Prospects of Application of Ultramicroelectrode Ensembles for Voltammetric Determination of Compounds with Close Standard Electrode Potentials and Different Diffusion Coefficients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Lithographic Production of Ultramicroelectrodes
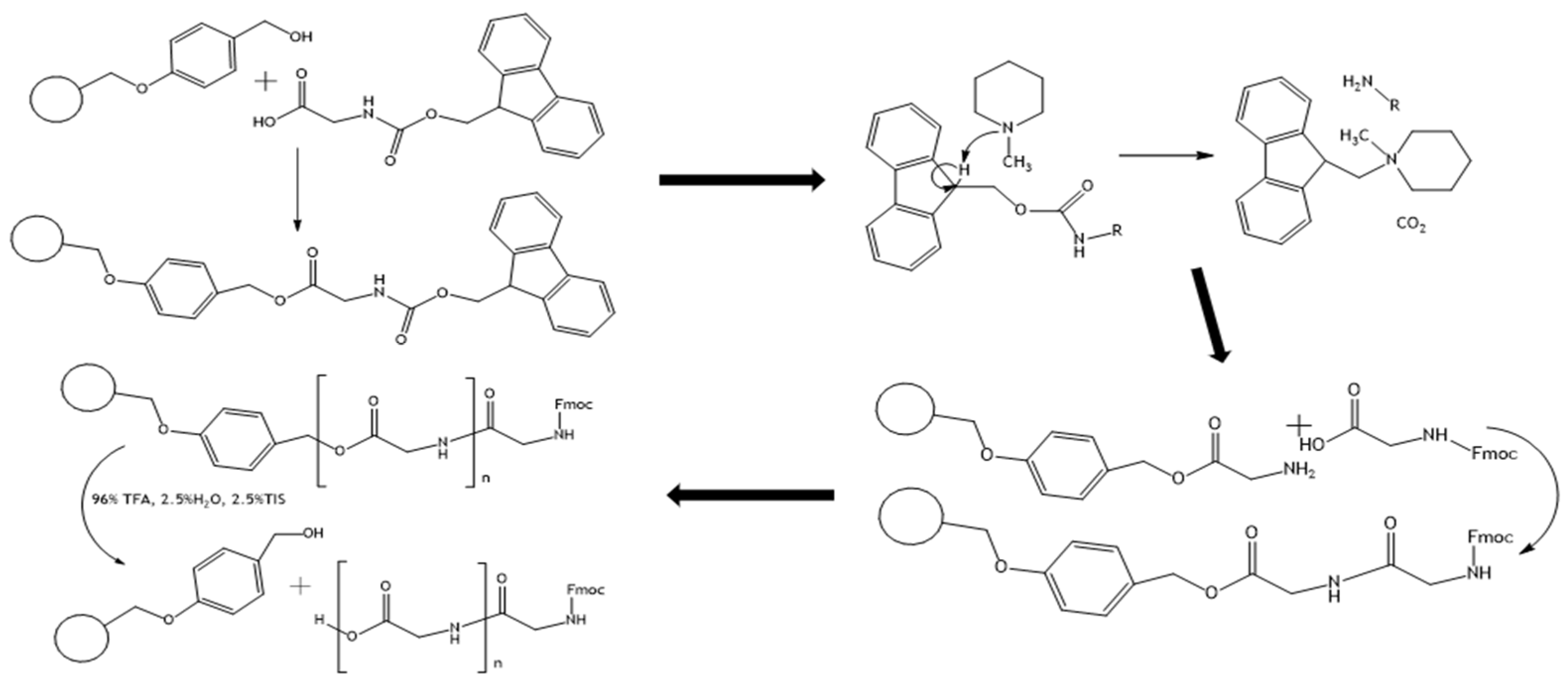
2.3. Triglycine Synthesis
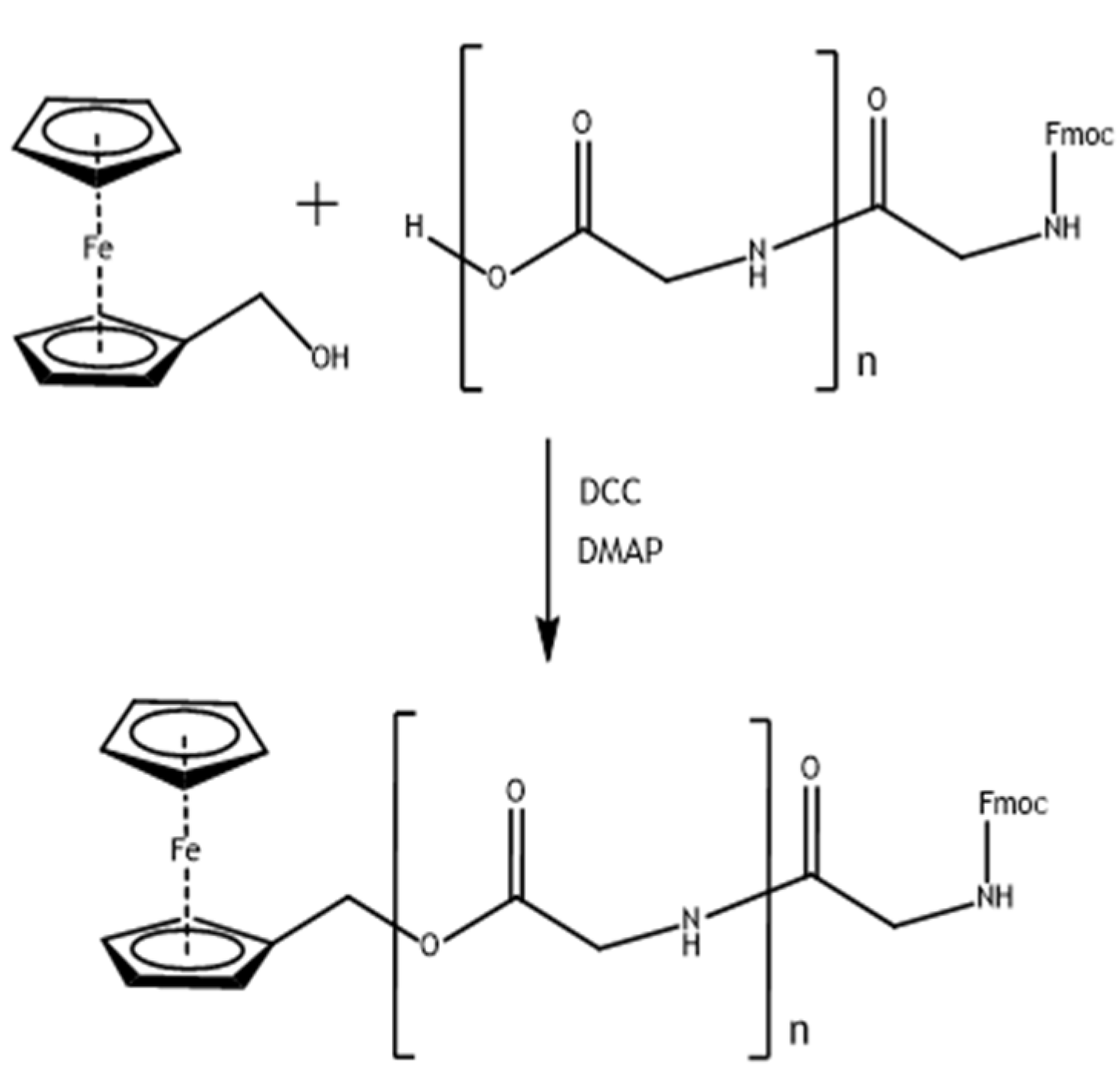
2.4. Synthesis of Glycine and Triglycine Esters of Ferrocenemethanol
2.5. Recording Cyclic Voltammograms
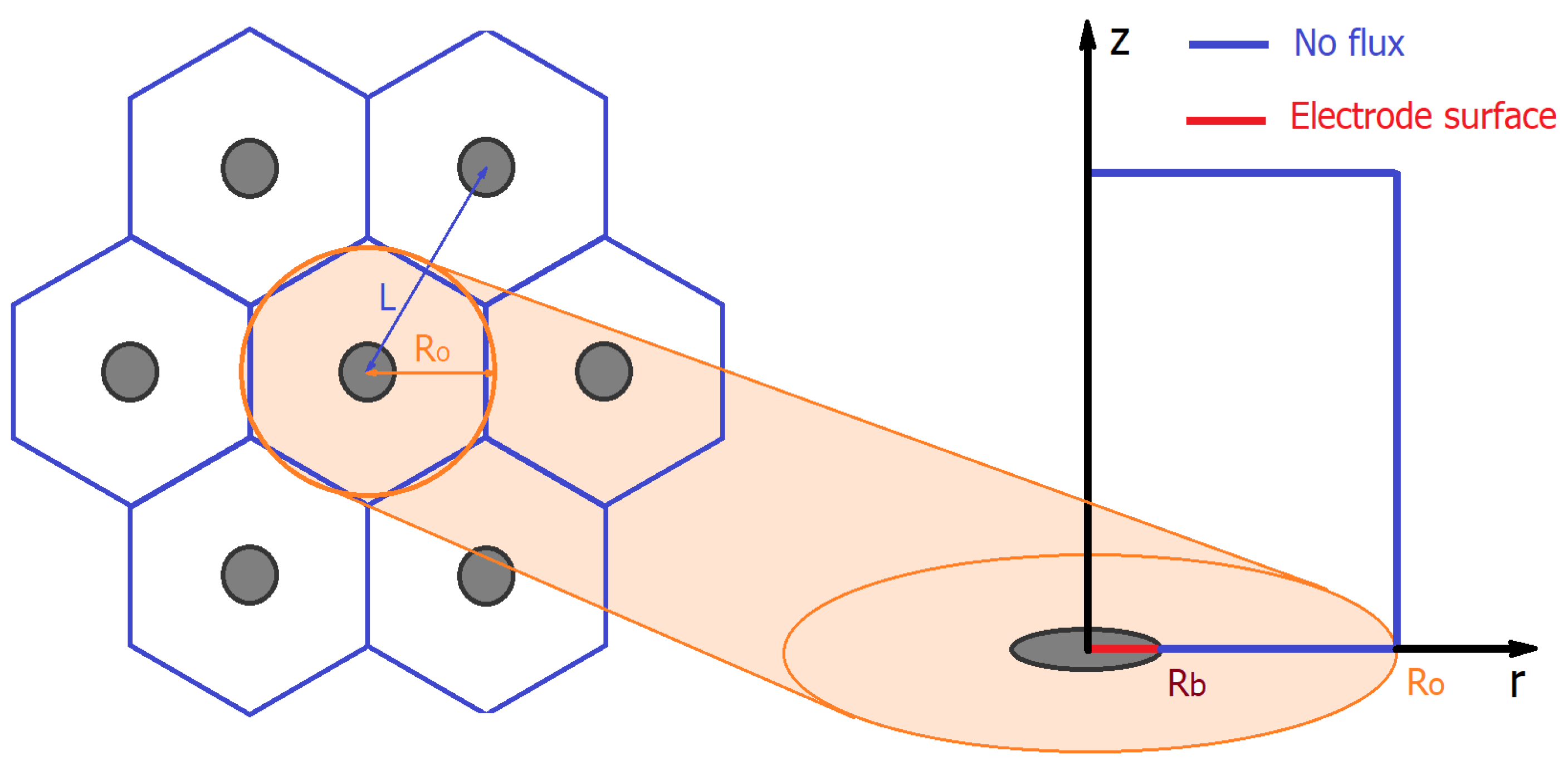
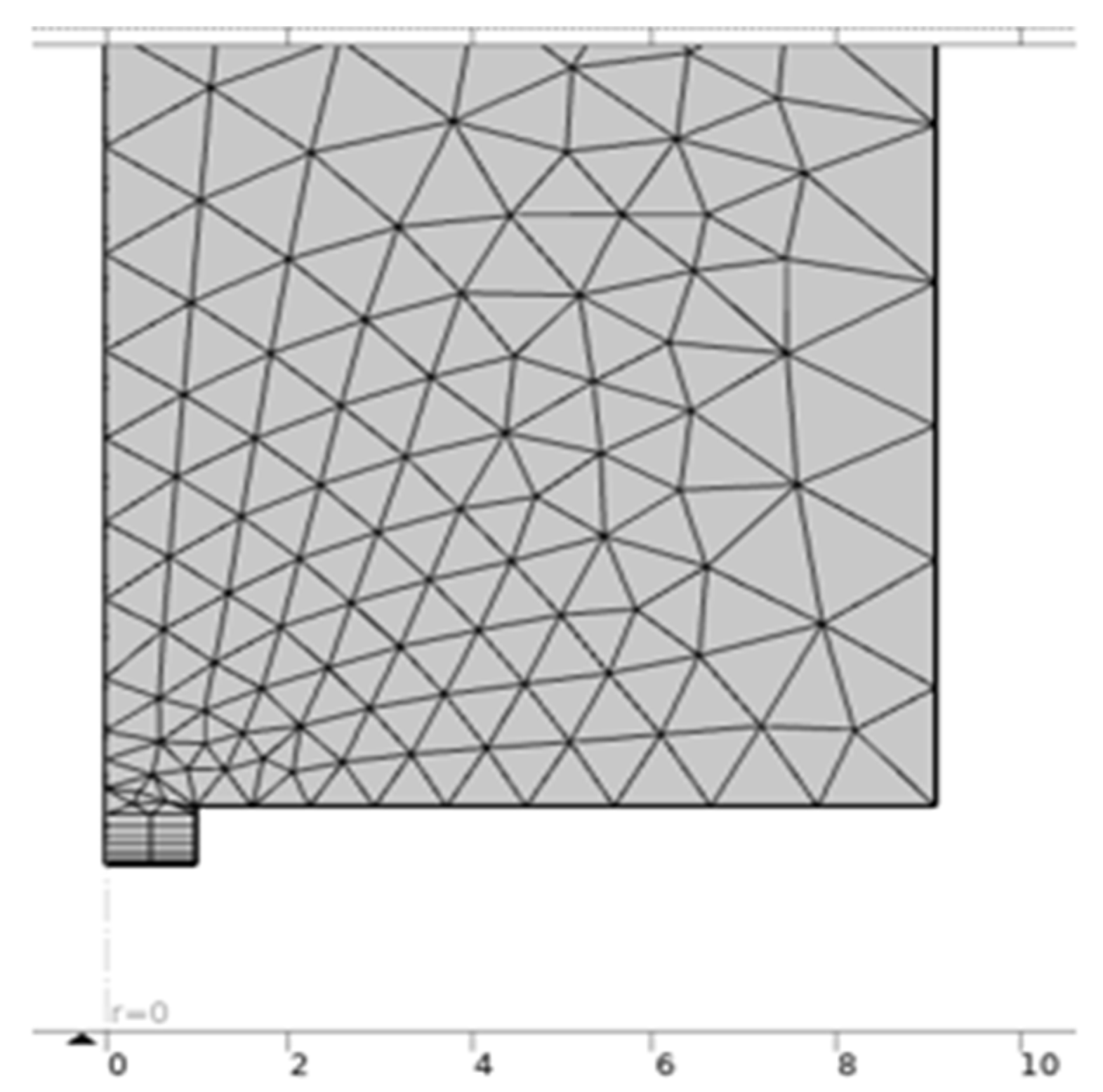
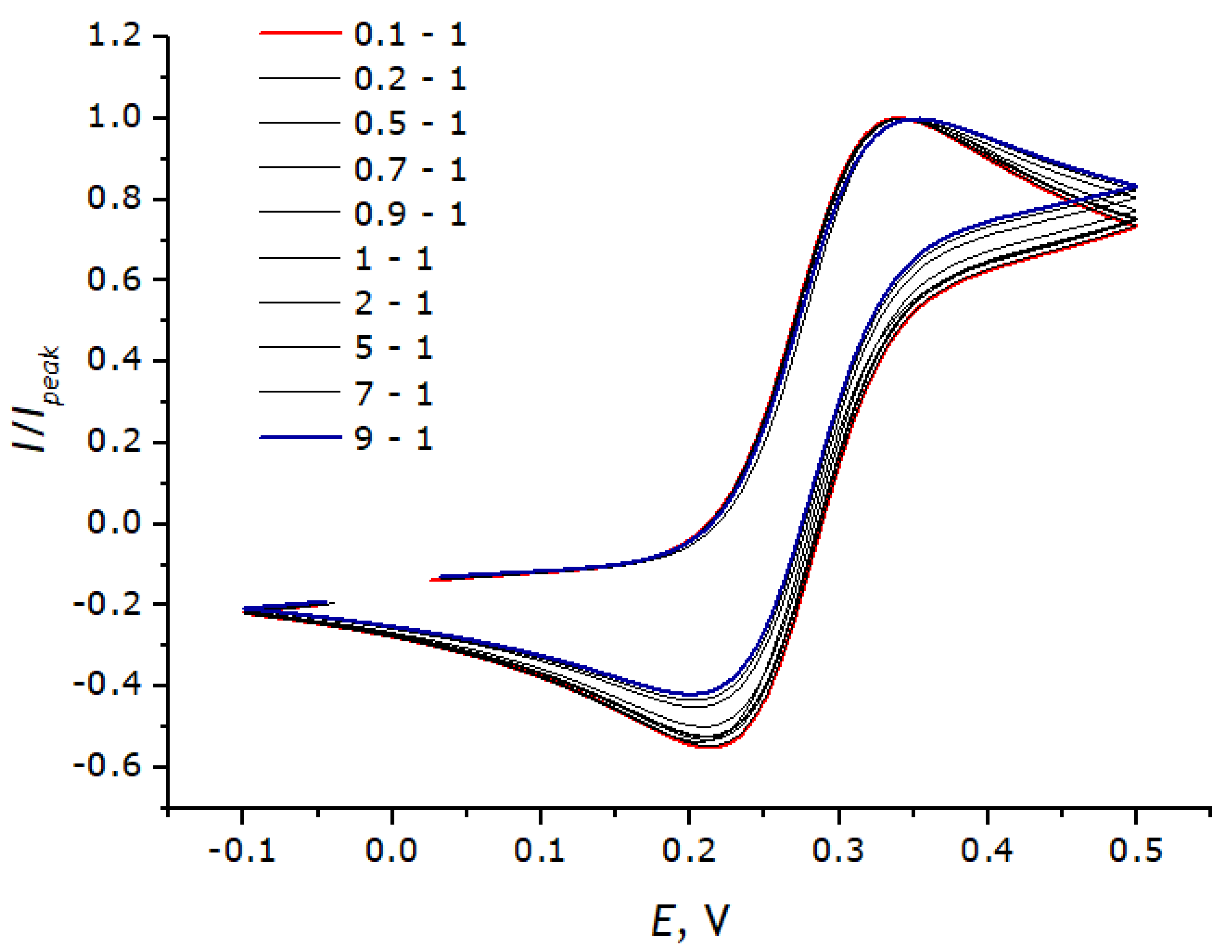
2.6. Modeling Cyclic Voltammograms
3. Results
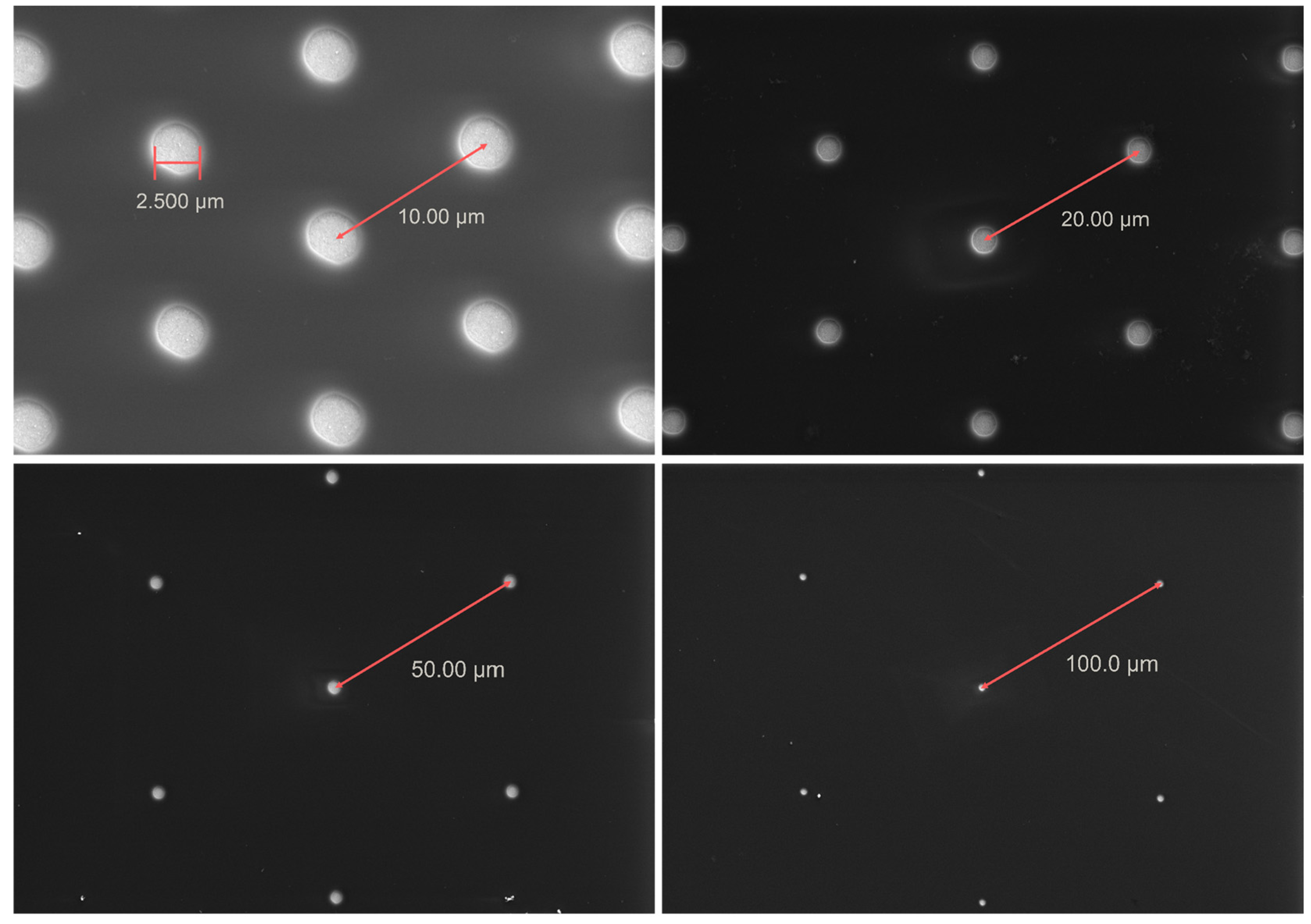
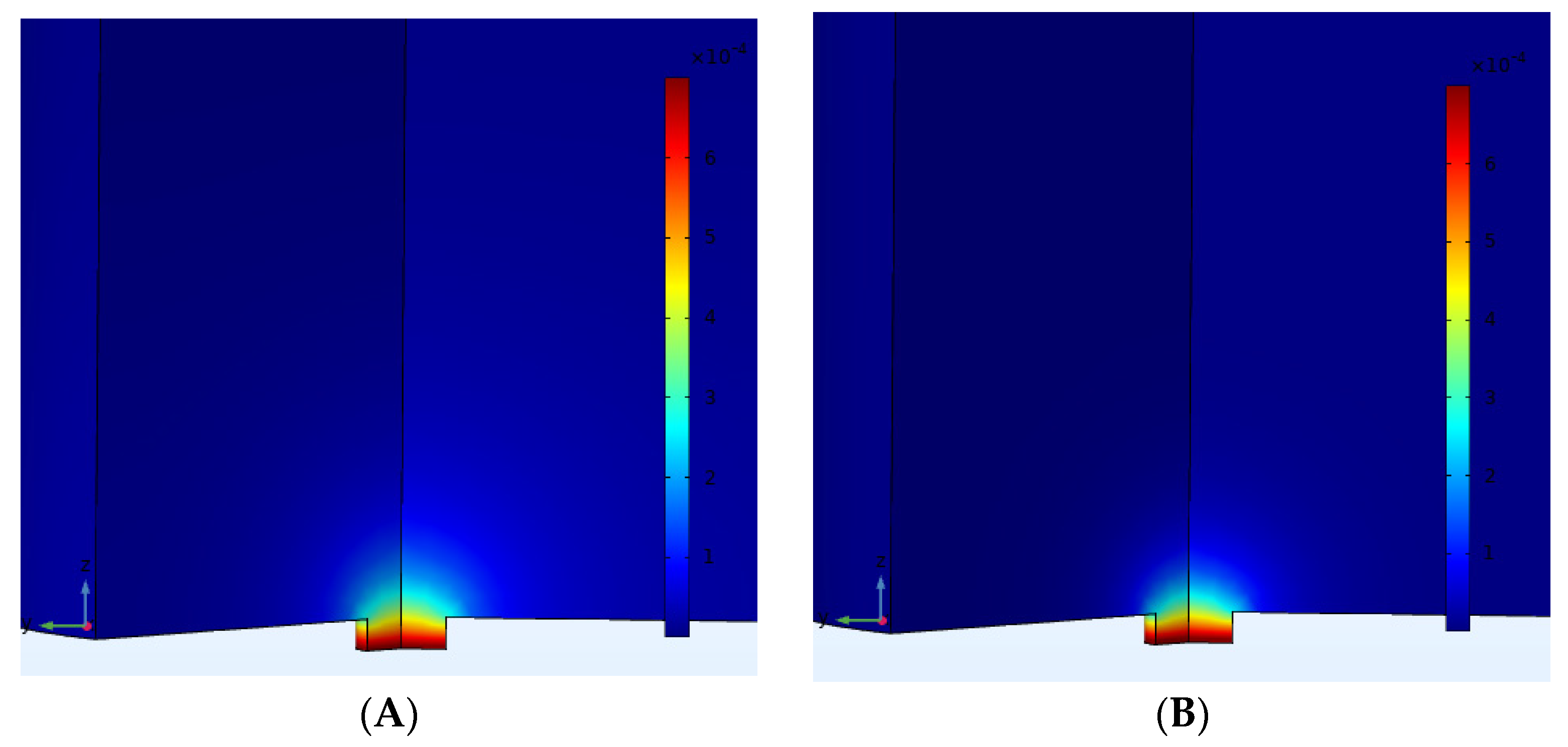
3.1. Microscopic Study of Microelectrode Arrays
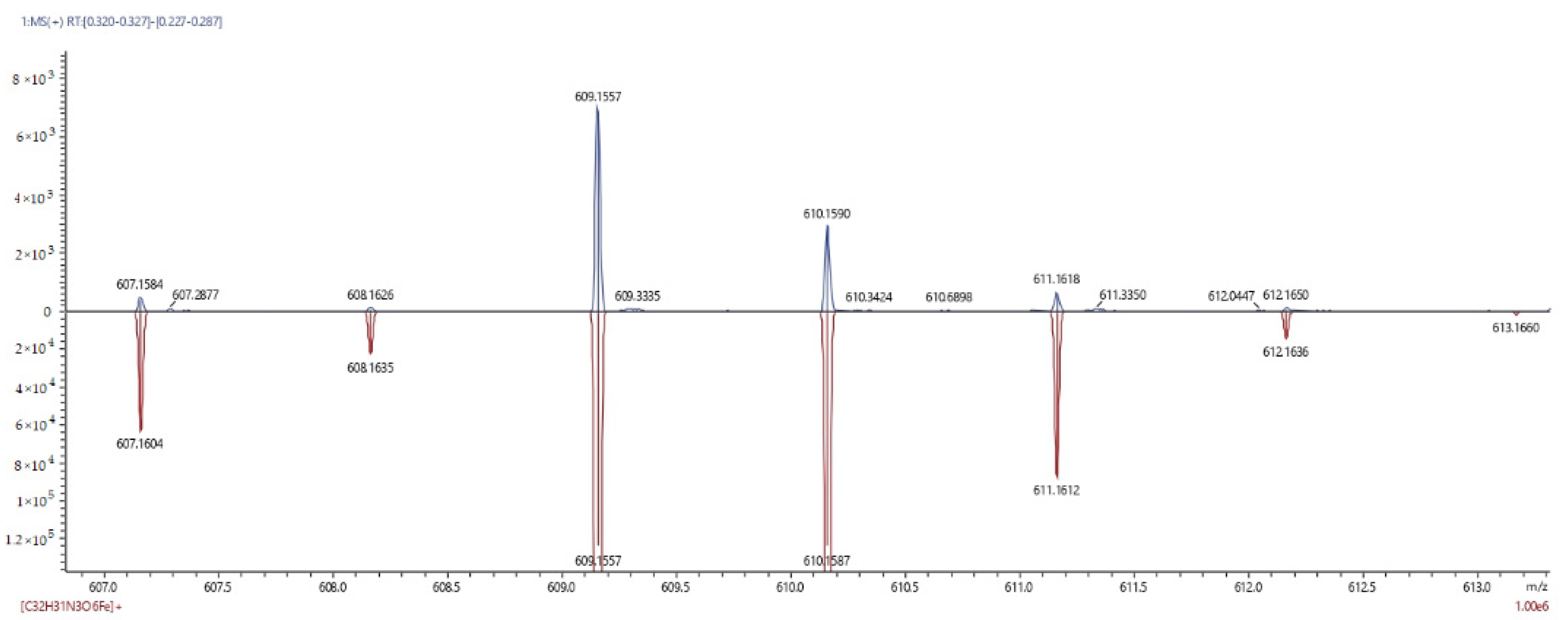
3.2. Determination of the Structure of Ferrocenemethanol Esters of Glycine and Triglycine
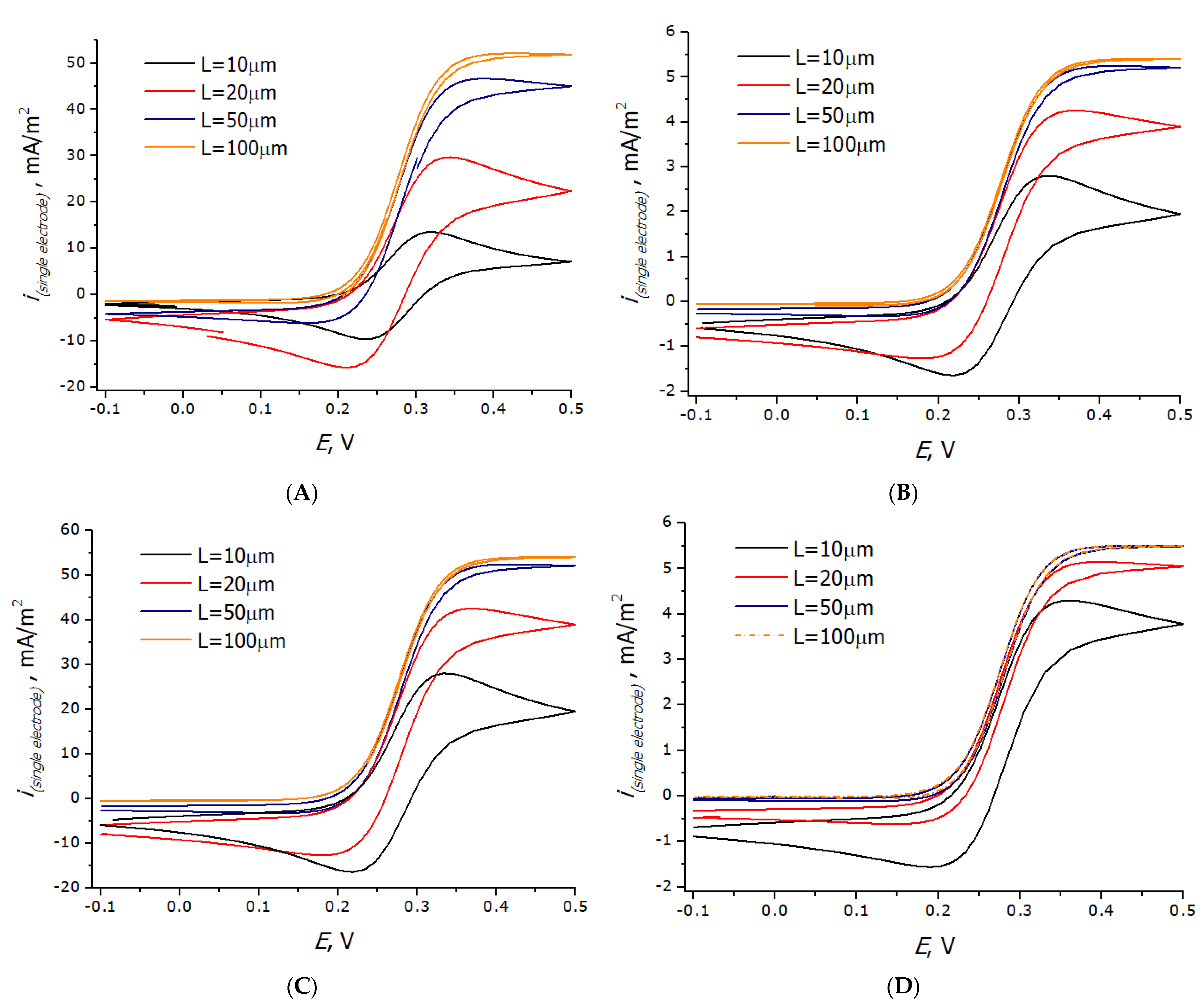
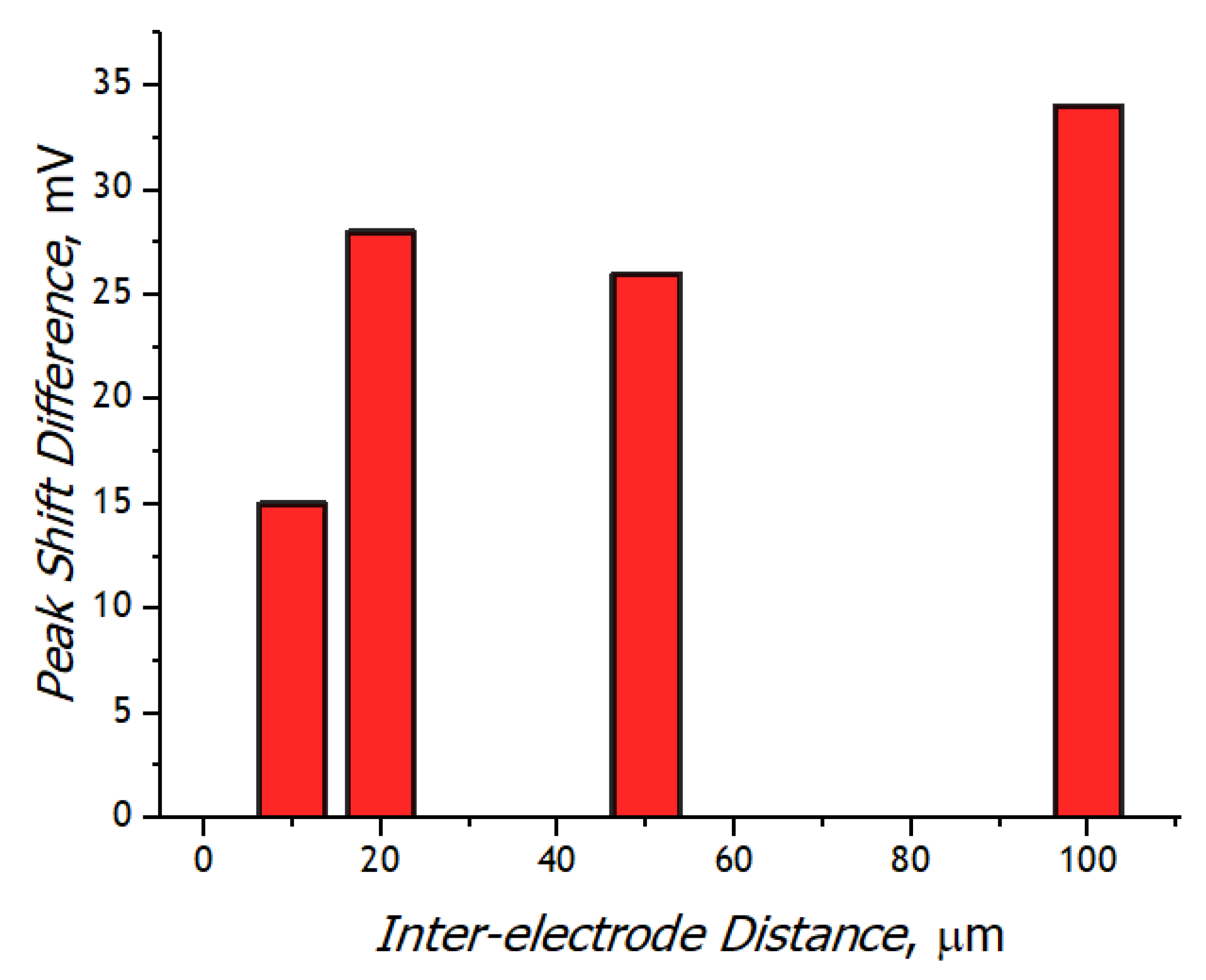
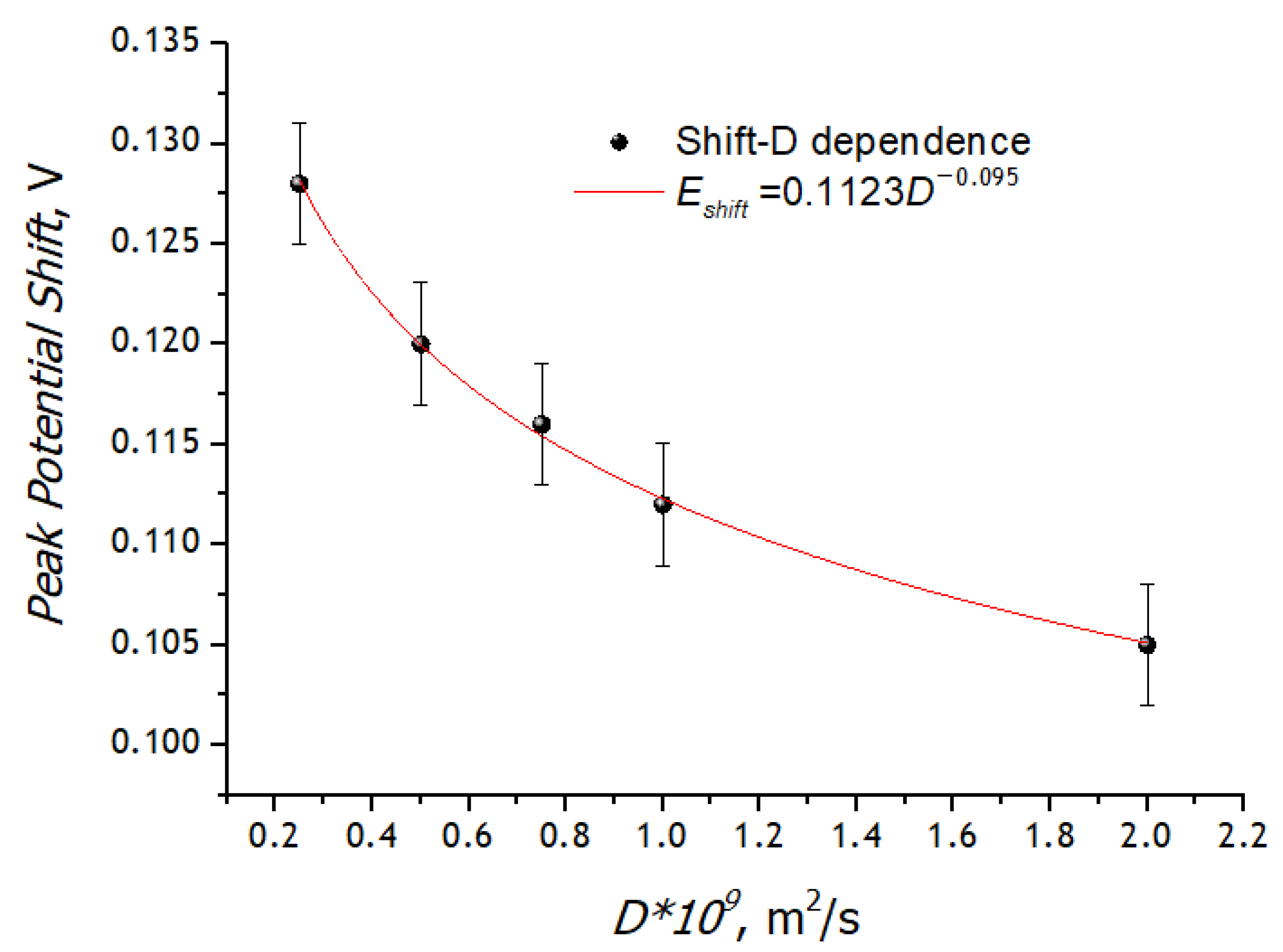
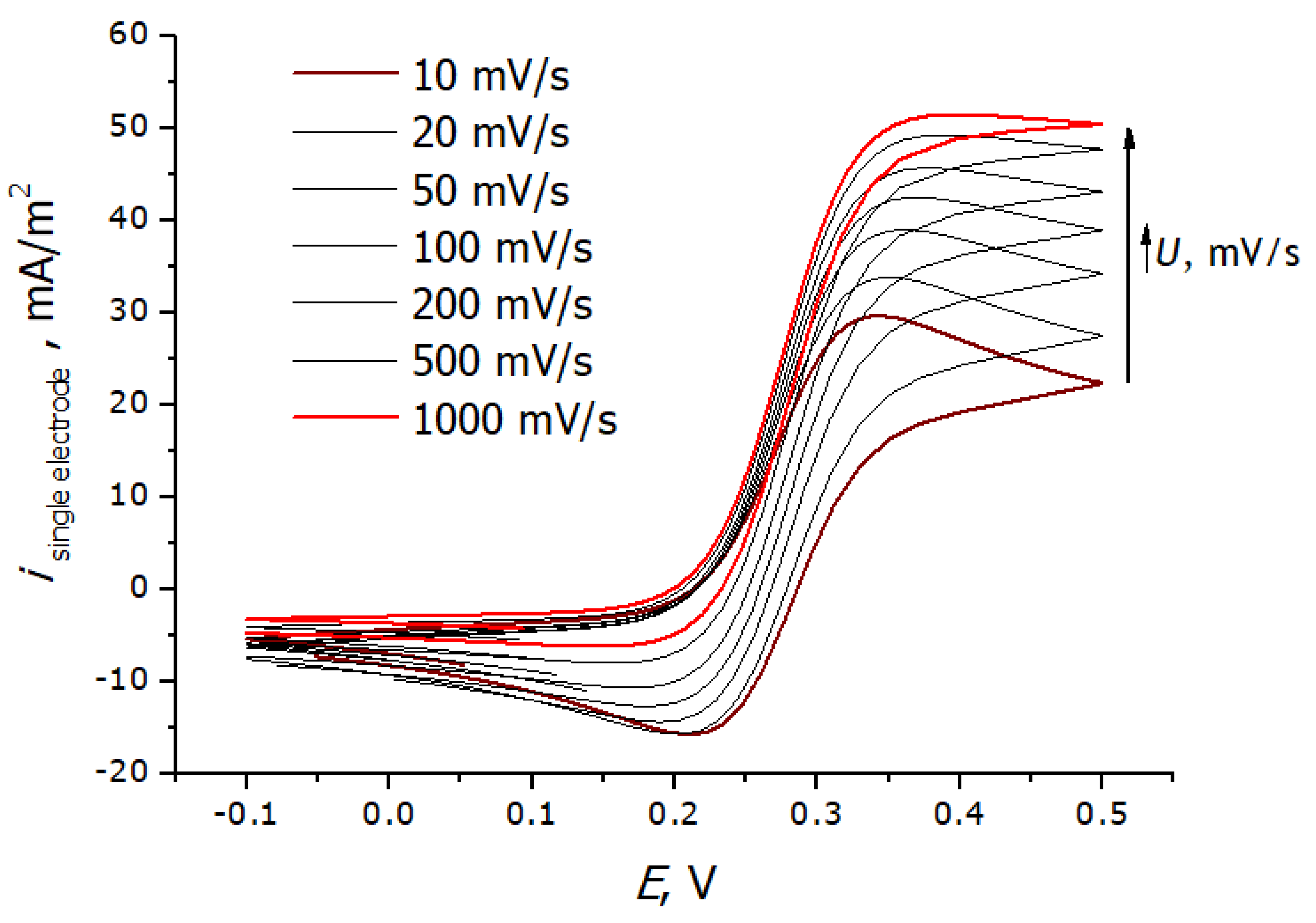
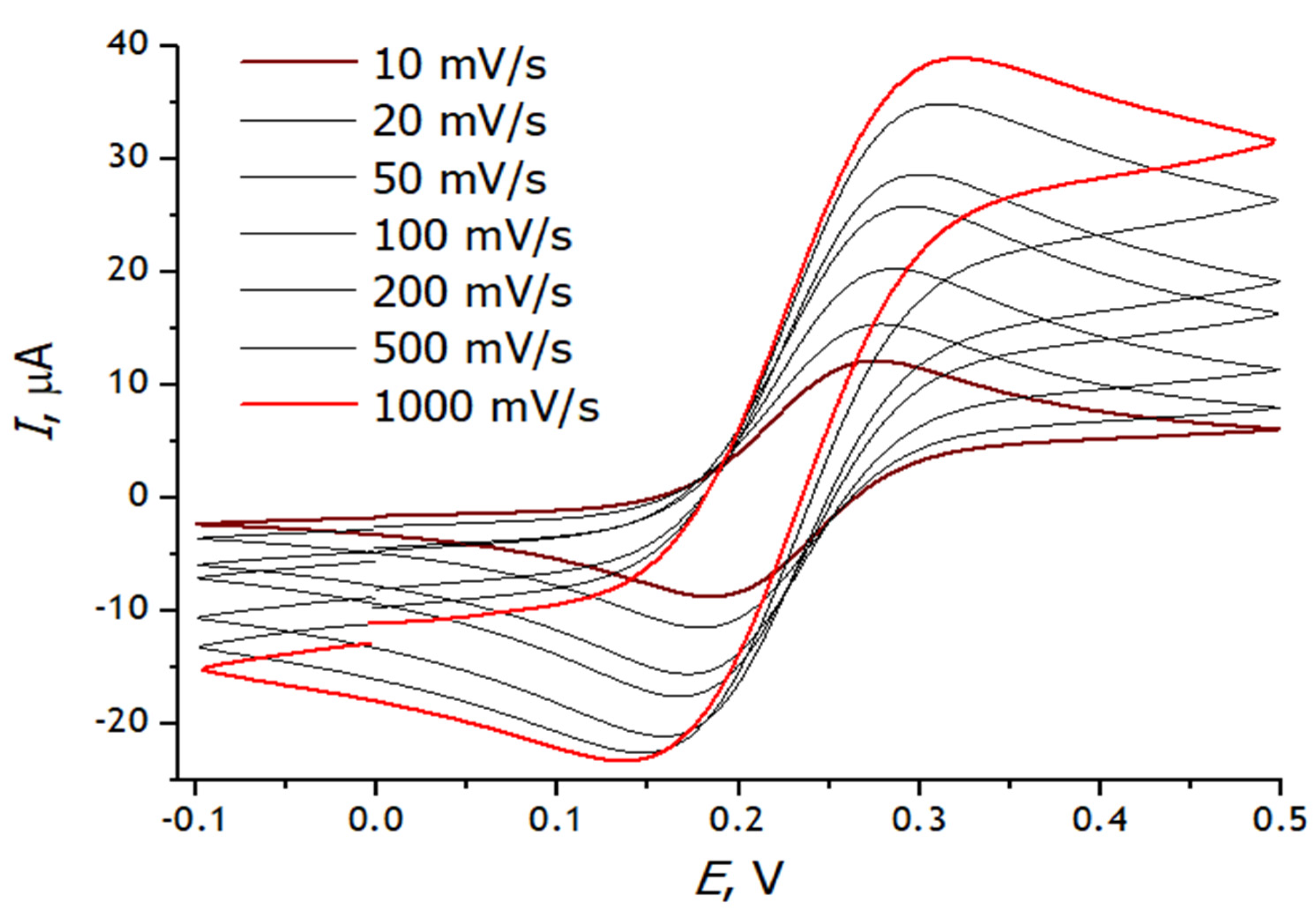
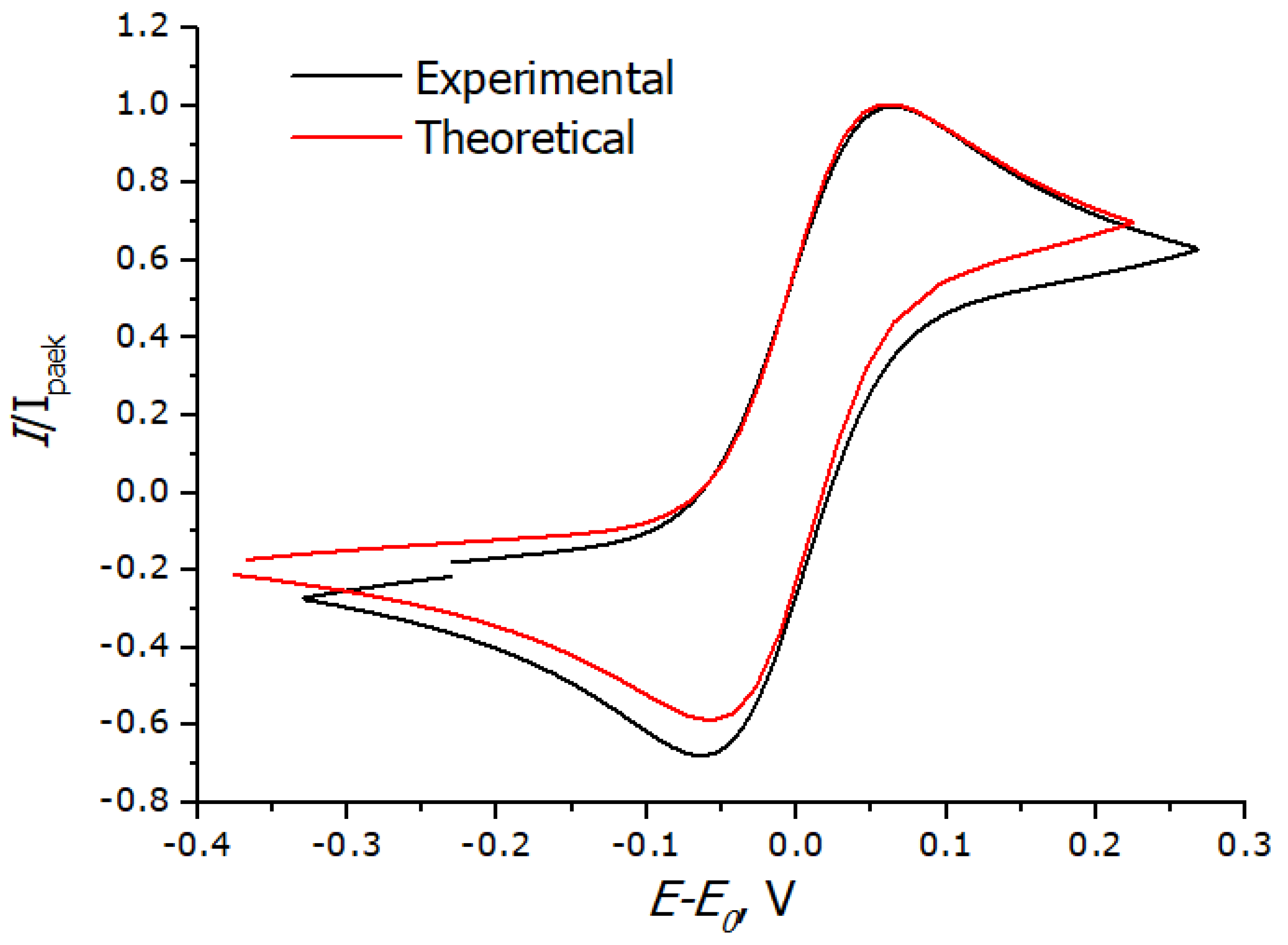
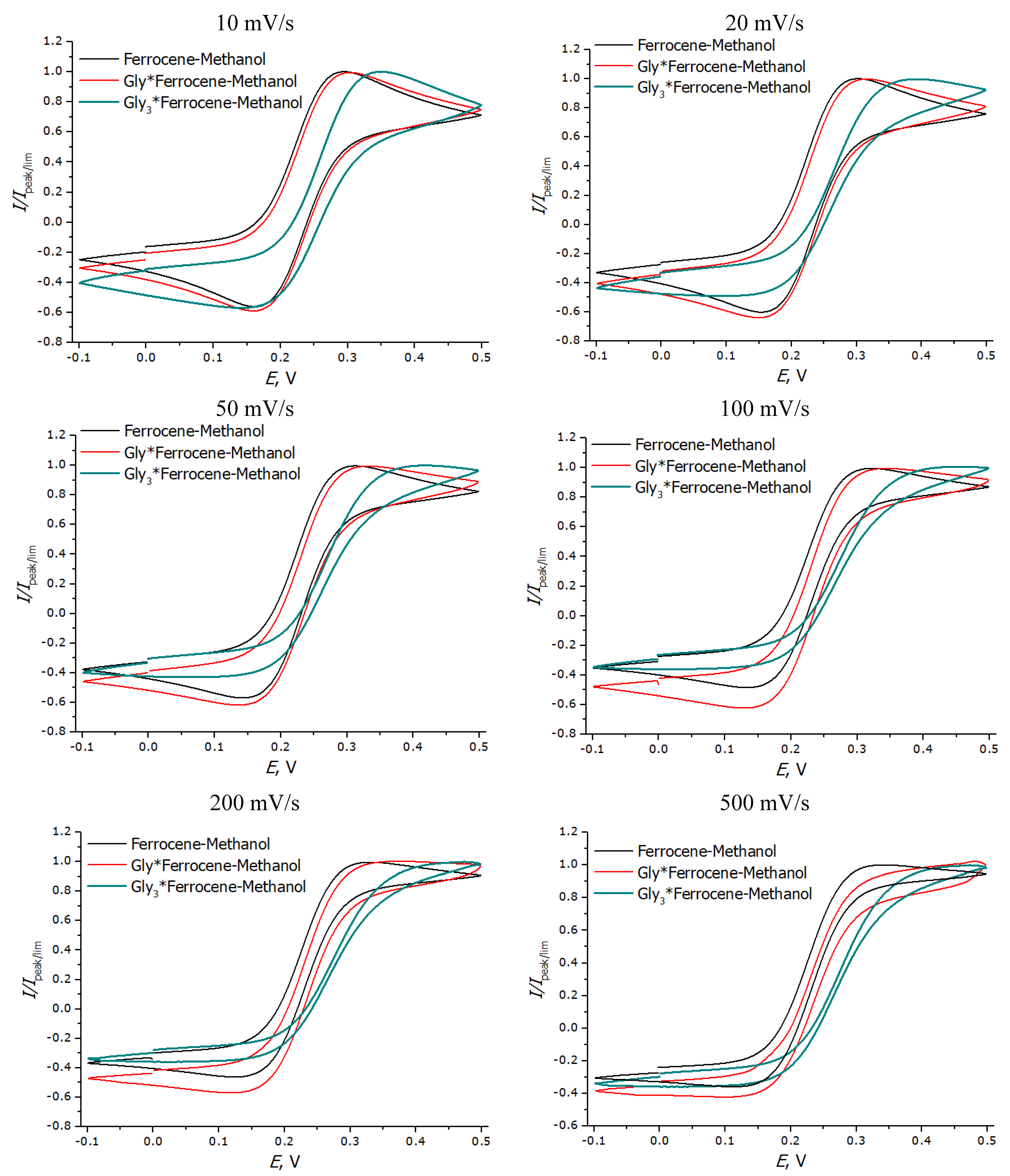
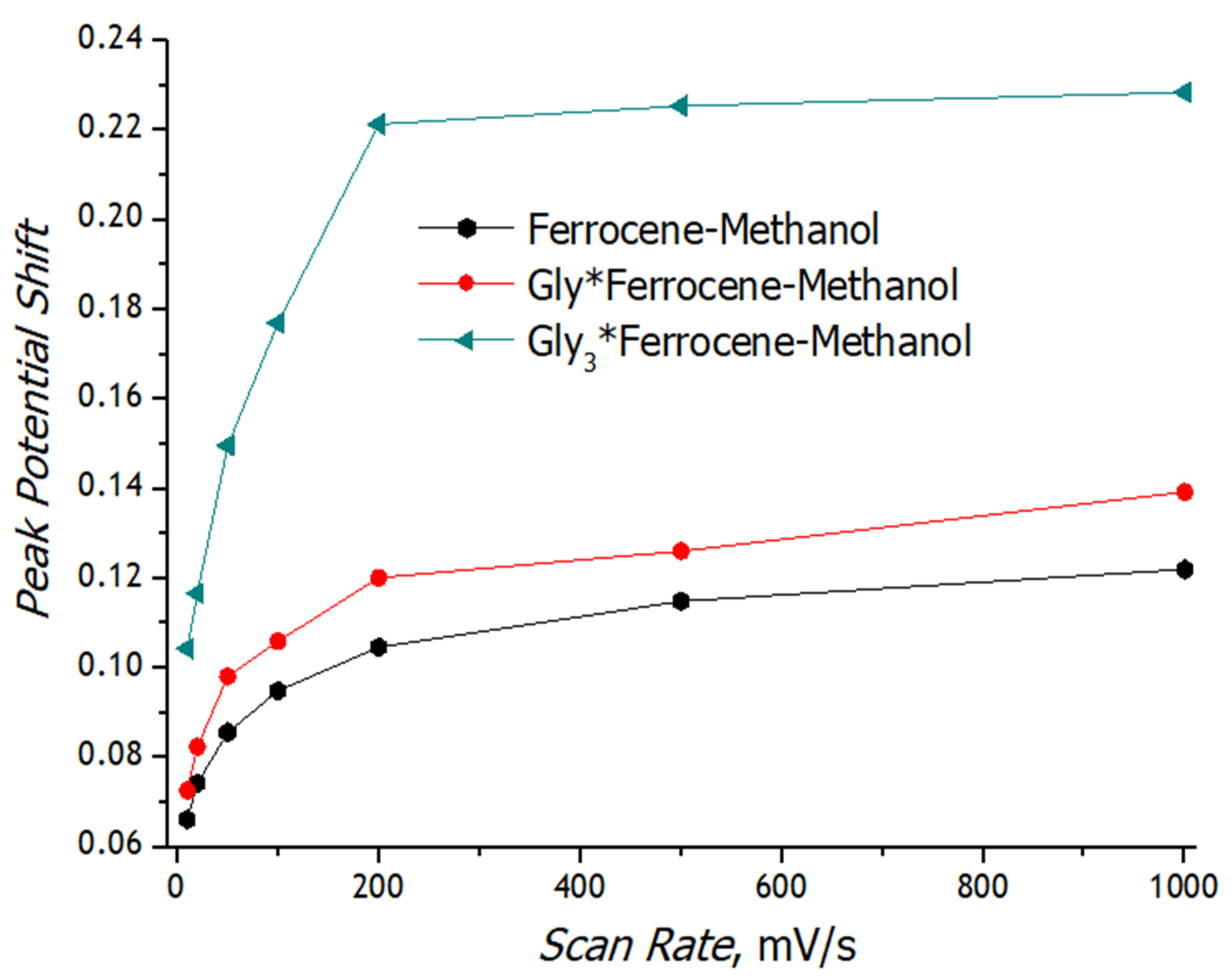
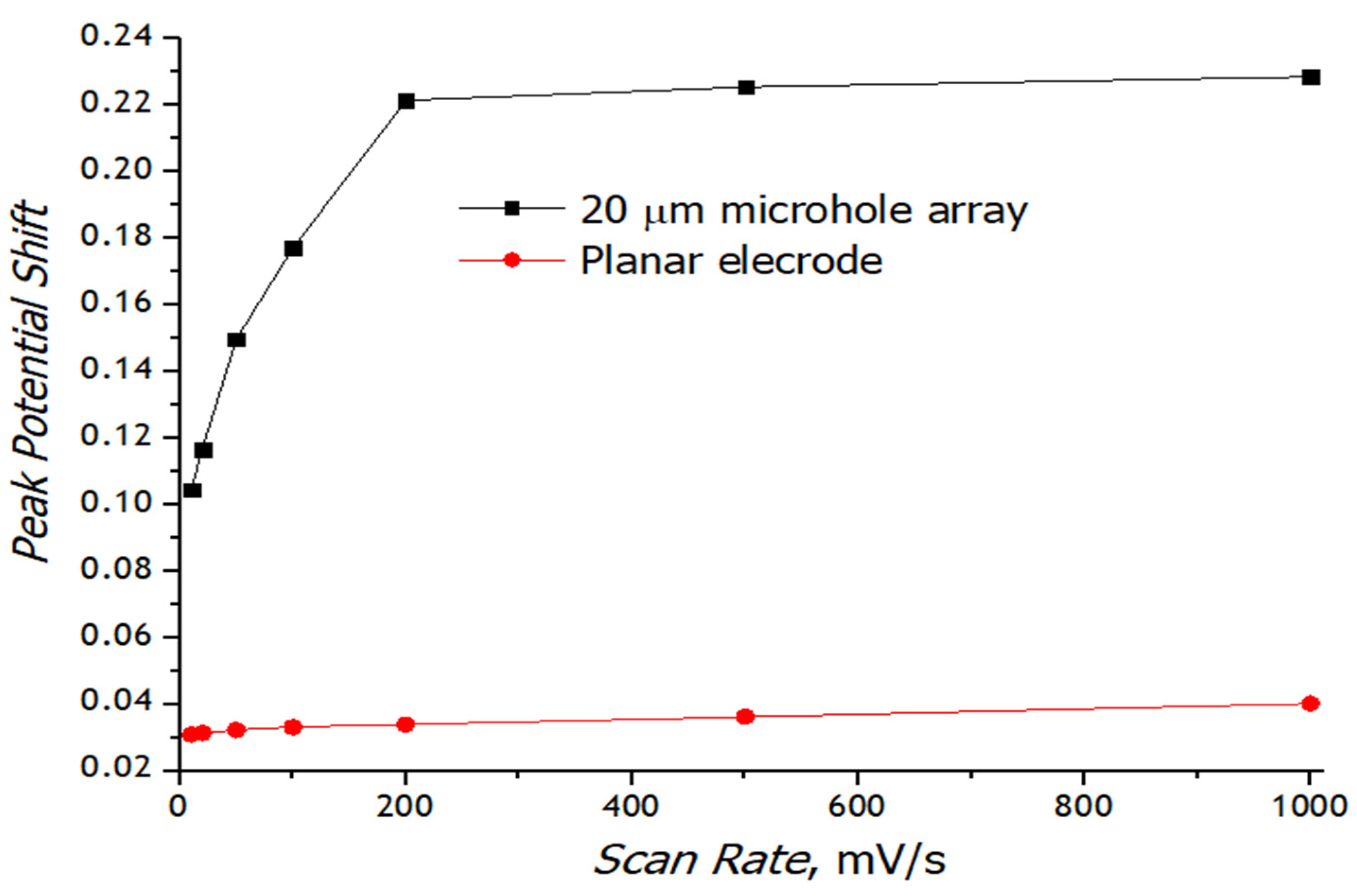
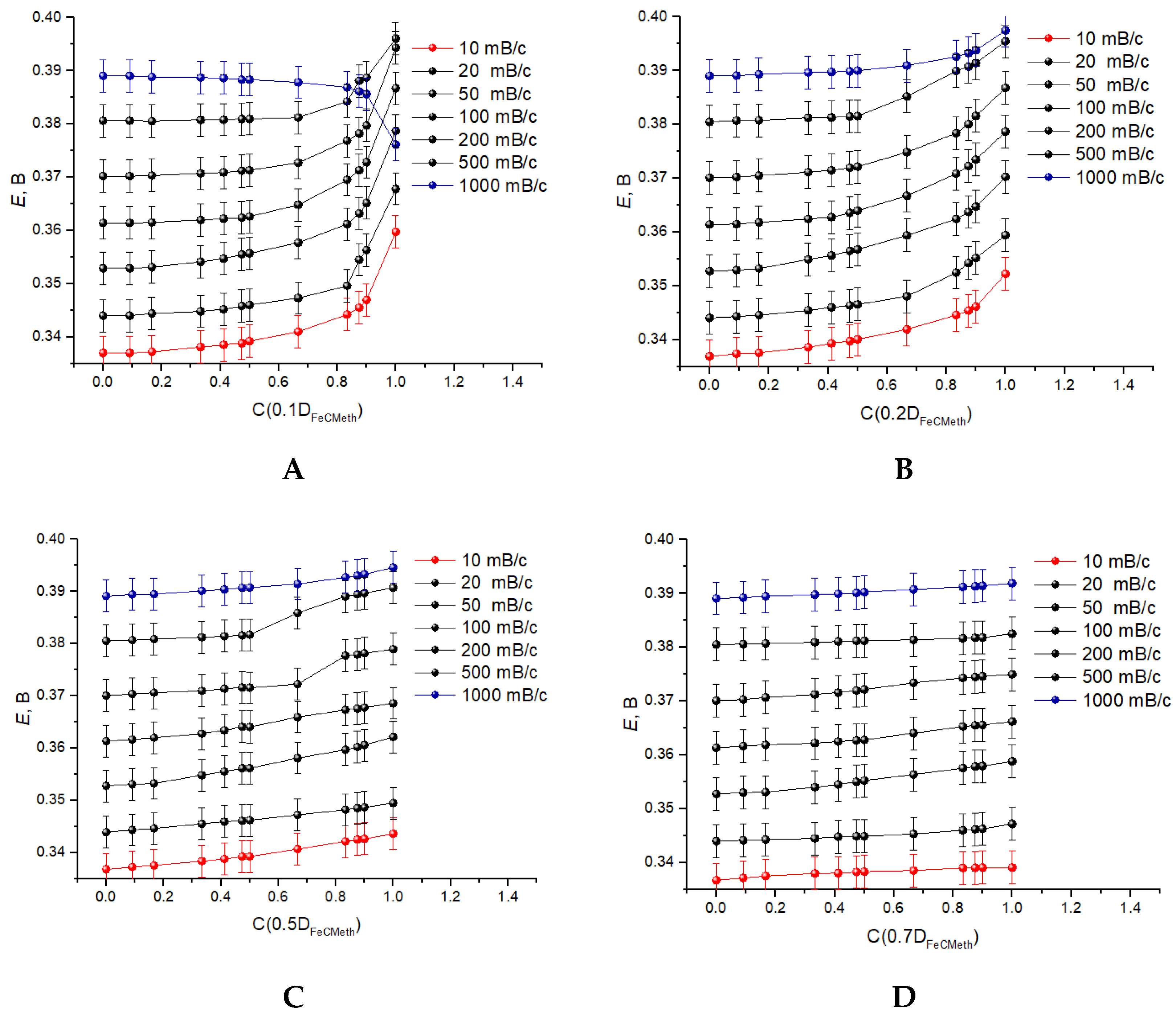
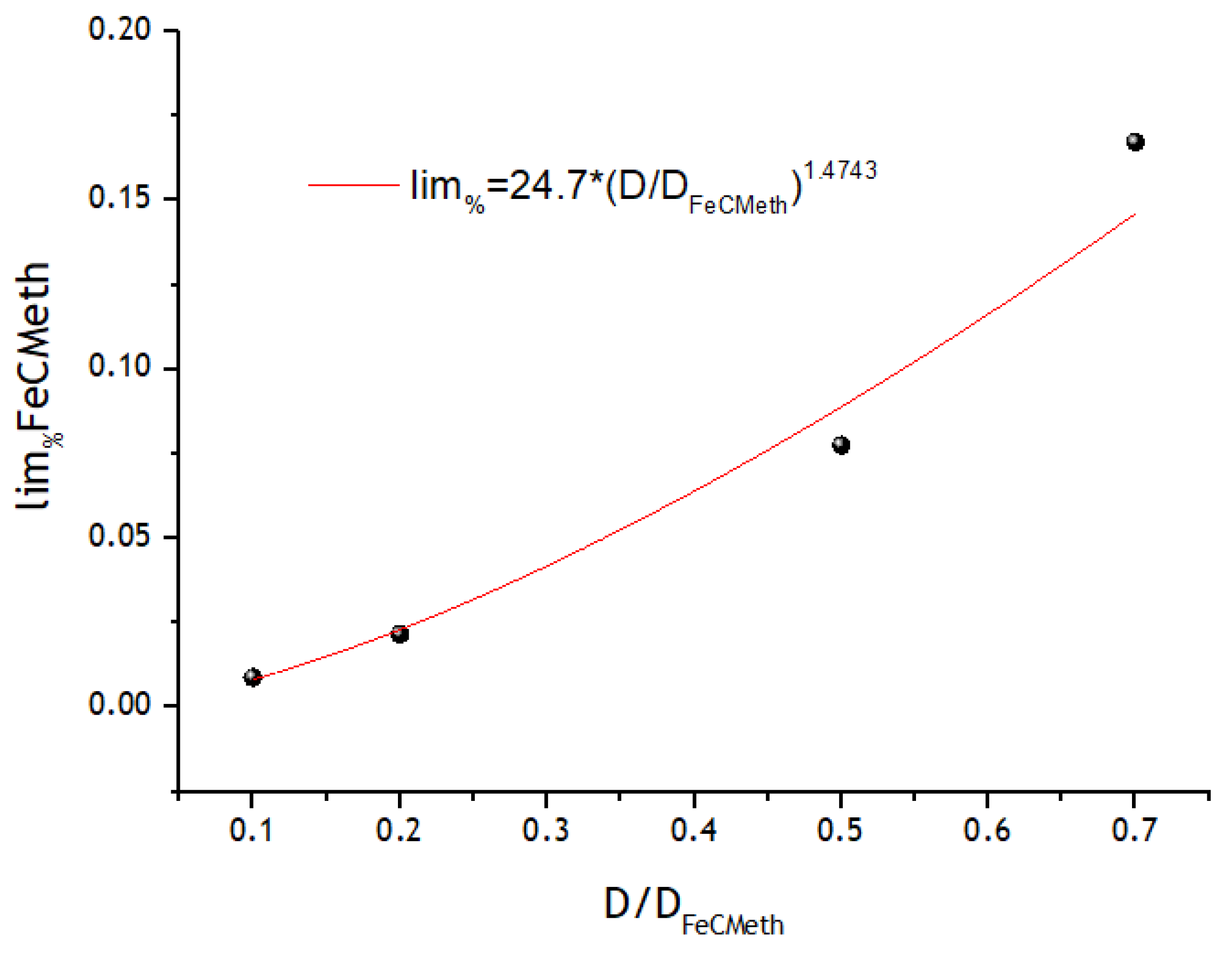
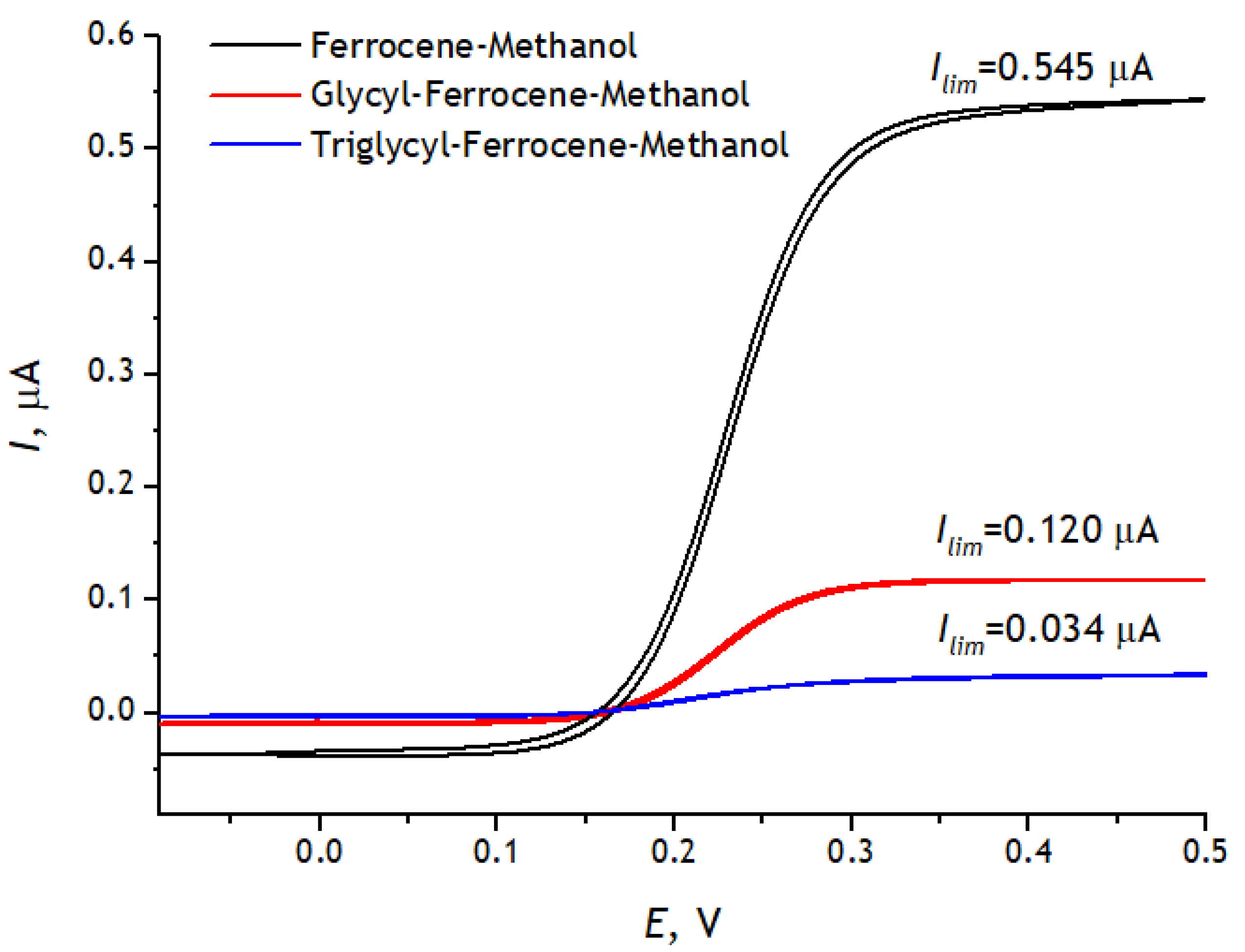
3.3. Registration of Voltammograms and Comparison of the Obtained Results with Theory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aikens, D.A. Electrochemical Methods, Fundamentals and Applications; ACS Publications: Washington, DC, USA, 1983. [Google Scholar]
- Zoski, C.G.; Wijesinghe, M. Electrochemistry at ultramicroelectrode arrays and nanoelectrode ensembles of macro- and ultramicroelectrode dimensions. Isr. J. Chem. 2010, 50, 347–359. [Google Scholar] [CrossRef]
- Zhiqiang, G.; Kok, S.S. Ultramicroelectrode ensembles based on self-assembled polymeric monolayers on gold electrodes. Electrochim. Acta 1997, 42, 315–321. [Google Scholar]
- Lei, S.; Ying, T.; Tong, S.; Wei, G.; Xueji, Z. Single-walled carbon nanotube ensembles modified gold ultramicroelectrodes prepared by self-assembly deposition method with 1-(1-pyrenyl)-1-methanethiol monolayer as an adhesion layer. Electrochem. Commun. 2012, 20, 163–166. [Google Scholar]
- Fernández, J.L.; Wijesinghe, M.; Zoski, C.G. Theory and experiments for voltammetric and SECM investigations and application to orr electrocatalysis at nanoelectrode ensembles of ultramicroelectrode dimensions. Anal. Chem. 2015, 87, 1066–1074. [Google Scholar] [CrossRef]
- Fernández, J.L.; Zoski, C.G. Voltammetric and scanning electrochemical microscopy investigations of the hydrogen evolution reaction in acid at nanostructured ensembles of ultramicroelectrode dimensions: Theory and experiment. J. Phys. Chem. C 2018, 122, 71–82. [Google Scholar] [CrossRef]
- Ortiz-Ledón, C.A.; Zoski, C.G. Fabrication of glass-insulated ultramicrometer to submicrometer carbon fiber electrodes to support a single nanoparticle and nanoparticle ensembles in electrocatalytic investigations. Anal. Chem. 2018, 90, 12616–12624. [Google Scholar] [CrossRef]
- Barham, A.S.; Akhtar, S.; Alkhatab, M.I.; Jaradat, S.Y.; Kennedy, B.M.; El Zein, B. Fabrication of microelectrode ensembles on thin-film single electrodes: The degradation of electropolymerized benzene-1,3-diol films in caustic solutions. Mater. Express 2018, 8, 305–315. [Google Scholar] [CrossRef]
- Wang, J.; Bian, C.; Tong, J.; Sun, J.; Li, Y.; Hong, W.; Xia, S. Modification of Graphene on Ultramicroelectrode Array and Its Application in Detection of Dissolved Oxygen. Sensors 2015, 15, 382–393. [Google Scholar] [CrossRef]
- Kiran, R.; Rousseau, L.; Lissorgues, G.; Scorsone, E.; Bongrain, A.; Yvert, B.; Picaud, S.; Mailley, P.; Bergonzo, P. Multichannel Boron Doped Nanocrystalline Diamond Ultramicroelectrode Arrays: Design, Fabrication and Characterization. Sensors 2012, 12, 7669–7681. [Google Scholar] [CrossRef]
- Miyata, M.; Kitazumi, Y.; Shirai, O.; Kataoka, K.; Kano, K. Diffusion-Limited Biosensing of Dissolved Oxygen by Direct Electron Transfer-Type Bioelectrocatalysis of Multi-Copper Oxidases Immobilized on Porous Gold Microelectrodes. J. Electroanal. Chem. 2020, 860, 113895. [Google Scholar] [CrossRef]
- Smirnov, W.; Yang, N.; Hoffmann, R.; Hees, J.; Obloh, H.; Müller-Sebert, W.; Nebel, C.E. Integrated all-diamond ultramicroelectrode arrays: Optimization of faradaic and capacitive currents. Anal. Chem. 2011, 83, 7438–7443. [Google Scholar] [CrossRef] [PubMed]
- Arbenin, A.Y.; Zemtsova, E.G.; Ermakov, S.S.; Gaskov, A.M.; Baburova, P.I.; Sokolova, D.N.; Smirnov, V.M. Three-component working electrode micron-sized Ag particles/TiO2 layer/Ti: Template electrochemical synthesis and potential use as electrochemical sensor for glutathione detection. Mater. Res. Express 2020, 7, 035401. [Google Scholar] [CrossRef]
- Gomez, H.; Riveros, G.; Ramirez, D. Chronoamperometric Cu (II) Analysis at Gold Ultramicroelectrodes in Concentrated Sulfuric Acid Solutions. Int. J. Electrochem. Sci 2017, 12, 985–993. [Google Scholar] [CrossRef]
- Davies, T.J.; Ward-Jones, S.; Banks, C.E.; del Campo, J.; Mas, R.; Muñoz, F.X.; Compton, R.G. The cyclic and linear sweep voltammetry of regular arrays of microdisc electrodes: Fitting of experimental data. J. Electroanal. Chem. 2005, 585, 51–62. [Google Scholar] [CrossRef]
- Heinze, J. Ultramicroelectrodes—A new dimension in electrochemistry? Angew. Chem. Int. Ed. Engl. 1991, 30, 170–171. [Google Scholar] [CrossRef]
- Guo, J.; Lindner, E. Cyclic voltammetry at shallow recessed microdisc electrode: Theoretical and experimental study. J. Electroanal. Chem. 2009, 629, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Kato, H.; Saito, T.; Matsuyama, S.; Kinugasa, S. Precise measurement of the self-diffusion coefficient for poly(ethylene glycol) in aqueous solution using uniform oligomers. J. Chem. Phys. 2005, 122, 244914. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Harfield, J.C.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical determination of glutathione: A review. Analyst 2012, 137, 2285. [Google Scholar] [CrossRef]
- Branham, M.L.; Singh, P.; Bisetty, K.; Sabela, M.; Govender, T. Preparation, spectrochemical, and computational analysis of L-carnosine (2-[(3-aminopropanoyl) amino]-3-(1H-imidazol-5-yl) propanoic acid) and its ruthenium (II) coordination complexes in aqueous solution. Molecules 2011, 16, 10269–10291. [Google Scholar] [CrossRef] [Green Version]
- Gooding, J.J.; Hibbert, D.B.; Yang, W. Electrochemical metal ion sensors. Exploiting amino acids and peptides as recognition elements. Sensors 2001, 1, 75–90. [Google Scholar]
- Galagedera, S.K.K.; Huynh, L.; Wachholz, F.; Jacobsen, M.; Haruehanroengra, P.; Sheng, J.; Flechsig, G.U. Voltammetric Detection of Thrombin by Labeling with Osmium Tetroxide Bipyridine and Binding with Aptamers on a Gold Electrode. Electroanalysis 2018, 30, 398–401. [Google Scholar] [CrossRef]
- Wang, S.S. p-Alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J. Am. Chem. Soc. 1973, 95, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 161–214. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbenin, A.Y.; Petrov, A.A.; Nazarov, D.V.; Serebryakov, E.; Kirichenko, S.O.; Vlasov, P.S.; Zemtsova, E.G.; Smirnov, V.M.; Danilova, E.E.; Ermakov, S.S.; et al. Prospects of Application of Ultramicroelectrode Ensembles for Voltammetric Determination of Compounds with Close Standard Electrode Potentials and Different Diffusion Coefficients. Chemosensors 2022, 10, 433. https://doi.org/10.3390/chemosensors10100433
Arbenin AY, Petrov AA, Nazarov DV, Serebryakov E, Kirichenko SO, Vlasov PS, Zemtsova EG, Smirnov VM, Danilova EE, Ermakov SS, et al. Prospects of Application of Ultramicroelectrode Ensembles for Voltammetric Determination of Compounds with Close Standard Electrode Potentials and Different Diffusion Coefficients. Chemosensors. 2022; 10(10):433. https://doi.org/10.3390/chemosensors10100433
Chicago/Turabian StyleArbenin, Andrey Yu., Alexey A. Petrov, Denis V. Nazarov, Evgeny Serebryakov, Sergey O. Kirichenko, Petr S. Vlasov, Elena G. Zemtsova, Vladimir M. Smirnov, Elena E. Danilova, Sergey S. Ermakov, and et al. 2022. "Prospects of Application of Ultramicroelectrode Ensembles for Voltammetric Determination of Compounds with Close Standard Electrode Potentials and Different Diffusion Coefficients" Chemosensors 10, no. 10: 433. https://doi.org/10.3390/chemosensors10100433






