Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation and Measurements
2.3. Synthesis of BSA–MSA Conjugate
2.4. Gold Nanoparticles Synthesis and Surface Modification
2.5. Fabrication of Lateral Flow Tests
2.6. Colorimetric Detection of Hg2+ Ion Using Lateral Flow Test
2.7. Analysis of Real Water
2.8. ICP-MS Detection Technique
3. Results and Discussion
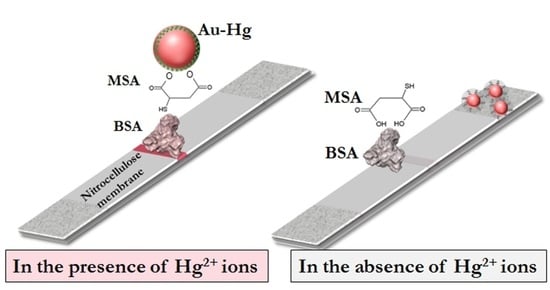
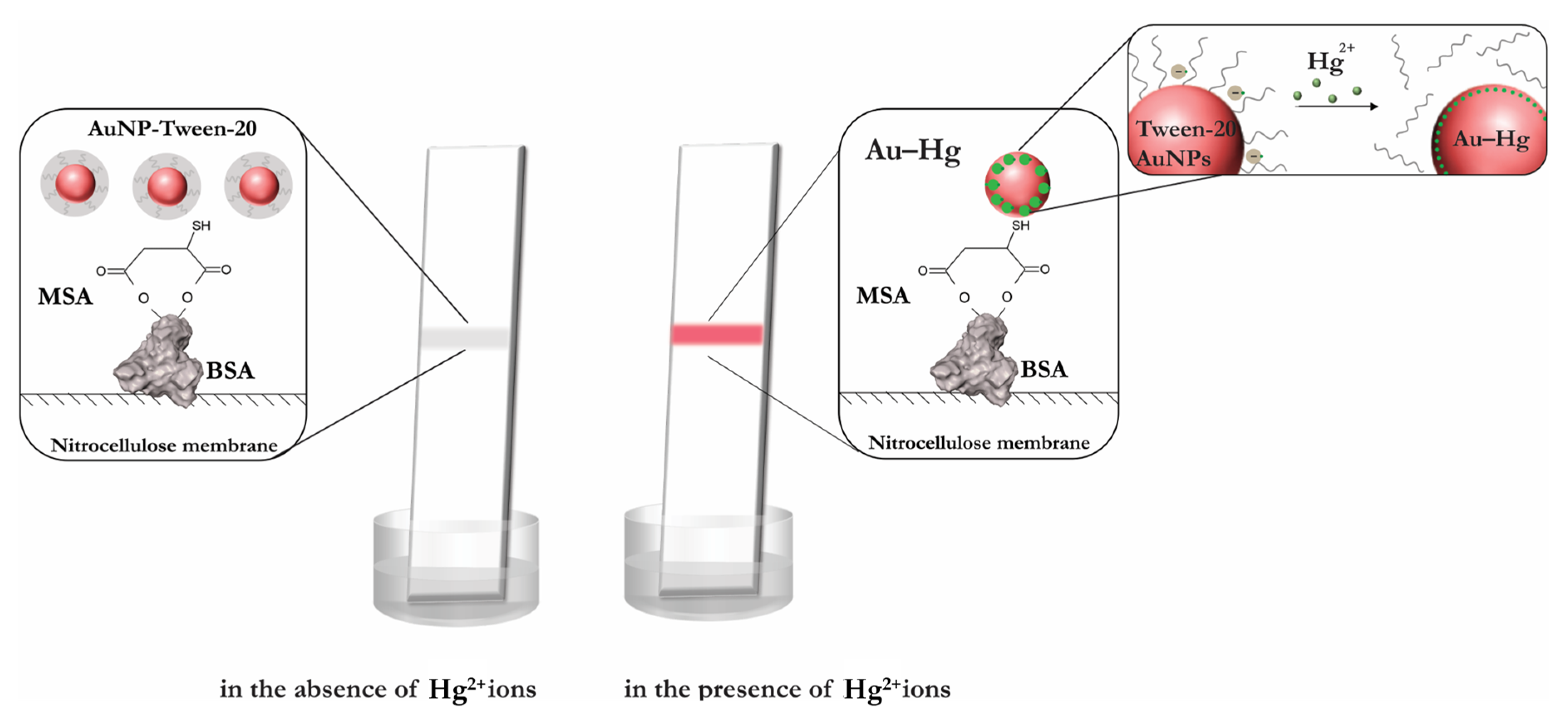
3.1. Principle of Hg2+ Sensing
3.2. Characterization of Detecting Agent
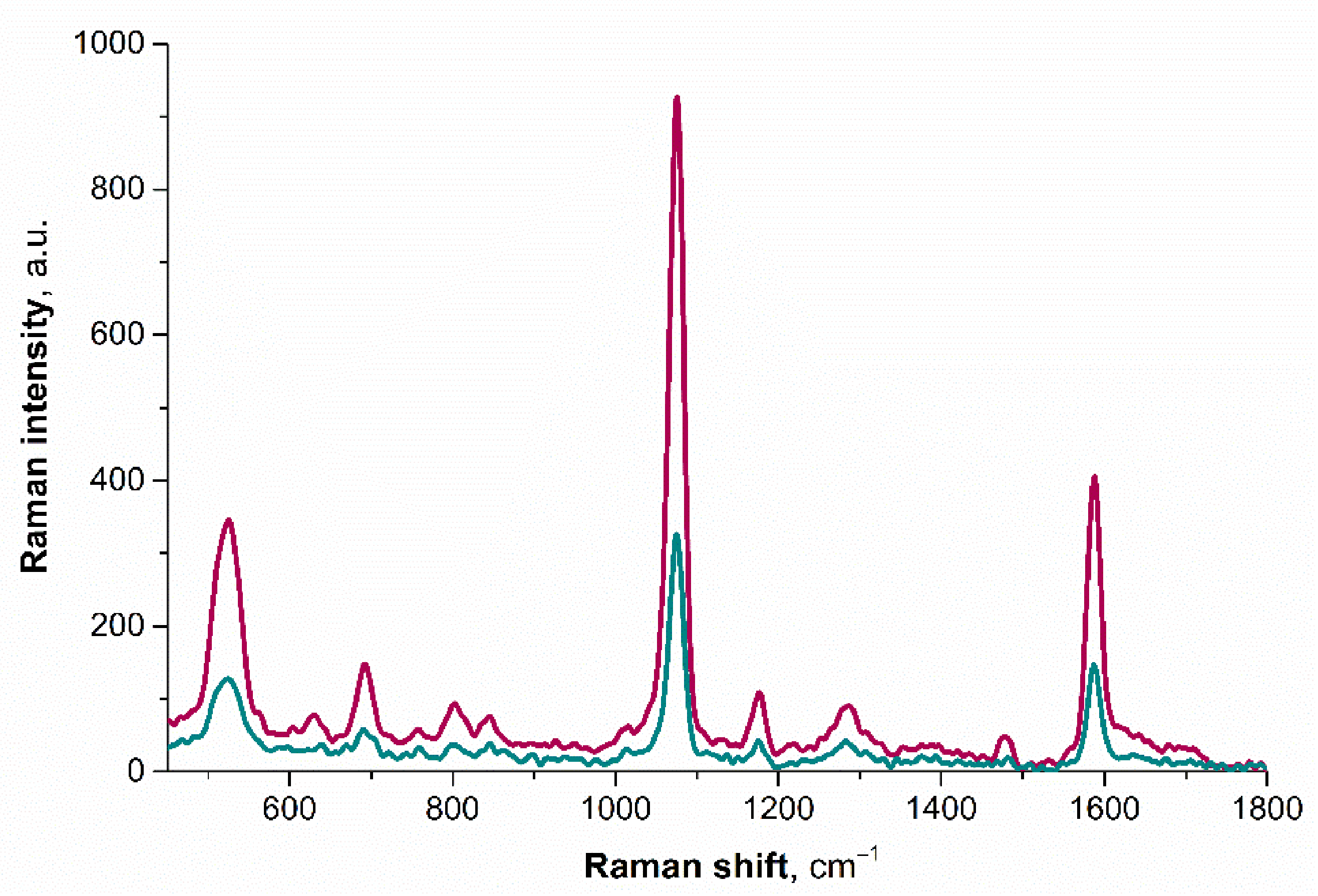
3.3. Confirmation of Au–Hg Nanoalloy Formation
3.4. Synthesis and Characterization of BSA–MSA Conjugate
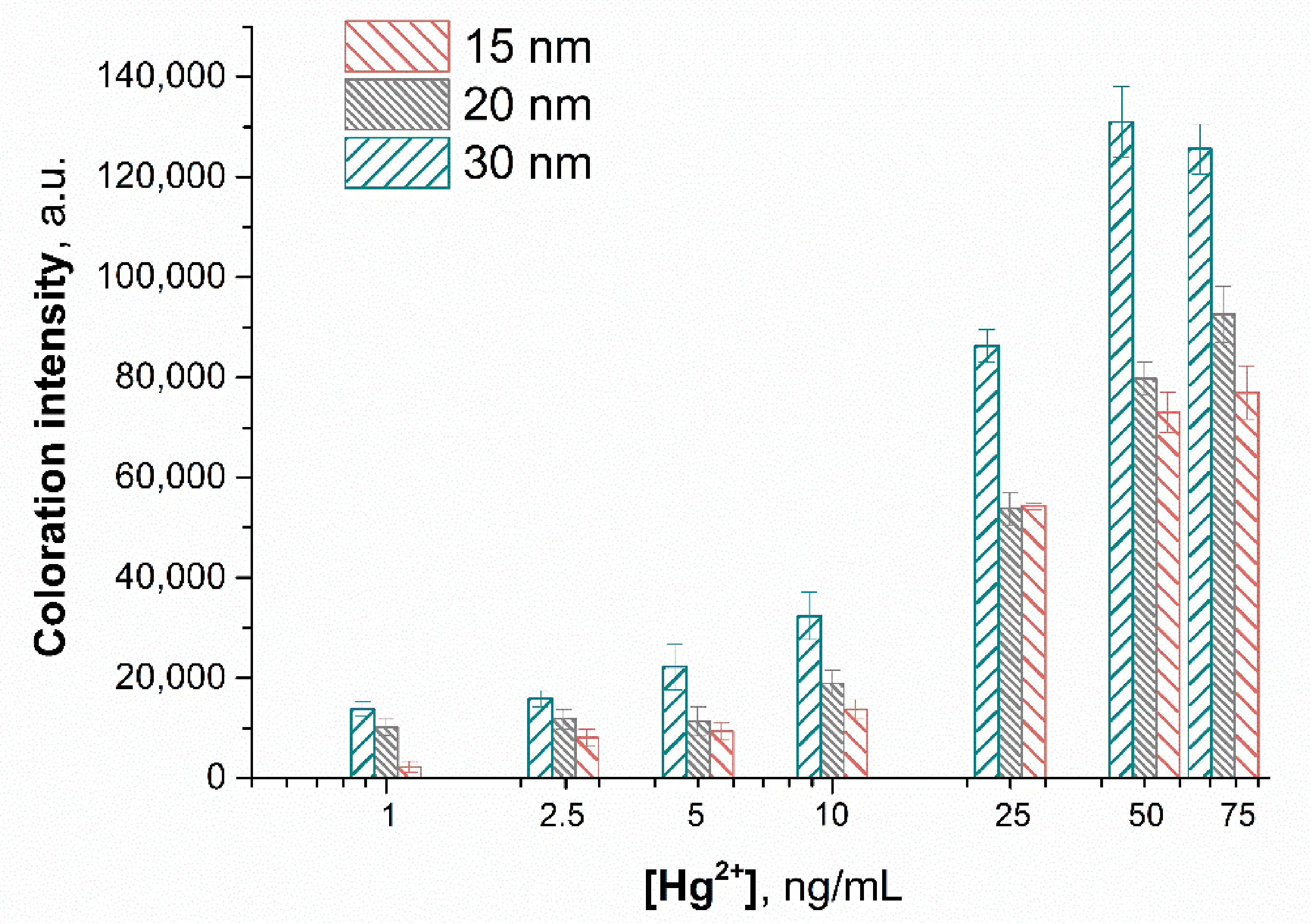
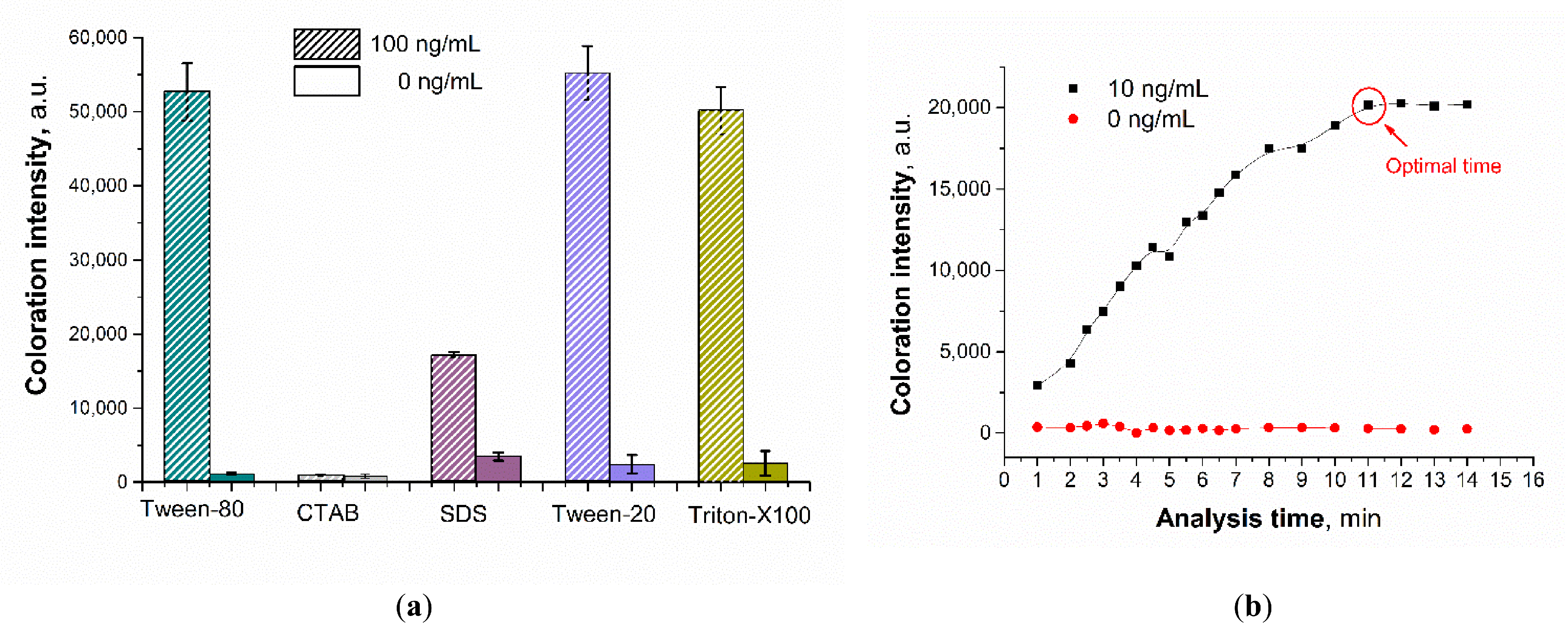
3.5. Optimization of the Sensing Parameters
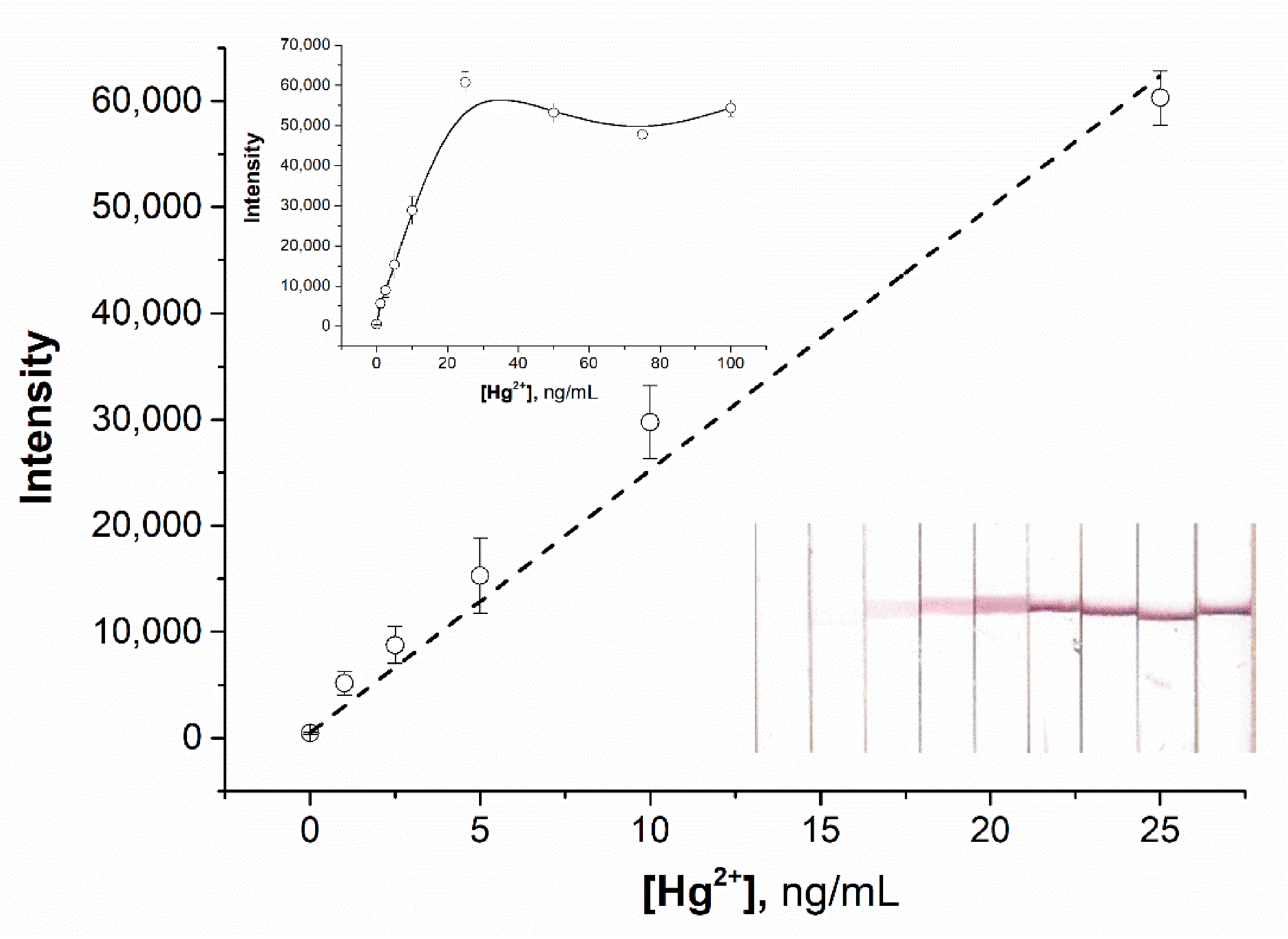
3.6. Sensitivity and Selectivity
3.7. Water Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef] [Green Version]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- US EPA. National Primary Drinking Water Regulations. Esdat Environmental Database Management Software. 2009. Available online: https://www.esdat.net/Environmental%20Standards/US/Federal/US%20Federal%20MLCs.pdf (accessed on 7 September 2022).
- GOST. Mineral Natural Nutritious Waters. General Specifications; Standartinform Publication: Moscow, Russia, 2011; p. 70. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jian, R.; Liao, J.; Cui, J.; Fang, P.; Zou, Z.; Huang, K. Recent development of non-chromatographic atomic spectrometry for speciation analysis of mercury. Appl. Spectrosc. Rev. 2021, 57, 441–460. [Google Scholar] [CrossRef]
- Zou, C.; Liu, Z.; Wang, X.; Liu, H.; Yang, M.; Huo, D.; Hou, C. A paper-based visualization chip based on nitrogen-doped carbon quantum dots nanoprobe for Hg(II) detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120346. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Dong, H.; Liu, K.; He, N. Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci. China Chem. 2016, 60, 329–337. [Google Scholar] [CrossRef]
- Mandil, A.; Idrissi, L.; Amine, A. Stripping voltammetric determination of mercury(II) and lead(II) using screen-printed electrodes modified with gold films, and metal ion preconcentration with thiol-modified magnetic particles. Microchim. Acta 2010, 170, 299–305. [Google Scholar] [CrossRef]
- Moutcine, A.; Chtaini, A. Electrochemical determination of trace mercury in water sample using EDTA-CPE modified electrode. Sens. Bio-Sens. Res. 2018, 17, 30–35. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.-Y.; Woo, M.-A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef]
- Hyder, A.; Buledi, J.A.; Nawaz, M.; Rajpar, D.B.; Shah, Z.-u.-H.; Orooji, Y.; Yola, M.L.; Karimi-Maleh, H.; Lin, H.; Solangi, A.R. Identification of heavy metal ions from aqueous environment through gold, Silver and Copper Nanoparticles: An excellent colorimetric approach. Environ. Res. 2022, 205, 112475. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Sethuraman, K.; Vashisht, D.; Vashisht, A.; Mehta, S.K.; Gupta, A. Colorimetric detection of mercury ions based on anti-aggregation of gold nanoparticles using 3, 5-dimethyl-1-thiocarboxamidepyrazole. Microchem. J. 2019, 148, 299–305. [Google Scholar] [CrossRef]
- Tian, K.; Siegel, G.; Tiwari, A. A simple and selective colorimetric mercury (II) sensing system based on chitosan stabilized gold nanoparticles and 2,6-pyridinedicarboxylic acid. Mater. Sci. Eng. C 2017, 71, 195–199. [Google Scholar] [CrossRef]
- Anh, N.H.; Doan, M.Q.; Dinh, N.X.; Huy, T.Q.; Tri, D.Q.; Ngoc Loan, L.T.; Van Hao, B.; Le, A.-T. Gold nanoparticle-based optical nanosensors for food and health safety monitoring: Recent advances and future perspectives. RSC Adv. 2022, 12, 10950–10988. [Google Scholar] [CrossRef]
- Ali, S.; Chen, X.; Shi, W.; Huang, G.; Yuan, L.-m.; Meng, L.; Chen, S.; Xie, Z.; Chen, X. Recent Advances in Silver and Gold Nanoparticles-Based Colorimetric Sensors for Heavy Metal Ions Detection: A Review. Crit. Rev. Anal. Chem. 2021, 1–33. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media using DNA-Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Politi, J.; Spadavecchia, J.; Iodice, M.; de Stefano, L. Oligopeptide–heavy metal interaction monitoring by hybrid gold nanoparticle based assay. Analyst 2015, 140, 149–155. [Google Scholar] [CrossRef]
- Berlina, A.N.; Sharma, A.K.; Zherdev, A.V.; Gaur, M.S.; Dzantiev, B.B. Colorimetric Determination of Lead Using Gold Nanoparticles. Anal. Lett. 2015, 48, 766–782. [Google Scholar] [CrossRef]
- Su, D.; Yang, X.; Xia, Q.; Chai, F.; Wang, C.; Qu, F. Colorimetric detection of Hg2+ using thioctic acid functionalized gold nanoparticles. RSC Adv. 2013, 3, 24618–24624. [Google Scholar] [CrossRef]
- Yu, C.-J.; Tseng, W.-L. Colorimetric Detection of Mercury(II) in a High-Salinity Solution Using Gold Nanoparticles Capped with 3-Mercaptopropionate Acid and Adenosine Monophosphate. Langmuir 2008, 24, 12717–12722. [Google Scholar] [CrossRef]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Pridvorova, S.M.; Zherdev, A.V.; Dzantiev, B.B. Mercaptosuccinic-Acid-Functionalized Gold Nanoparticles for Highly Sensitive Colorimetric Sensing of Fe(III) Ions. Chemosensors 2021, 9, 290. [Google Scholar] [CrossRef]
- Chadha, R.; Das, A.; Debnath, A.K.; Kapoor, S.; Maiti, N. 2-thiazoline-2-thiol functionalized gold nanoparticles for detection of heavy metals, Hg(II) and Pb(II) and probing their competitive surface reactivity: A colorimetric, surface enhanced Raman scattering (SERS) and x-ray photoelectron spectroscopic (XPS) study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126279. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Meelapsom, R.; Pencharee, S.; Obma, A.; Amatatongchai, M.; Ditcharoen, N.; Chairam, S.; Tamuang, S. Use of a Smartphone as a Colorimetric Analyzer in Paper-based Devices for Sensitive and Selective Determination of Mercury in Water Samples. Anal. Sci. 2018, 34, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Mertens, S.F.L.; Gara, M.; Sologubenko, A.S.; Mayer, J.; Szidat, S.; Krämer, K.W.; Jacob, T.; Schiffrin, D.J.; Wandlowski, T. Au@Hg Nanoalloy Formation Through Direct Amalgamation: Structural, Spectroscopic, and Computational Evidence for Slow Nanoscale Diffusion. Adv. Funct. Mater. 2011, 21, 3259–3267. [Google Scholar] [CrossRef]
- Park, J.-H.; Byun, J.-Y.; Yim, S.-Y.; Kim, M.-G. A Localized Surface Plasmon Resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system. J. Hazard. Mater. 2016, 307, 137–144. [Google Scholar] [CrossRef]
- Jin, L.-H.; Han, C.-S. Eco-friendly colorimetric detection of mercury(II) ions using label-free anisotropic nanogolds in ascorbic acid solution. Sens. Actuators B Chem. 2014, 195, 239–245. [Google Scholar] [CrossRef]
- Apilux, A.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Simple and rapid colorimetric detection of Hg(II) by a paper-based device using silver nanoplates. Talanta 2012, 97, 388–394. [Google Scholar] [CrossRef]
- Meelapsom, R.; Jarujamrus, P.; Amatatongchai, M.; Chairam, S.; Kulsing, C.; Shen, W. Chromatic analysis by monitoring unmodified silver nanoparticles reduction on double layer microfluidic paper-based analytical devices for selective and sensitive determination of mercury(II). Talanta 2016, 155, 193–201. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Brennan, J.D. β-Galactosidase-Based Colorimetric Paper Sensor for Determination of Heavy Metals. Anal. Chem. 2011, 83, 8772–8778. [Google Scholar] [CrossRef]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead(ii) by lateral flow test strip. Anal. Chim. Acta 2021, 1155, 338318. [Google Scholar] [CrossRef]
- Song, S.; Zou, S.; Zhu, J.; Liu, L.; Kuang, H. Immunochromatographic paper sensor for ultrasensitive colorimetric detection of cadmium. Food Agric. Immunol. 2018, 29, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.L.; Wang, J.F.; Li, Z.Y.; Liang, S.Z.; Wang, X.N. Immunoassay for cadmium detection and quantification. Biomed Env. Sci 2009, 22, 188–193. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Yang, S.; Sun, C. Preparation of Anti-Cadmium-EDTA Complex Polyclonal Antibody and Its Application for Determination of Cadmium in Aqueous Solution. Anal. Lett. 2009, 42, 409–424. [Google Scholar] [CrossRef]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Membrane analytical test system for highly sensitive determination of Hg2+ ions in natural waters. Limnol. Freshw. Biol. 2022, 5, 3. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Liu, H.; Gan, Z.; Song, C.; Xu, B.; Guo, Z.; Zhou, X.; Chen, Z. Use of a smartphone for intelligent detection of cyromazine based on Tween 20 modified gold nanoparticles. Anal. Methods 2020, 12, 1469–1477. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Zeng, G.; Li, Z.; Chen, A.; Yan, M.; Liu, L.; Huang, D. Ultrasensitive detection and co-stability of mercury(ii) ions based on amalgam formation with Tween 20-stabilized silver nanoparticles. RSC Adv. 2014, 4, 59275–59283. [Google Scholar] [CrossRef]
- Schopf, C.; Wahl, A.; Martín, A.; O’Riordan, A.; Iacopino, D. Direct Observation of Mercury Amalgamation on Individual Gold Nanorods Using Spectroelectrochemistry. J. Phys. Chem. C 2016, 120, 19295–19301. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Jing, X.; Zhou, H.; Wang, Y.; Ye, J.; Chu, Q. Tween 20-capped gold nanoparticles for selective extraction of free low-molecular-weight thiols in saliva followed by capillary electrophoresis with contactless conductivity detection. J. Chromatogr. B 2021, 1176, 122756. [Google Scholar] [CrossRef]
- Ojea-Jiménez, I.; López, X.; Arbiol, J.; Puntes, V. Citrate-Coated Gold Nanoparticles As Smart Scavengers for Mercury(II) Removal from Polluted Waters. ACS Nano 2012, 6, 2253–2260. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Wagers, K.; Chui, T.; Adem, S.M. Effect of pH on the Stability of Gold Nanoparticles and Their Application for Melamine Detection in Infant Formula. IOSR J. Appl. Chem. 2014, 7, 15–20. [Google Scholar] [CrossRef]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold Nanoparticles: Synthesis, Stability Test, and Application for the Rice Growth. J. Nanomater. 2014, 2014, 451232. [Google Scholar] [CrossRef] [Green Version]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef]
- Xu, D.; Yu, S.; Yin, Y.; Wang, S.; Lin, Q.; Yuan, Z. Sensitive Colorimetric Hg2+ Detection via Amalgamation-Mediated Shape Transition of Gold Nanostars. Front. Chem. 2018, 6, 566. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yang, P.-C.; Wu, T.; Lin, Y.-W. Determination of mercury (II) ions based on silver-nanoparticles-assisted growth of gold nanostructures: UV–Vis and surface enhanced Raman scattering approaches. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 301–307. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, H.; Zhang, L.; Wu, T.; Sun, L.; Jiang, D.; Du, Y. In situ preparation of Ag nanoparticles by laser photoreduction as SERS substrate for determination of Hg2+. J. Raman Spectrosc. 2017, 48, 399–404. [Google Scholar] [CrossRef]
- Kong, X.; Cheng, C.; Wang, Q.; Meng, M.; Chen, C.; Wang, X.; Huang, S.; Gan, W. Surface-enhanced Raman scattering of trace Hg2+ on a 4-mercaptobenzoic acid-modified nanoporous Au substrate. Opt. Mater. Express 2021, 11, 3015–3022. [Google Scholar] [CrossRef]
- Retnakumari, A.; Setua, S.; Menon, D.; Ravindran, P.; Muhammed, H.; Pradeep, T.; Nair, S.; Koyakutty, M. Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 2010, 21, 055103. [Google Scholar] [CrossRef]
- Arivarasan, A.; Bharathi, S.; Ezhilarasi, S.; Arunpandiyan, S.; Jayavel, R. Photovoltaic Performances of Yb Doped CdTe QDs Sensitized TiO2 Photoanodes for Solar cell Applications. J. Inorg. Organomet. Polym. Mater. 2019, 29, 859–868. [Google Scholar] [CrossRef]
- Askari, E. A Novel Approach to Facile Synthesis and Biosensing of the Protein-Regulated Graphene. Int. J. Electrochem. Sci. 2018, 13, 886–897. [Google Scholar] [CrossRef]
- Safenkova, I.; Zherdev, A.; Dzantiev, B. Factors influencing the detection limit of the lateral-flow sandwich immunoassay: A case study with potato virus X. Anal. Bioanal. Chem. 2012, 403, 1595–1605. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, L.; Jia, H. Facile control of the self-assembly of gold nanoparticles by changing the capping agent structures. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 9–14. [Google Scholar] [CrossRef]
- Xu, S.; Yuan, H.; Xu, A.; Wang, J.; Wu, L. Rapid Synthesis of Stable and Functional Conjugates of DNA/Gold Nanoparticles Mediated by Tween 80. Langmuir 2011, 27, 13629–13634. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Gul, Z.; Ullah, S.; Khan, S.; Ullah, H.; Khan, M.U.; Ullah, M.; Ali, S.; Altaf, A.A. Recent Progress in Nanoparticles Based Sensors for the Detection of Mercury (II) Ions in Environmental and Biological Samples. Crit. Rev. Anal. Chem. 2022. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G.; Ciptawati, E.; Khaled, M. Analytical methods for mercury speciation, detection, and measurement in water, oil, and gas. TrAC Trends Anal. Chem. 2020, 132, 116016. [Google Scholar] [CrossRef]
- Yang, F.; Duan, J.; Li, M.; Wang, Z.; Guo, Z. Visual and On-site Detection of Mercury(II) Ions on Lateral Flow Strips Using DNA-functionalized Gold Nanoparticles. Anal. Sci. 2012, 28, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.-H.; Wu, C.-S.; Huang, C.-C.; Liang, J.-C.; Wang, H.-T.; Tang, P.-T.; Lin, L.-Y.; Ko, F.-H. A rapid and portable sensor based on protein-modified gold nanoparticle probes and lateral flow assay for naked eye detection of mercury ion. Microelectron. Eng. 2012, 97, 294–296. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, H.; Hu, J.; Fu, Q.; Yao, C.; Yu, S.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Chen, J.; Ge, C.; Fang, Z.; Wang, L.; Xing, X.; Zeng, L. An autonomous T-rich DNA machine based lateral flow biosensor for amplified visual detection of mercury ions. Anal. Methods 2014, 6, 2024–2027. [Google Scholar] [CrossRef]
- Yao, L.; Teng, J.; Zhu, M.; Zheng, L.; Zhong, Y.; Liu, G.; Xue, F.; Chen, W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens. Bioelectron. 2016, 85, 331–336. [Google Scholar] [CrossRef]
- Azmi, N.A.; Ahmad, S.H.; Low, S.C. Detection of mercury ions in water using a membrane-based colorimetric sensor. RSC Adv. 2018, 8, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef]





| Method | LOD, ng/mL | Time | Ref. |
|---|---|---|---|
| Lateral flow test using DNA-functionalized AuNPs | 1.2 | 5 min | [61] |
| Lateral flow test using AuNPs functionalized with carboxylic modified protein | 200 | n/a | [62] |
| Fluorescence-quenching lateral flow assay using aptamer-modified AuNPs | 0.13 | 15 min | [63] |
| Lateral flow assay using DNA-functionalized AuNPs | 1 | 60 min | [64] |
| Lateral flow assay using nucleic acid-modified multi-walled carbon nanotubes | 0.05 | 15 min | [65] |
| Membrane-based colorimetric sensor using dithizone as chromophore | 3000 | 1 min | [66] |
| Microfluidic paper-based assay using single-stranded DNA-modified AuNPs | 10 | 40 min | [67] |
| Lateral flow test using Tween-20-stabilized AuNPs | 0.13 | 11 min | This work |
| Sample | pH | Added, ng/mL | Found, ng/mL | Recovery, % |
|---|---|---|---|---|
| Spring water | 6.85 | 5.00 | 5.67 ± 0.09 | 113.4 |
| 2.50 | 3.35 ± 0.20 | 134.0 | ||
| 1.00 | 1.2 ± 0.1 | 120.0 | ||
| Waterfall water | 9.33 | 6.25 | 6.26 ± 0.06 | 100.2 |
| 3.12 | 2.49 ± 0.01 | 79.7 | ||
| 1.56 | 1.1 ± 0.1 | 70.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment. Chemosensors 2022, 10, 413. https://doi.org/10.3390/chemosensors10100413
Komova NS, Serebrennikova KV, Berlina AN, Zherdev AV, Dzantiev BB. Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment. Chemosensors. 2022; 10(10):413. https://doi.org/10.3390/chemosensors10100413
Chicago/Turabian StyleKomova, Nadezhda S., Kseniya V. Serebrennikova, Anna N. Berlina, Anatoly V. Zherdev, and Boris B. Dzantiev. 2022. "Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment" Chemosensors 10, no. 10: 413. https://doi.org/10.3390/chemosensors10100413
APA StyleKomova, N. S., Serebrennikova, K. V., Berlina, A. N., Zherdev, A. V., & Dzantiev, B. B. (2022). Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment. Chemosensors, 10(10), 413. https://doi.org/10.3390/chemosensors10100413