Novel Hydrazone Chromophore Sensor for Metallochromic Determination of Cadmium Ions
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
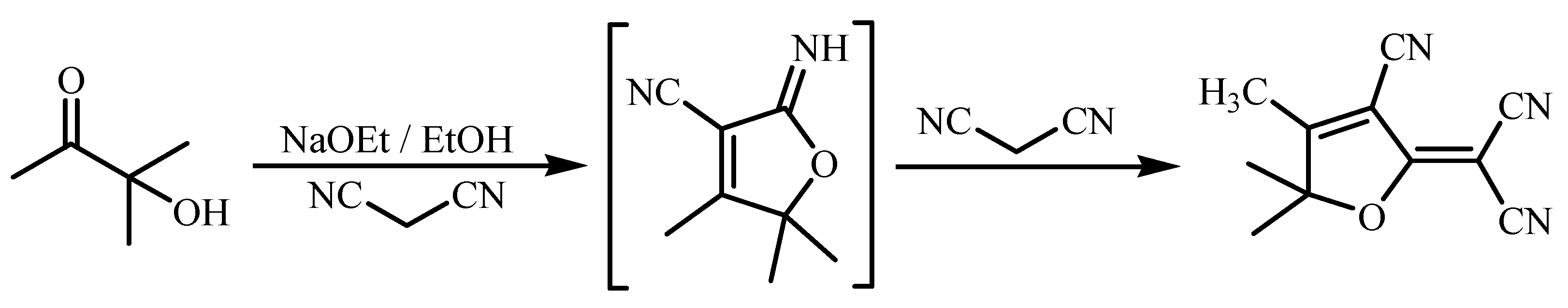
2.2. Synthesis of DCDHF
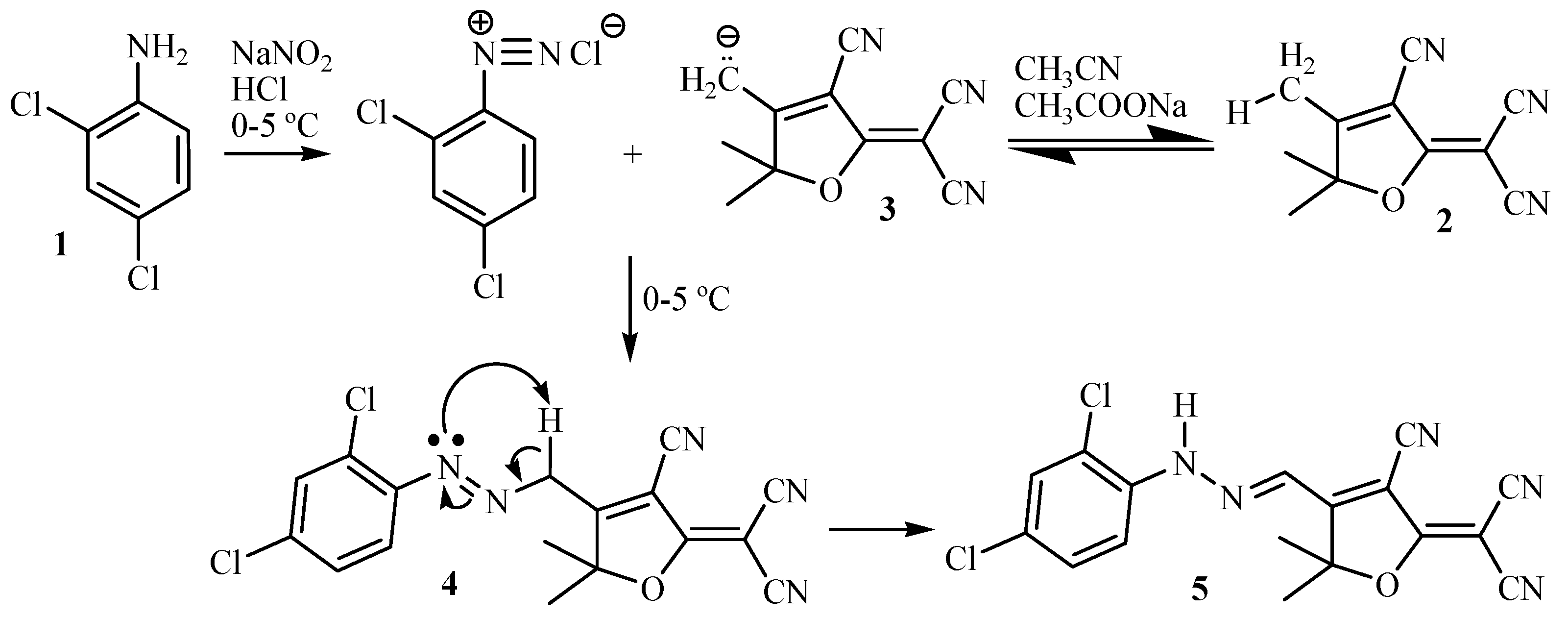
2.3. Preparation of Hydrazone Chromophore
2.4. Preparation of Strip Sensor
2.5. Quantitative and Qualitative Determination of Cd(II)
2.6. Methods
2.7. pH Measurements of DCDHFH Solution
2.8. pH Effect of Cadmium Solution on Strip Detection
3. Results and Discussion
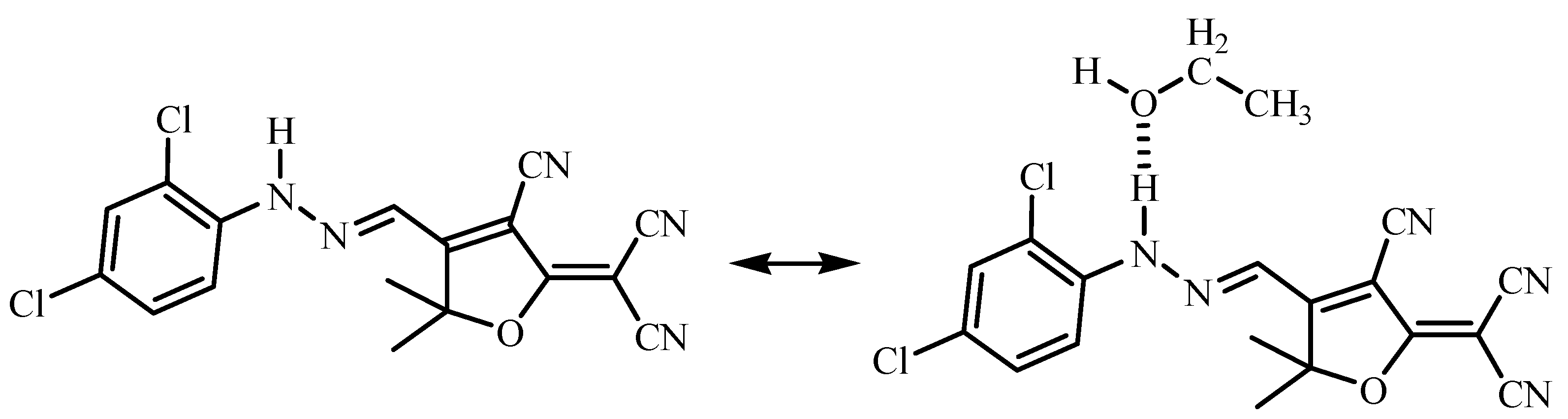
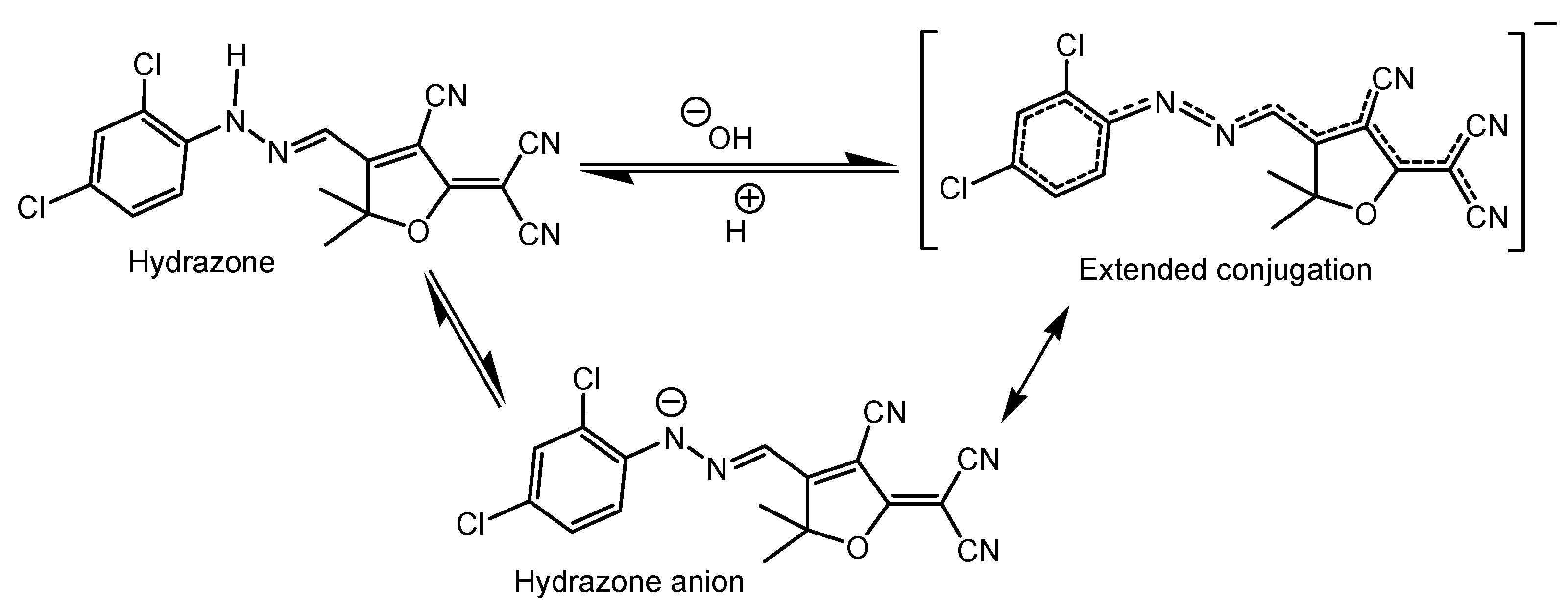
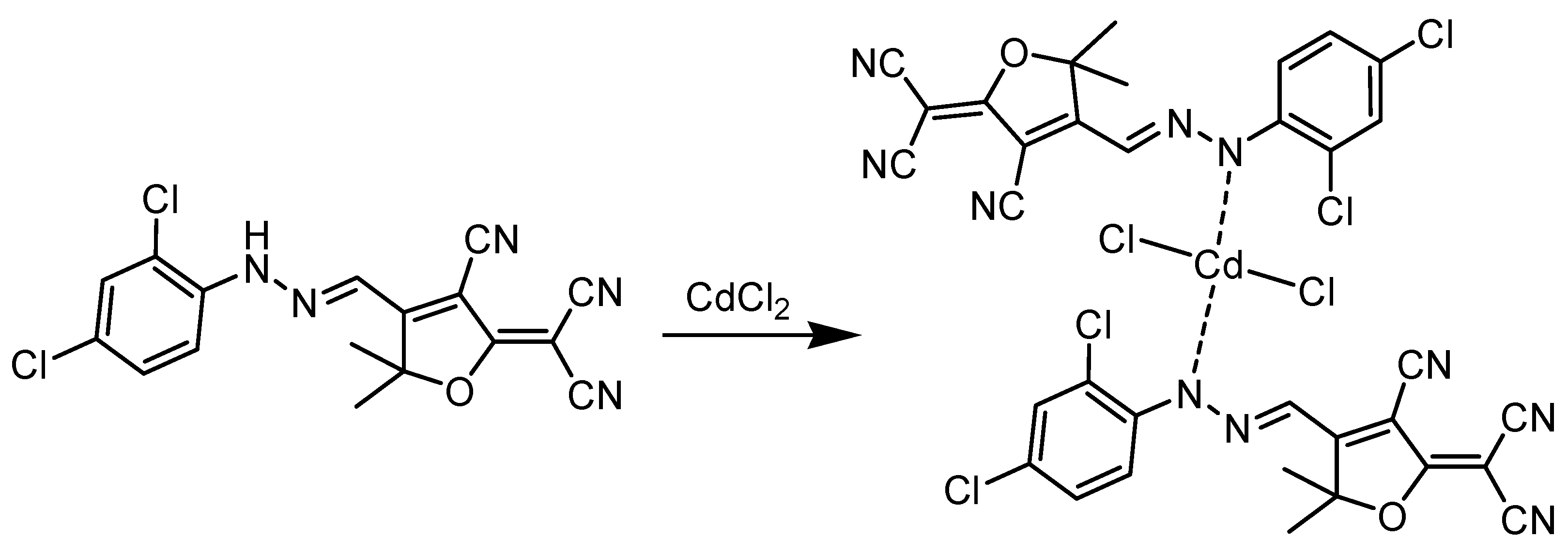
3.1. Chemistry of Chemosensor
3.2. Solvatochromic Behavior
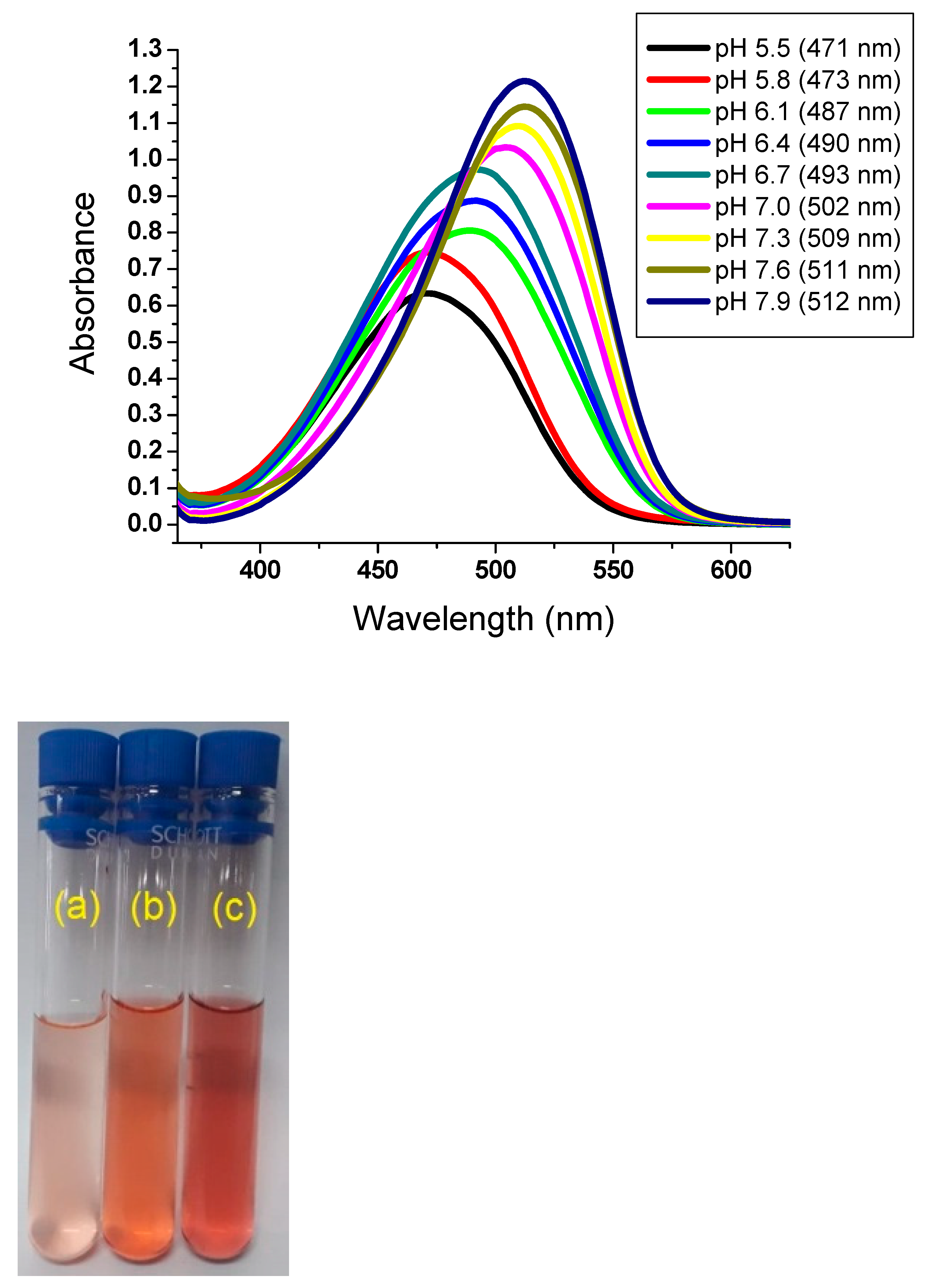
3.3. pH-Sensory of DCDHFH Solution
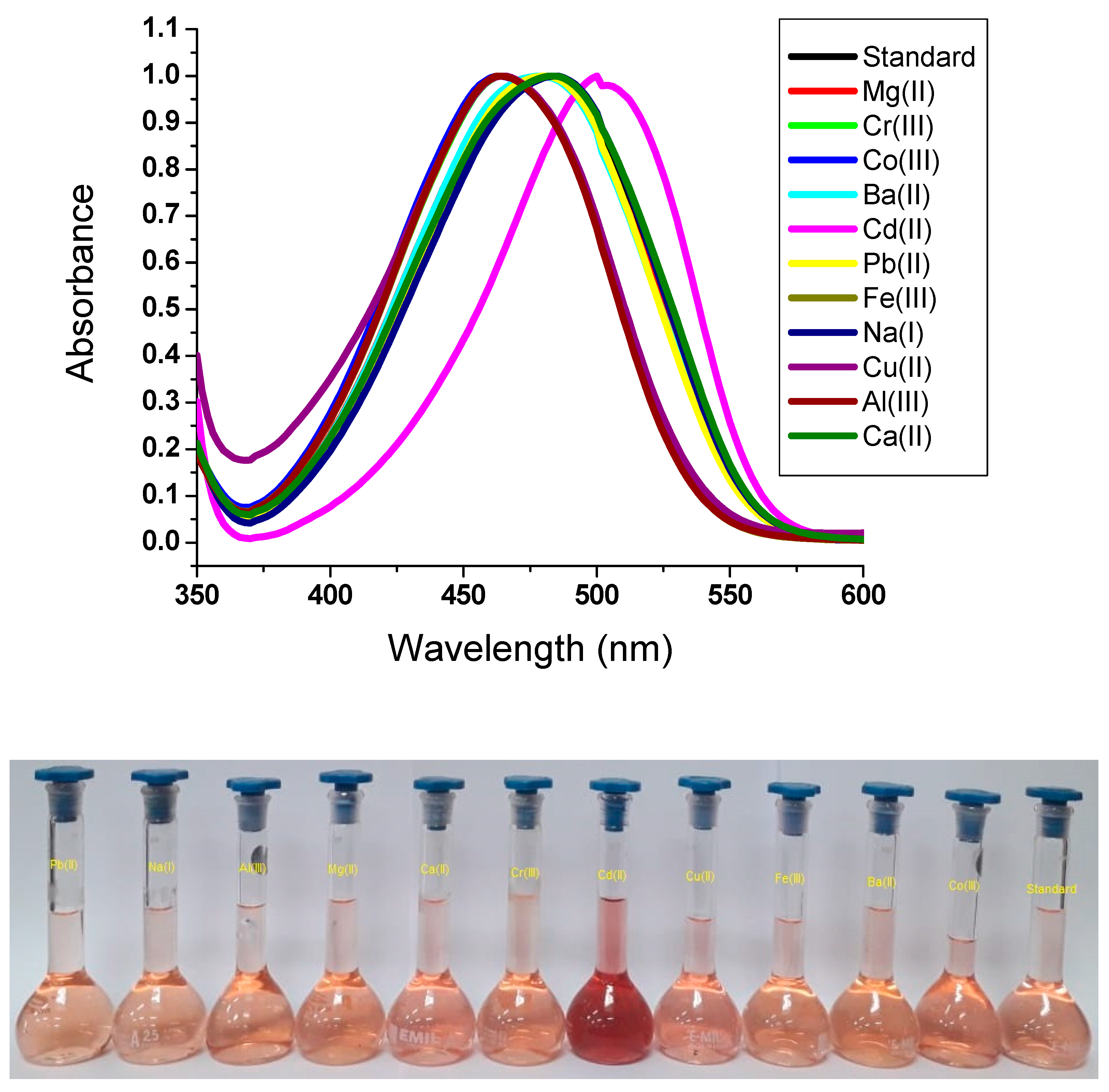
3.4. Solution-Based Determination of Cd(II)
3.5. Preparation of Colorimetric Dipstick
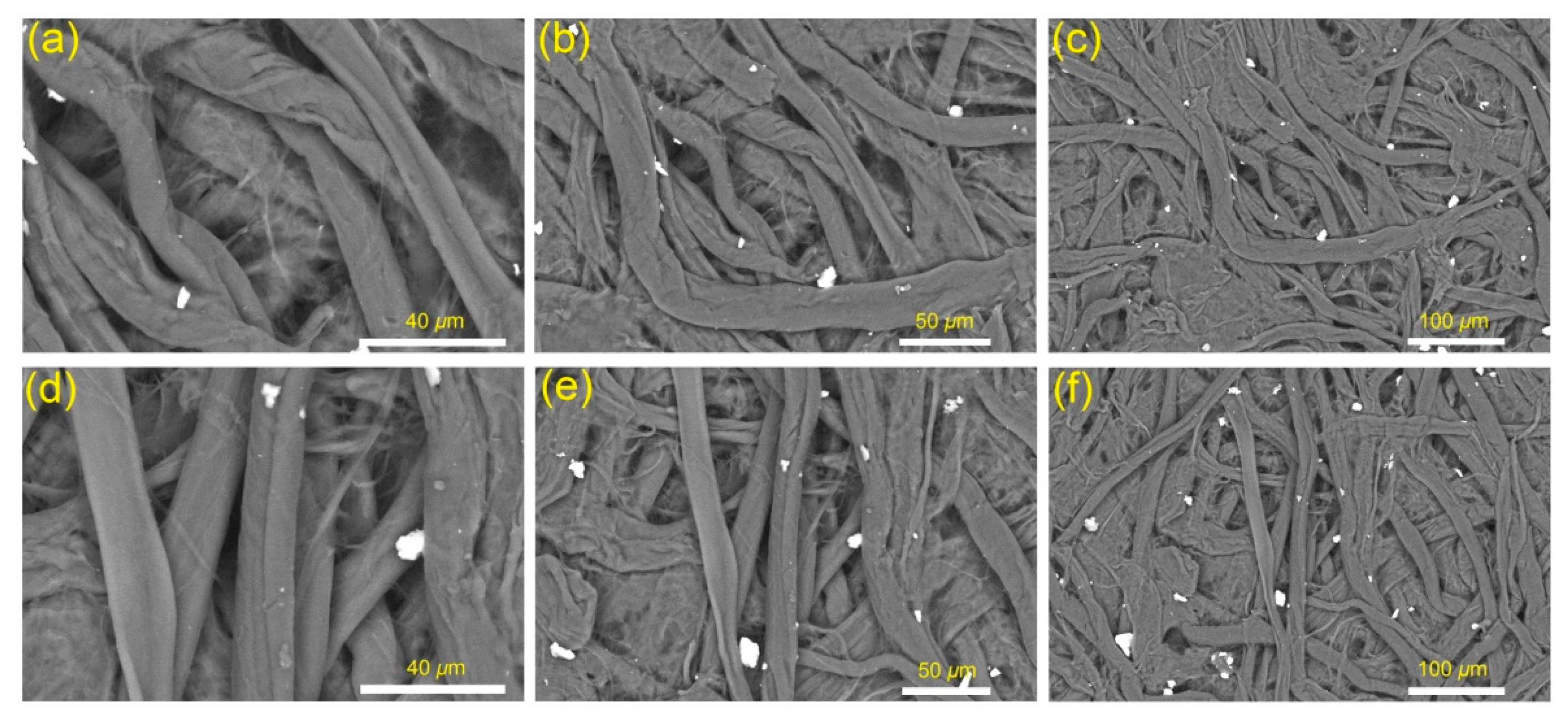
3.6. Morphological Properties
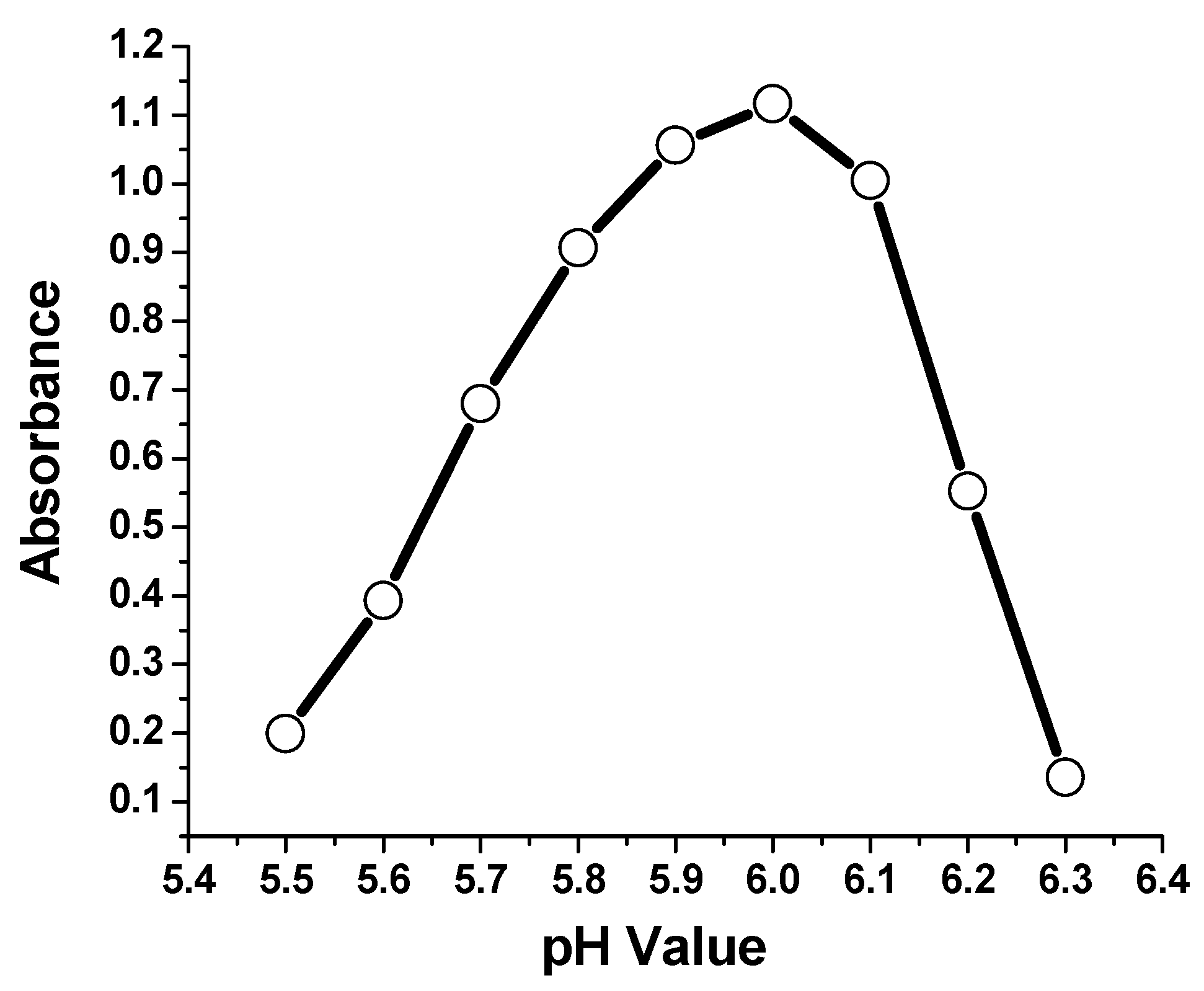
3.7. Effect of pH
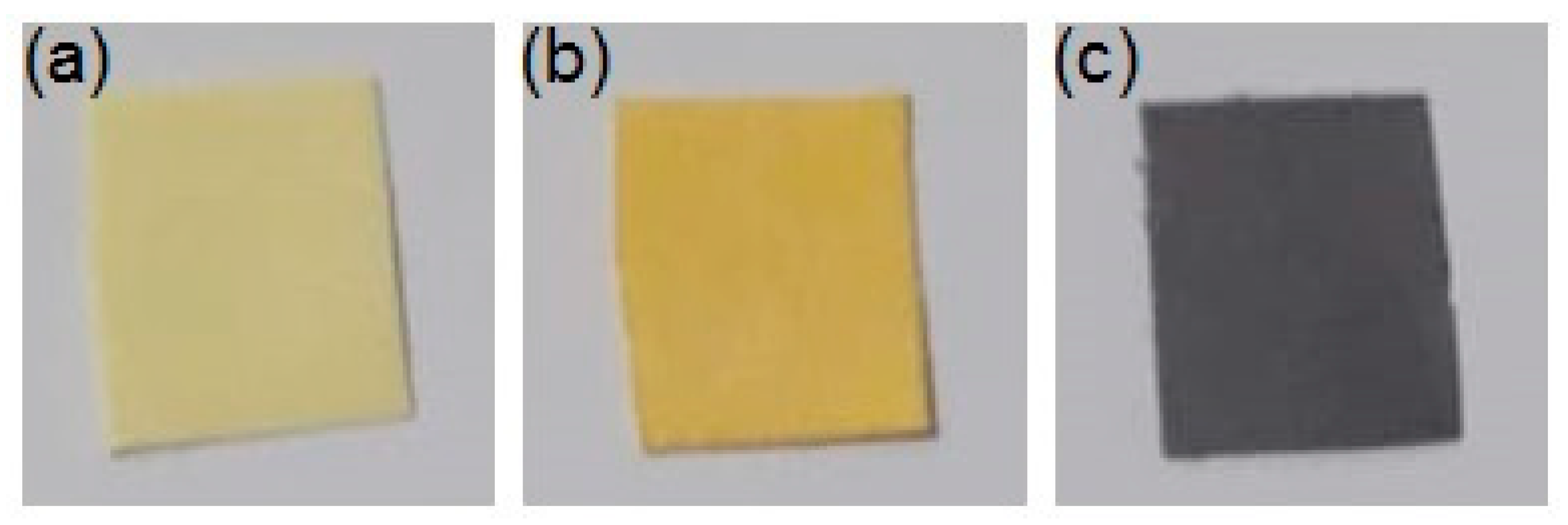
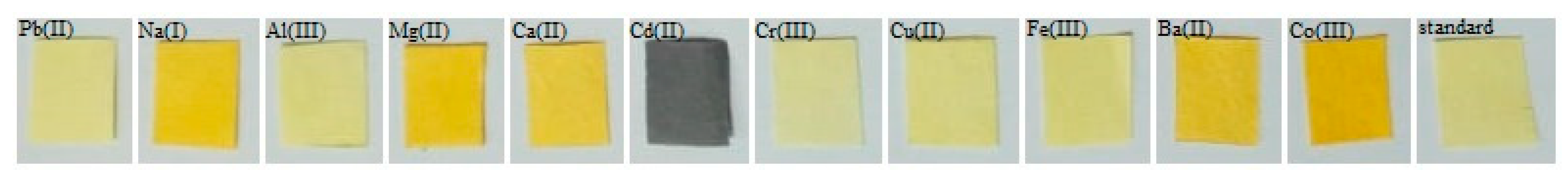
3.8. Colorimetric Properties of Dipstick
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P.; Nguyen, V.-H. Tailoring cadmium sulfide-based photocatalytic nanomaterials for water decontamination: A review. Environ. Chem. Lett. 2021, 19, 271–306. [Google Scholar]
- Zhao, X.-M.; Yao, L.-A.; Ma, Q.-L.; Zhou, G.-J.; Wang, L.; Fang, Q.-L.; Xu, Z.-C. Distribution and ecological risk assessment of cadmium in water and sediment in Longjiang River, China: Implication on water quality management after pollution accident. Chemosphere 2018, 194, 107–116. [Google Scholar] [CrossRef]
- Mikhailenko, A.V.; Ruban, D.A.; Ermolaev, V.A.; van Loon, A.J. Cadmium pollution in the tourism environment: A literature review. Geosciences 2020, 10, 242. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhou, Y.; Tsang, D.C.W.; Liu, J.; Yang, X.; Yin, M.; Wu, S.; Wang, J.; Xiao, T.; Zhang, Z. Cadmium isotopes as tracers in environmental studies: A review. Sci. Total Environ. 2020, 736, 139585. [Google Scholar] [CrossRef]
- Xing, C.; Kuang, H.; Hao, C.; Liu, L.; Wang, L.; Xu, C. A silver enhanced and sensitive strip sensor for Cadmium detection. Food Agric. Immunol. 2014, 25, 287–300. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Li, Z.; Huang, X.; Zhang, W.; Zhang, D.; Zou, X.; Shi, J. A smartphone-integrated ratiometric fluorescence sensor for visual detection of cadmium ions. J. Hazard. Mater. 2021, 408, 124872. [Google Scholar] [CrossRef]
- Hasan, N.; Salman, S.; Islam, A.; Znad, H.; Hasan, M. Sustainable composite sensor material for optical cadmium (II) monitoring and capturing from wastewater. Microchem. J. 2021, 161, 105800. [Google Scholar] [CrossRef]
- Shahat, A.; Kubra, K.T.; Salman, S.; Hasan, N.; Hasan, M. Novel solid-state sensor material for efficient cadmium (II) detection and capturing from wastewater. Microchem. J. 2021, 164, 105967. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Shin, W.-R.; Sekhon, S.S.; Rhee, S.-K.; Ko, J.H.; Ahn, J.-Y.; Min, J.; Kim, Y.-H. Aptamer-based paper strip sensor for detecting Vibrio fischeri. ACS Comb. Sci. 2018, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Leichner, J.; Naja, G.M.; Li, C.-Z. Smart-phone, paper-based fluorescent sensor for ultra-low inorganic phosphate detection in environmental samples. Microsyst. Nanoeng. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, H.; Zhao, Q.; Xing, L.; Zheng, X.; Wang, W.; Zhao, Y.; Yu, Y.; Hu, L.; Yao, W. Recent developments of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst 2020, 145, 3173–3187. [Google Scholar] [CrossRef]
- He, W.; Luo, L.; Liu, Q.; Chen, Z. Colorimetric sensor array for discrimination of heavy metal ions in aqueous solution based on three kinds of thiols as receptors. Anal. Chem. 2018, 90, 4770–4775. [Google Scholar] [CrossRef]
- Tamizselvi, R.; Napoleon, A.A. Fluorescent and Colorimetric Chemosensor for the Detection of Toxic Metal Ions of Mercury and Lead–A Mini Review. ECS Trans. 2022, 107, 16489. [Google Scholar]
- Ali, S.; Chen, X.; Shi, W.; Huang, G.; Yuan, L.-M.; Meng, L.; Chen, S.; Zhonghao, X.; Chen, X. Recent Advances in Silver and Gold Nanoparticles-Based Colorimetric Sensors for Heavy Metal Ions Detection: A Review. Crit. Rev. Anal. Chem. 2021, 1–33. [Google Scholar] [CrossRef]
- Moon, S.; Kim, C. Chalcone-Based Colorimetric Chemosensor for Detecting Ni2+. Chemosensors 2022, 10, 151. [Google Scholar] [CrossRef]
- Khattab, T.A.; El-Naggar, M.E.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Simple Development of Novel Reversible Colorimetric Thermometer Using Urea Organogel Embedded with Thermochromic Hydrazone Chromophore. Chemosensors 2020, 8, 132. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Khattab, T.A.; Youssef, Y.A.; Al-Balakocy, N.; Kamel, S. Novel cellulose-based halochromic test strips for naked-eye detection of alkaline vapors and analytes. Talanta 2017, 170, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, Y.M.; Chao, J.B.; Wang, H.; Wang, Y.; Shuang, S.M. A simple but efficient fluorescent sensor for ratiometric sensing of Cd2+ and bio-imaging studies. Sens. Actuator B Chem. 2020, 303, 127216. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Lu, R.; Liu, G.; Pu, S. A multi-functional hydrazinobenzothiazole-based diarylethene derivative: Highly efficient discrimination cadmium ion from zinc ion and near-infrared absorption detection of hydroxide ion. Dyes Pigm. 2017, 146, 305–315. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.; Shi, F.; Pu, S. A diarylethene based multi-functional sensor for fluorescent detection of Cd2+ and colorimetric detection of Cu2+. Dyes Pigm. 2019, 161, 34–43. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A.; Kamel, S. Development of a novel colorimetric thermometer based on poly (N-vinylcaprolactam) with push–π–pull tricyanofuran hydrazone anion dye. New J. Chem. 2021, 45, 5382–5390. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Hamshary, H.; Salem, W.M. Solution blowing spinning technology towards green development of urea sensor nanofibers immobilized with hydrazone probe. Polymers 2021, 13, 531. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Habeebullah, T.M.; Alsoliemy, A.; Alzahrani, H.K.; Shah, R.; Alfi, A.A.; El-Metwaly, N.M. Preparation of polyvinyl alcohol reinforced with microcrystalline cellulose to function as test strips immobilized with a hydrazone chromophore for colorimetric identification of toxic ammonia. Mater. Chem. Phys. 2022, 275, 125218. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Al-Qahtani, S.D.; Bayazeed, A.; Munshi, A.M.; Alsoliemy, A.; Alqarni, S.A.; El-Metwaly, N.M. Cellulose Acetate–Cellulose Nanowhisker Nanocomposite Immobilized with a DCDHF-Hydrazone Chromophore toward a Smart Test Strip for Colorimetric Detection of Diethyl Chlorophosphate as a Nerve Agent Mimic. ACS Omega 2022, 7, 5595–5604. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Abdelrahman, M.S.; Shaban, E.; Khattab, T.A. Metallochromic Hydrazone-Based Chemosensor with Application in a Colorimetric Paper Strip for Selective Detection of Cu2+. ChemistrySelect 2022, 7, e202200811. [Google Scholar] [CrossRef]
- Al-Azmi, A.; John, E. Synthesis and characterization of novel tricyanofuran hydrazone probe: Solvatochromism, density-functional theory calculation and selective fluorescence, and colorimetric determination of iron (III). Luminescence 2021, 36, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; El-Newehy, M.H.; Aldalbahi, A.; Salem, W.M.; Khattab, T.A. Immobilization of anthocyanin extract from red-cabbage into electrospun polyvinyl alcohol nanofibers for colorimetric selective detection of ferric ions. J. Environ. Chem. Eng. 2021, 9, 105072. [Google Scholar] [CrossRef]
- Khattab, T.A.; El-Naggar, M.E.; Pannipara, M.; Wageh, S.; Abou Taleb, M.F.; Abu-Saied, M.A.; El-Tantawy El Sayed, I. Green metallochromic cellulose dipstick for Fe (III) using chitosan nanoparticles and cyanidin-based natural anthocyanins red-cabbage extract. Int. J. Biol. Macromol. 2022, 202, 269–277. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A.; Kamel, S. Hydrazone-Based Supramolecular Organogel for Selective Chromogenic Detection of Organophosphorus Nerve Agent Mimic. ChemistrySelect 2021, 6, 2002–2009. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alnoman, R.B.; Snari, R.M.; Aljuhani, E.; Bayazeed, A.; Qurban, J.; El-Metwaly, N.M. Synthesis and Characterization of Novel Ionochromic Tricyanofuran-Based Phenothiazine Fluorophore: Cellulose-Based Xerogel for Colorimetric Detection of Toxic Cyanides. J. Polym. Environ. 2022, 30, 3107–3118. [Google Scholar] [CrossRef]
- Śmigiel-Kamińska, D.; Pośpiech, J.; Makowska, J.; Stepnowski, P.; Wąs-Gubała, J.; Kumirska, J. The identification of polyester fibers dyed with disperse dyes for forensic purposes. Molecules 2019, 24, 613. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Snari, R.M.; Al-Ahmed, Z.A.; Hossan, A.; Munshi, A.M.; Alfi, A.A.; El-Metwaly, N.M. Novel halochromic hydrazonal chromophore immobilized into rice-straw based cellulose aerogel for vapochromic detection of ammonia. J. Mol. Liq. 2022, 350, 118539. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Bidin, N.; Salim, A.A.; Riaz, S.; Krishnan, G.; Naseem, S. Crack-free high surface area silica-titania nanocomposite coating as opto-chemical sensor device. Sens. Actuator A Phys. 2018, 270, 153–161. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.-C.; Choi, S.Y.; Kim, Y.; Seo, S.-Y.; Park, T.-E.; Kwon, S.-H.; Cho, B.; Ahn, J.-H. Self-formed channel devices based on vertically grown 2D materials with large-surface-area and their potential for chemical sensor applications. Small 2018, 14, 1704116. [Google Scholar] [CrossRef]
- Hu, T.; Xu, K.; Qiu, S.; Han, Y.; Chen, J.; Xu, J.; Chen, K.; Sun, Z.; Yi, H.; Ni, Z. Colorimetric detection of urine glucose using a C/CdTe QDs–GOx aerogel based on a microfluidic assay sensor. J. Mater. Chem. B 2020, 8, 7160–7165. [Google Scholar] [CrossRef]


| Solvent | λmax (nm) |
|---|---|
| Methanol | 466 |
| Cyclohexanone | 473 |
| Ethyl acetate | 455 |
| n-Propanol | 500 |
| iso-Propanol | 466 |
| Dimethylformamide | 508 |
| Chloroform | 468 |
| Acetonitrile | 460 |
| 1,4-Dioxane | 457 |
| Dimethyl sulfoxide | 471 |
| Metal | λmax (nm) |
|---|---|
| Free | 482 |
| CrCl3 | 464 |
| CuCl2 | 464 |
| FeCl3 | 464 |
| AlCl3 | 464 |
| PbCl2 | 480 |
| CaCl2 | 484 |
| CdCl2 | 507 |
| NaCl | 484 |
| CoCl3 | 464 |
| BaCl2 | 478 |
| MgCl2 | 480 |
| Cd(II) (ppm) | Paper Color | L* | a* | b* | K/S |
|---|---|---|---|---|---|
| Zero | Yellow | 68.60 | −4.88 | 15.94 | 1.86 |
| 1 | Yellow | 67.10 | −2.91 | 14.03 | 2.02 |
| 10 | Orange | 65.86 | −1.96 | 11.48 | 2.46 |
| 25 | Orange | 63.27 | 5.21 | 10.29 | 2.82 |
| 50 | Orange | 59.38 | 5.97 | 10.40 | 3.32 |
| 100 | Orange | 55.02 | 6.91 | 9.19 | 3.76 |
| 150 | Red | 50.09 | 8.33 | 7.97 | 5.45 |
| 200 | Red | 47.21 | 10.65 | 6.71 | 7.53 |
| 250 | Red | 45.95 | 13.24 | 5.88 | 7.99 |
| 300 | Red | 44.90 | 16.01 | 5.24 | 8.14 |
| 350 | Red | 44.63 | 17.51 | 4.97 | 8.58 |
| Metals | Paper Color | L* | a* | b* | K/S |
|---|---|---|---|---|---|
| CrCl3 | Yellow | 68.73 | −4.88 | 15.32 | 2.69 |
| CuCl2 | Yellow | 66.29 | −3.35 | 13.99 | 2.80 |
| FeCl3 | Yellow | 66.86 | −3.25 | 13.17 | 2.54 |
| AlCl3 | Yellow | 68.02 | −4.89 | 14.46 | 2.62 |
| PbCl2 | Yellow | 68.71 | −4.74 | 15.04 | 1.98 |
| CaCl2 | Yellow | 65.32 | −2.22 | 12.83 | 1.93 |
| CoCl3 | Yellow | 59.29 | −0.58 | 9.44 | 2.12 |
| NaCl | Yellow | 62.41 | −1.02 | 10.96 | 2.27 |
| CdCl2 | Red | 45.95 | 13.24 | 5.88 | 7.99 |
| BaCl2 | Yellow | 63.45 | −1.92 | 12.21 | 2.81 |
| MgCl2 | Yellow | 61.33 | −1.11 | 11.49 | 2.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nagar, I.; Youssef, A.M.; Abd El-Hakim, A.A.; Kenawy, E.-R.; Mandour, H.S.A.; Khattab, T.A. Novel Hydrazone Chromophore Sensor for Metallochromic Determination of Cadmium Ions. Chemosensors 2022, 10, 451. https://doi.org/10.3390/chemosensors10110451
El-Nagar I, Youssef AM, Abd El-Hakim AA, Kenawy E-R, Mandour HSA, Khattab TA. Novel Hydrazone Chromophore Sensor for Metallochromic Determination of Cadmium Ions. Chemosensors. 2022; 10(11):451. https://doi.org/10.3390/chemosensors10110451
Chicago/Turabian StyleEl-Nagar, Islam, Ahmed M. Youssef, A. A. Abd El-Hakim, El-Refaie Kenawy, Hamada S. A. Mandour, and Tawfik A. Khattab. 2022. "Novel Hydrazone Chromophore Sensor for Metallochromic Determination of Cadmium Ions" Chemosensors 10, no. 11: 451. https://doi.org/10.3390/chemosensors10110451
APA StyleEl-Nagar, I., Youssef, A. M., Abd El-Hakim, A. A., Kenawy, E.-R., Mandour, H. S. A., & Khattab, T. A. (2022). Novel Hydrazone Chromophore Sensor for Metallochromic Determination of Cadmium Ions. Chemosensors, 10(11), 451. https://doi.org/10.3390/chemosensors10110451






