 2003, 51, 5996–6005. [Google Scholar] [CrossRef]
- Xu, G.; Xiao, Y.; Cheng, L.; Zhou, R.; Xu, H.; Chai, Y.; Lang, M. Synthesis and rheological investigation of self-healable deferoxamine grafted alginate hydrogel. J. Polym. Sci. B Polym. Phys. 2017, 55, 856–865. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Cappai, R.; Spano, N.; Sanna, G. A Friendly Complexing Agent for Spectrophotometric Determination of Total Iron. Molecules 2021, 26, 3071. [Google Scholar] [CrossRef]
- Alberti, G.; Emma, G.; Colleoni, R.; Nurchi, V.M.; Pesavento, M.; Biesuz, R. Simple solid-phase spectrophotometric method for free iron(III) determination. Arab. J. Chem. 2019, 12, 573–579. [Google Scholar] [CrossRef]
- Emma, G.; Guiso, M.G.; Alberti, G.; Dacarro, G.; Taglietti, A.; Biesuz, R. Chemically modified mesoporous silica for free iron sensing. In Acta of the XXII International Symposium on Metal Complexes, Proceedings of the ISMEC 2011, Giardini Naxos, Italy, 13–16 June 2011; Università degli Studi di Messina: Messina, Italy, 2011. [Google Scholar]
- Alberti, G.; Quattrini, F.; Colleoni, R.; Nurchi, V.M.; Biesuz, R. Deferoxamine–paper for iron(III) and vanadium(V) sensing. Chem. Pap. 2015, 69, 1024–1032. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Disposable and Low-Cost Colorimetric Sensors for Environmental Analyses. Int. J. Environ. Res. Public Health 2020, 17, 8331. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Santos, M.A.; Nurchi, V.M.; Biesuz, R. DFO@EVOH and 3,4-HP@EVOH: Towards New Polymeric Sorbents for Iron(III). Chemosensors 2020, 8, 111. [Google Scholar] [CrossRef]
- de Silva, A.P.; Vance, T.P.; West, M.E.; Wright, G.D. Bright molecules with sense, logic, numeracy and utility. Org. Biomol. Chem. 2008, 6, 2468–2480. [Google Scholar] [CrossRef]
- Delattre, F.; Cazier-Dennin, F.; Leleu, L.; Dewaele, D.; Landy, D.; Mallard, I.; Danjou, P.E. Recognition of iron ions by carbazole–desferrioxamine fluorescent sensor and its application in total iron detection in airbone particulate matter. Talanta 2015, 144, 451–455. [Google Scholar] [CrossRef]
- Su, B.L.; Moniotte, N.; Nivarlet, N.; Chen, L.H.; Fu, Z.Y.; Desmet, J.; Li, J. Fl–DFO molecules@ mesoporous silica materials: Highly sensitive and selective nanosensor for dosing with iron ions. J. Colloid Interface Sci. 2011, 358, 136–145. [Google Scholar] [CrossRef]
- Cheung, W.; Patel, M.; Ma, Y.; Chen, Y.; Xie, Q.; Lockard, J.V.; Gao, Y.; He, H. π-Plasmon absorption of carbon nanotubes for the selective and sensitive detection of Fe3+ ions. Chem. Sci. 2016, 7, 5192–5199. [Google Scholar] [CrossRef]
- Kindra, L.R.; Eggers, C.J.; Liu, A.T.; Mendoza, K.; Mendoza, J.; Klein Myers, A.R.; Penner, R.M. Lithographically patterned PEDOT nanowires for the detection of iron (III) with nanomolar sensitivity. Anal. Chem. 2015, 87, 11492–11500. [Google Scholar] [CrossRef] [PubMed]
- Galinetto, P.; Taglietti, A.; Pasotti, L.; Pallavicini, P.; Dacarro, G.; Giulotto, E.; Grandi, M.S. SERS activity of silver nanoparticles functionalized with a desferrioxamine B derived ligand for Fe(III) binding and sensing. J. Appl. Spectrosc. 2016, 82, 1052–1059. [Google Scholar] [CrossRef]
- Cennamo, N.; Alberti, G.; Pesavento, M.; D’Agostino, G.; Quattrini, F.; Biesuz, R.; Zeni, L. A Simple Small Size and Low Cost Sensor Based on Surface Plasmon Resonance for Selective Detection of Fe(III). Sensors 2014, 14, 4657–4671. [Google Scholar] [CrossRef] [PubMed]
- Coale, K.H.; Johnson, K.S.; Chavez, F.P.; Buesseler, K.O.; Barber, R.T.; Brzezinski, M.A.; Cochlan, W.P.; Millero, F.J.; Falkowski, P.G.; Bauer, J.E.; et al. Southern Ocean iron enrichment experiment: Carbon cycling in high-and low-Si waters. Science 2004, 304, 408–414. [Google Scholar] [CrossRef]
- Ussher, S.J.; Worsfold, P.J.; Achterberg, E.P.; Laes, A.; Blain, S.; Laan, P.; de Baar, H.J.W. Distribution and redox speciation of dissolved iron on the European continental margin. Limnol. Oceanogr. 2007, 52, 2530–2539. [Google Scholar] [CrossRef][Green Version]
- Roy, E.G.; Jiang, C.; Wells, M.L.; Tripp, C. Determining Subnanomolar Iron Concentrations in Oceanic Seawater Using a Siderophore-Modified Film Analyzed by Infrared Spectroscopy. Anal. Chem. 2008, 80, 4689–4695. [Google Scholar] [CrossRef]
- Arrigan, D.W.M.; Deasy, B.; Glennon, J.D.; Johnston, B.; Svehla, G. Incorporation of Hydroxamic Acid Ligands Into Nafion Film Electrodes. Analyst 1993, 118, 355–359. [Google Scholar] [CrossRef]
- Norocel, L.; Gutt, G. Development and performance testing of an electrochemical sensor for determination of iron ions in wine. Aust. J. Grape Wine Res. 2019, 25, 161–164. [Google Scholar] [CrossRef]
- Ruzik, L. Speciation of challenging elements in food by atomic spectrometry. Talanta 2012, 93, 18–31. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in alcoholic beverages: A review of sources, effects, concentrations, removal, speciation, and analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Neto, J.H.S.; Porto, I.S.; Schneider, M.P.; Dos Santos, A.M.; Gomes, A.A.; Ferreira, S.L. Speciation analysis based on digital image colorimetry: Iron (II/III) in white wine. Talanta 2019, 194, 86–89. [Google Scholar] [CrossRef]
- Rousseva, M.; Kontoudakis, N.; Schmidtke, L.M.; Scollary, G.R.; Clark, A.C. Impact of wine production on the fractionation of copper and iron in Chardonnay wine: Implications for oxygen consumption. Food Chem. 2016, 203, 440–447. [Google Scholar] [CrossRef]
- de Campos Costa, R.C.; Araújo, A.N. Determination of Fe(III) and total Fe in wines by sequential injection analysis and flame atomic absorption spectrometry. Anal. Chim. Acta 2001, 438, 227–233. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Ferreira, H.S.; de Jesus, R.M.; Santos, J.V.; Brandao, G.C.; Souza, A.S. Development of method for the speciation of inorganic iron in wine samples. Anal. Chim. Acta 2007, 602, 89–93. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Albendin, G.; Arufe, M.I.; Mánuel-Vez, M.P. Simplification of iron speciation in wine samples: A spectrophotometric approach. J. Agric. Food Chem. 2015, 63, 4545–4550. [Google Scholar] [CrossRef] [PubMed]
- Capitán-Vallvey, L.F.; Lopez-Ruiz, N.; Martinez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef]
- Lao, M.; Companys, E.; Weng, L.; Puy, J.; Galceran, J. Speciation of Zn, Fe, Ca and Mg in wine with the Donnan membrane technique. Food Chem. 2018, 239, 1143–1150. [Google Scholar] [CrossRef]
- Camara-Martos, F.; da Costa, J.; Justino, C.I.; Cardoso, S.; Duarte, A.C.; Rocha-Santos, T. Disposable biosensor for detection of iron (III) in wines. Talanta 2016, 154, 80–84. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Akrami, Z. Gold–deferrioxamine nanometric interface for selective recognition of Fe(III) using square wave voltammetry and electrochemical impedance spectroscopy methods. Biosens. Bioelectron. 2013, 39, 31–36. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Akrami, Z.; Sabzyan, H. Nanostructure molecular assemblies constructed based on ex-situ and in-situ layer-by-layer ferrioxamation characterized by electrochemical and scanning tunneling microscopy methods. J. Phys. Chem. C 2011, 115, 8042–8055. [Google Scholar] [CrossRef]
- See, W.P.; Heng, L.Y.; Nathan, S. Highly Sensitive Aluminium(III) Ion Sensor Based on a Self-assembled Monolayer on a Gold Nanoparticles Modified Screen-printed Carbon Electrode. Anal. Sci. 2015, 31, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, V.M. New electroanalytical applications of self-assembled monolayers. TrAC—Trends Anal. Chem. 2002, 21, 439–450. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Rovertoni, S.; Magnaghi, L.R.; Biesuz, R. Screen-Printed Gold Electrode Functionalized with Deferoxamine for Iron(III) Detection. Chemosensors 2022, 10, 214. [Google Scholar] [CrossRef]

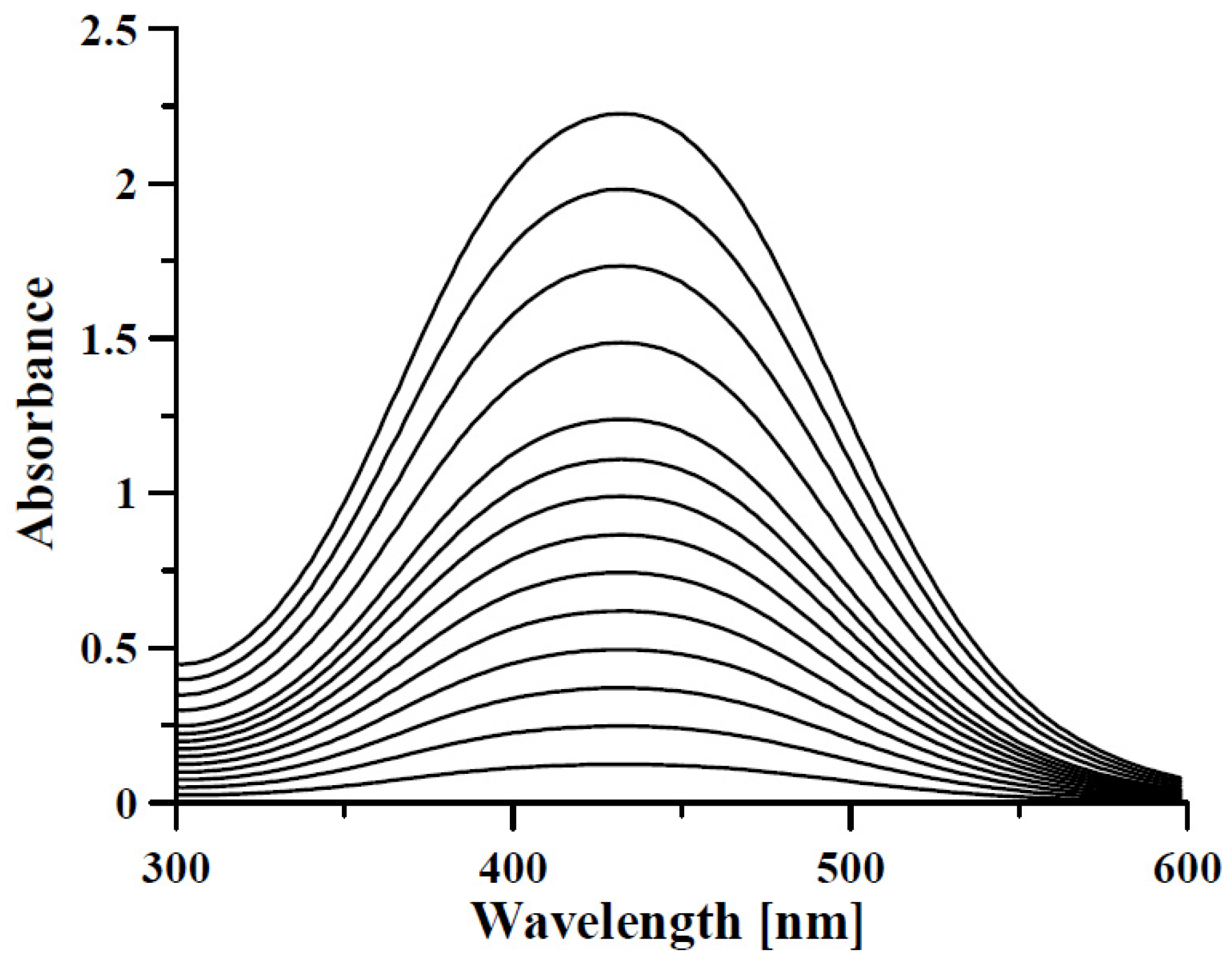
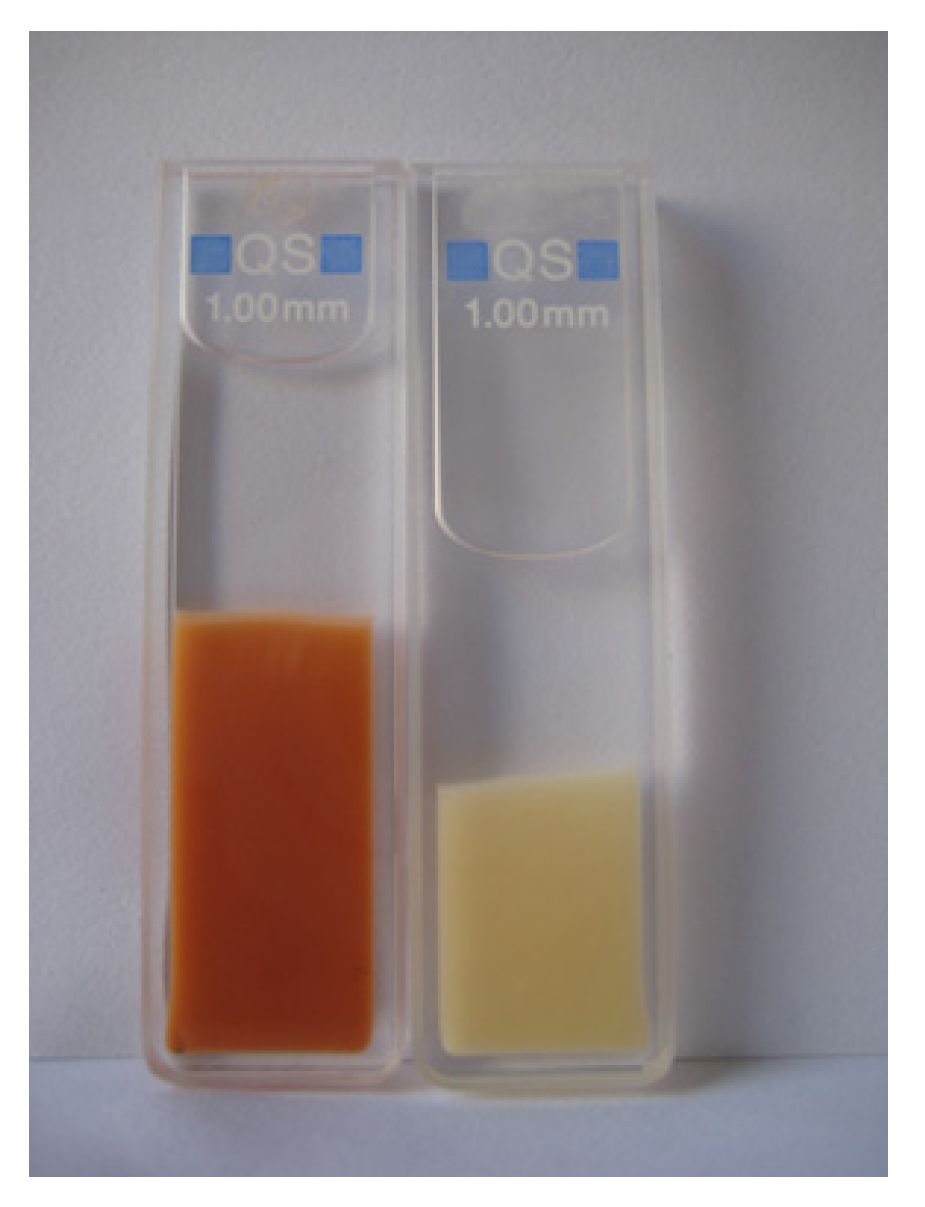
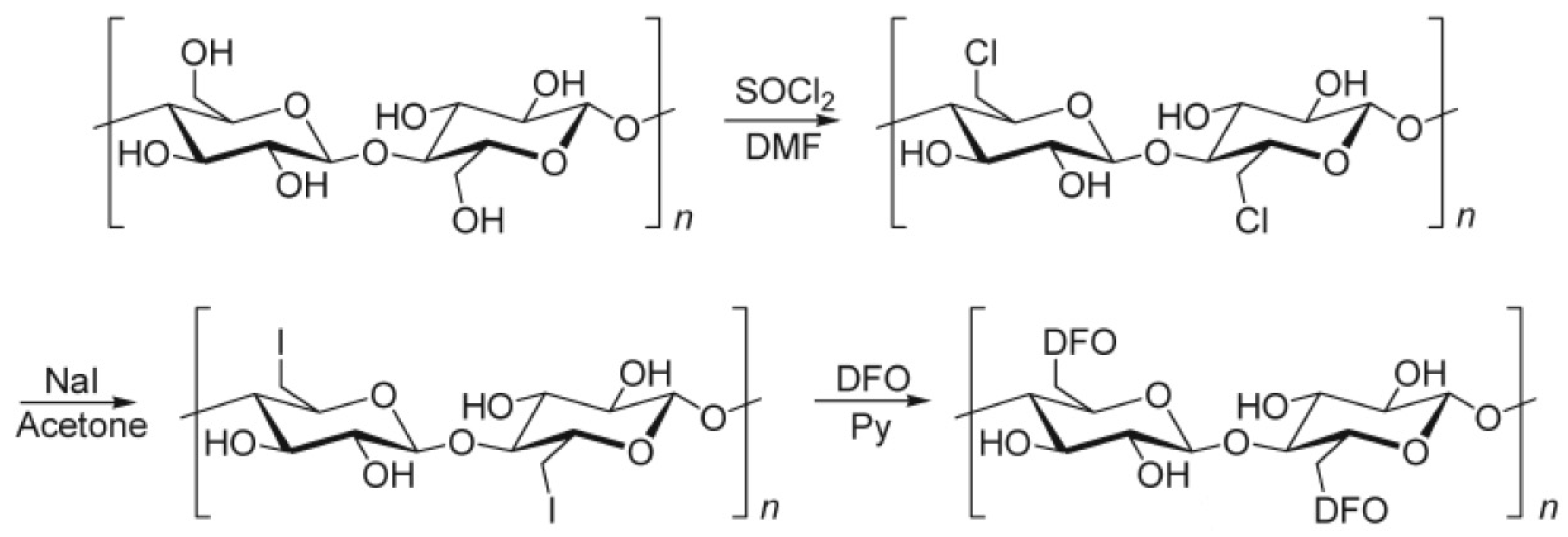
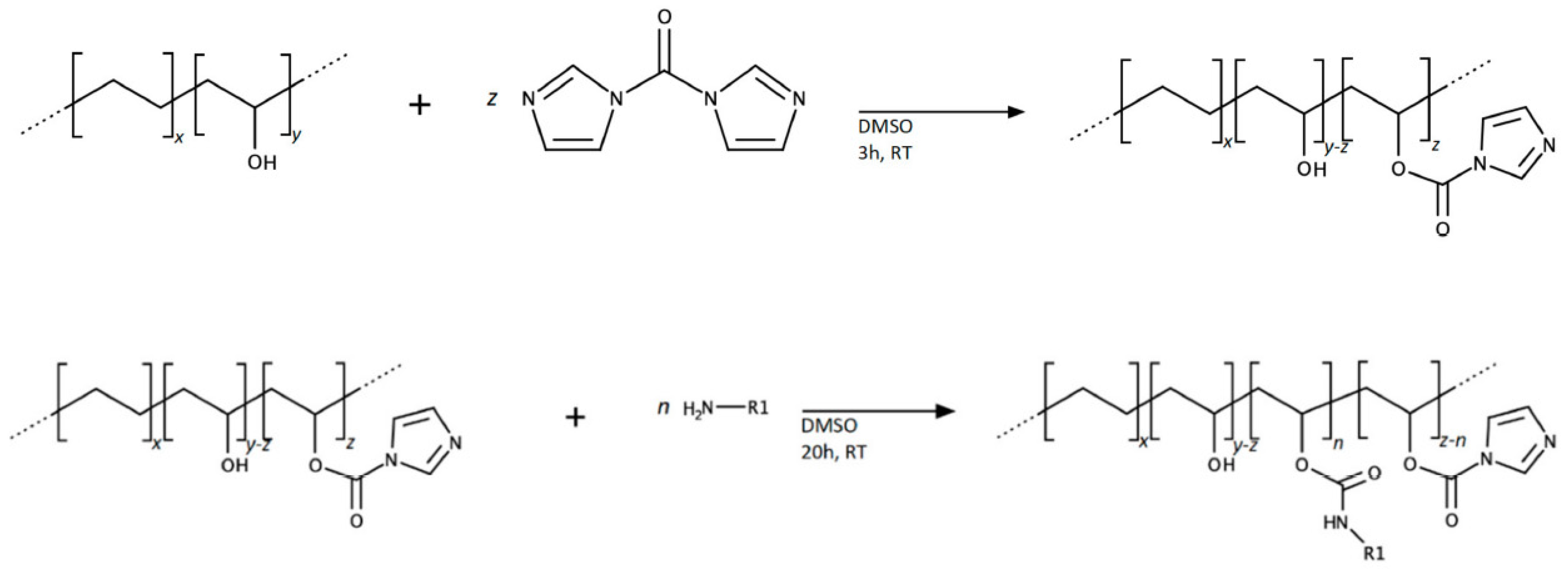

| Material | DFO Concentration | Trials | Ref. |
|---|---|---|---|
| Polyphenol-DFO nanoparticles | 80% w/w | in vitro and in vivo | [22] |
| Unilamellar and multilamellar liposomes encapsulated DFO | 10–12% w/w | in vitro and in vivo | [24] |
| Unilamellar and multilamellar liposomes encapsulated DFO | 5–28% w/w | in vitro and in vivo | [25] |
| Liposomes encapsulated DFO | 28.5% w/w | in vivo | [26] |
| DFO-loaded polymeric micelles | 4.5–6.1% w/w | in vitro and in vivo | [27] |
| DFO- Tetraphenylethene encapsulated polymeric micelles | 12.5% w/w | in vitro | [28] |
| Starch-conjugated DFO | >50% w/w | in vivo | [29] |
| DFO-loaded chitosan-nanoparticles | 20, 45 and 75 w/w | in vitro | [30] |
| DFO-nanogel | 8.5% w/w | in vitro | [31] |
| Chitosan/alginate hydrogel encapsulated DFO | 10–25% w/w | in vitro | [33] |
| Polyrotaxane–DFO conjugates | 35.7% w/w | in vitro and in vivo | [36,37] |
| DFO-based copolymers polyethylene glycol methacrylate | 5.5–15% w/w | in vitro | [39] |
| Star Like Eight-Arm Polyethylene Glycol-DFO Conjugate | ~100% w/w | in vitro and in vivo | [45] |
| Material | Equilibration Time (min) | Sorption Capacity, qmax (mmol Fe(III)/g) | Ref. |
|---|---|---|---|
| DFO-SAM on mesoporous silica MCM41 | 200 | 0.3 | [47] |
| DFO-grafted stellate silica nanoparticles | 30 | 0.48 | [49] |
| DFO-grafted amorphous silica | 300–600 | 1.5–2 | [50] |
| DFO-immobilized Sepharose gel | 1440 | 0.14 | [51] |
| DFO-grafted alginate hydrogel | n.d. 1 | n.d. 1 | [52] |
| Receptor | Analytical Method | Sensitivity | RDS % | LOD (M) | Ref. |
|---|---|---|---|---|---|
| Deferoxamine (aqueous solution) | UV–vis spectroscopy | 2764.8 cm−1 M−1 | 2 | 1.4 × 10−4 | [53] |
| DFO-SAM on mesoporous silica | UV–vis spectroscopy | 23,337 M−1 | 10–15 | 1 × 10−5 | [48,54] |
| DFO immobilized on filter paper | Colorimetry | n.d. 1 | n.d. 1 | 3 × 10−6 | [56,57] |
| DFO@Ethylene-vinyl alcohol copolymer | Colorimetry | n.d. 1 | n.d. 1 | n.d. 1 | [58] |
| carbazole–desferrioxamine | Fluorescence | n.d. 1 | n.d. 1 | n.d. 1 | [60] |
| fluoresceine–DFO@mesoporous silica | Fluorescence | n.d. 1 | n.d. 1 | 1 × 10−7 | [61] |
| DFO-modified carbon nanotubes | UV–vis–NIR spectroscopy | 0.0128 pM−1 | n.d. 1 | 1 × 10−11 | [62] |
| DFO-PEDOT | Impedance spectroscopy | n.d. 1 | n.d. 1 | 1 × 10−8 | [63] |
| DFO-functionalized Ag nanoparticles | surface-enhanced raman scattering (SERS) | n.d. 1 | n.d. 1 | n.d. 1 | [64] |
| DFO-SAM on Au surface | Plastic optical fiber–Surface plasmon resonance (POF-SPR) | 1.5 × 10−5 nm M−1 | n.d. 1 | 1 × 10−6 | [65] |
| DFO on nanostructured silica | IR spectroscopy | 0.0372 pM−1 | 15 | 5 × 10−11 | [68] |
| DFO-immobilized Nafion-coated glassy carbon electrode | Differential pulse voltammetry (DPV) | n.d. 1 | n.d. 1 | 5 × 10−7 | [69] |
| DFO-modified graphite screen-printed electrode | Cyclic voltammetry (CV) | 2.93 × 10−5 µA cm2 mmol−1 | 6–13 | 1.6 × 10−5 | [70] |
| DFO-SAM on gold disk electrode | Square wave voltammetry (SWV) | 3.7 µA (p[Fe(III)/µM])−1 | 2.2 | 2 × 10−11 | [81] |
| DFO-SAM on gold screen-printed electrode | Differential pulse voltammetry (DPV) | 0.421 µA nM−1 | n.d. 1 | 5 × 10−10 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Deferoxamine-Based Materials and Sensors for Fe(III) Detection. Chemosensors 2022, 10, 468. https://doi.org/10.3390/chemosensors10110468
Alberti G, Zanoni C, Magnaghi LR, Biesuz R. Deferoxamine-Based Materials and Sensors for Fe(III) Detection. Chemosensors. 2022; 10(11):468. https://doi.org/10.3390/chemosensors10110468
Chicago/Turabian StyleAlberti, Giancarla, Camilla Zanoni, Lisa Rita Magnaghi, and Raffaela Biesuz. 2022. "Deferoxamine-Based Materials and Sensors for Fe(III) Detection" Chemosensors 10, no. 11: 468. https://doi.org/10.3390/chemosensors10110468
APA StyleAlberti, G., Zanoni, C., Magnaghi, L. R., & Biesuz, R. (2022). Deferoxamine-Based Materials and Sensors for Fe(III) Detection. Chemosensors, 10(11), 468. https://doi.org/10.3390/chemosensors10110468









