Advanced Sensing of Antibiotics with Sr@Se Flower-Like Structure on Phosphorus-Doped g-C3N4 Composite: Application towards Detection of Chloramphenicol in Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
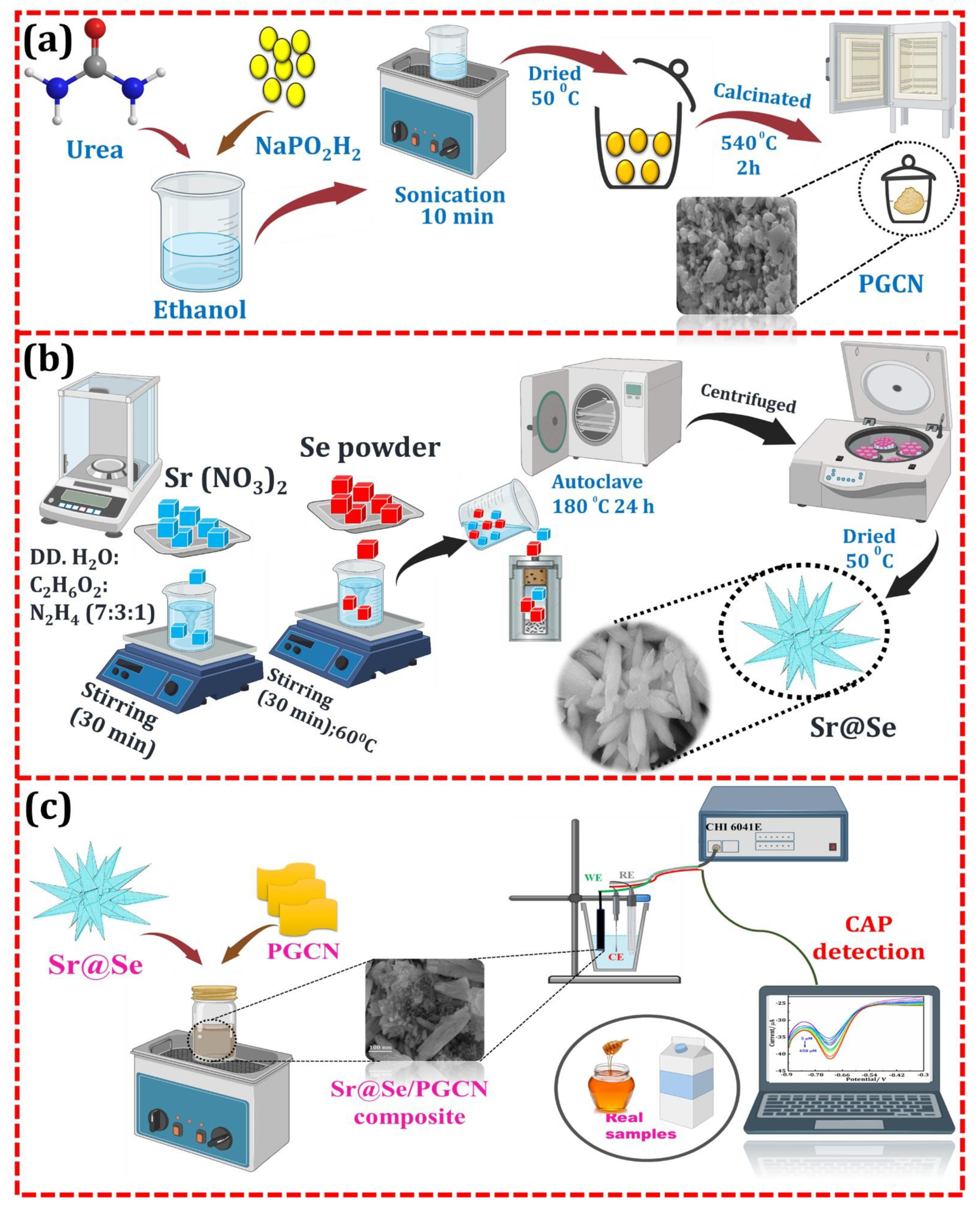
2.3. Synthesis of Sr@Se
2.4. Preparation of PGCN
2.5. Fabrication of the Sr@Se/PGCN/GCE
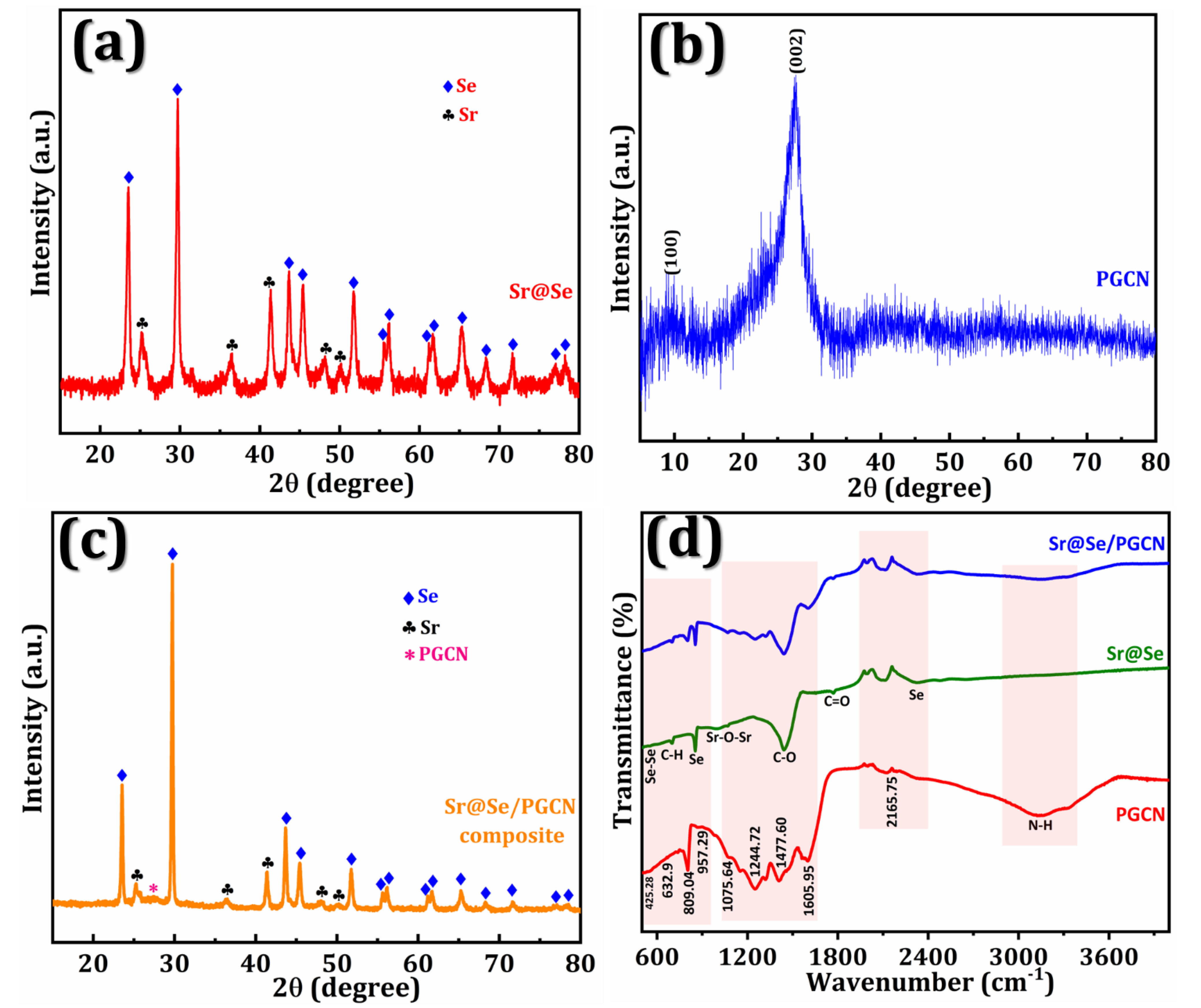
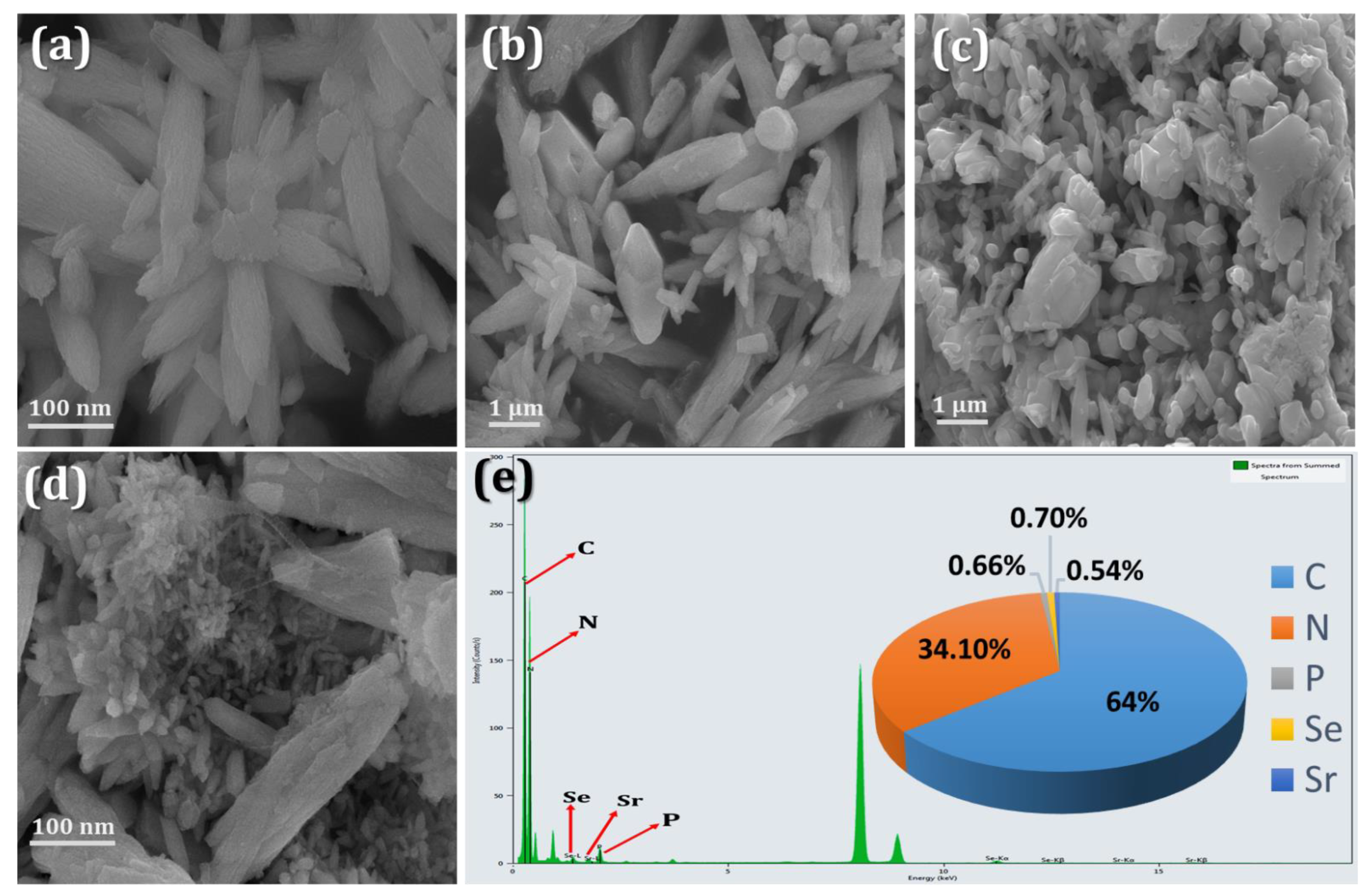
3. Results
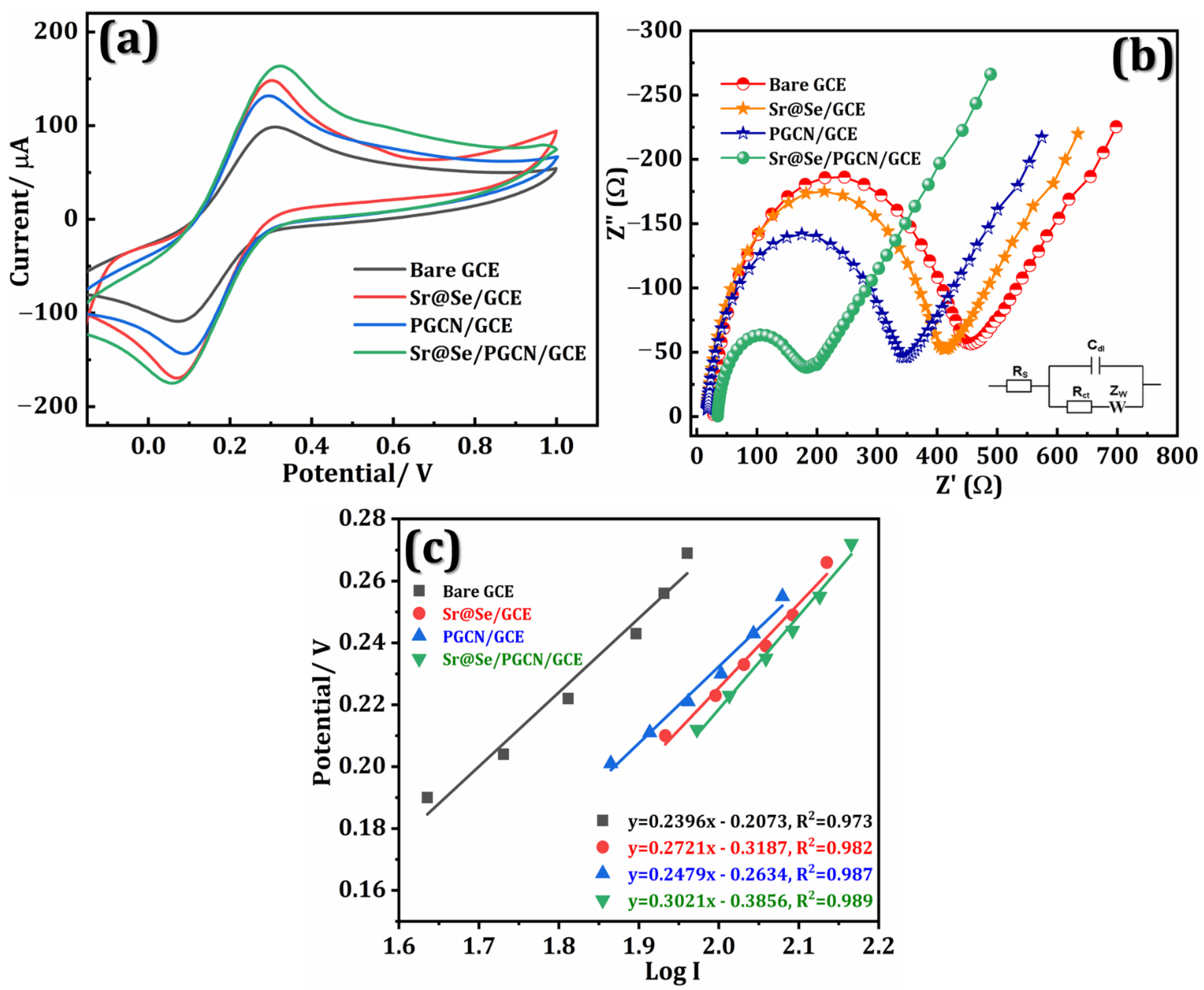
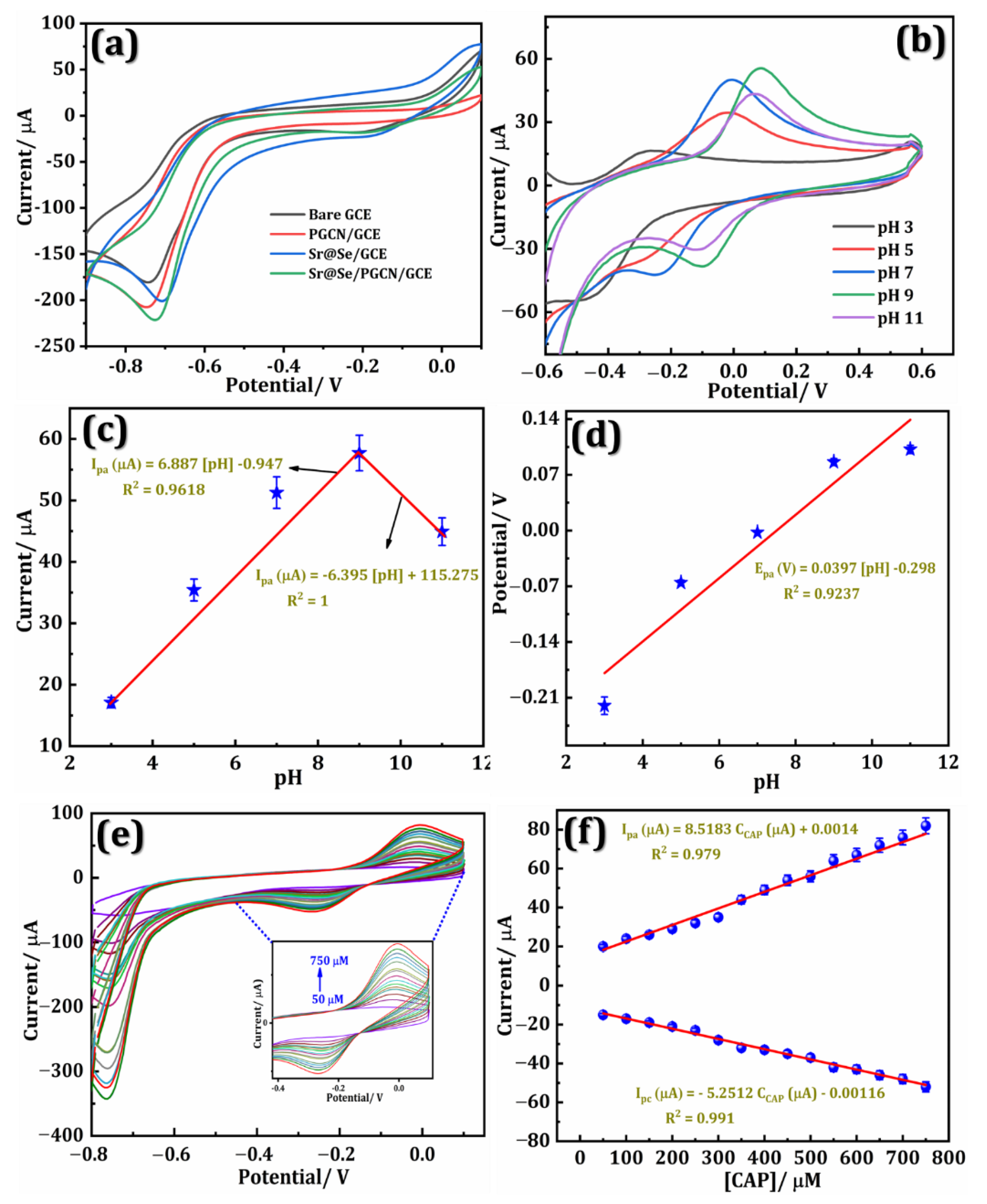
3.1. Electrochemical Performance of the Sr@Se/PGCN Nanocomposite
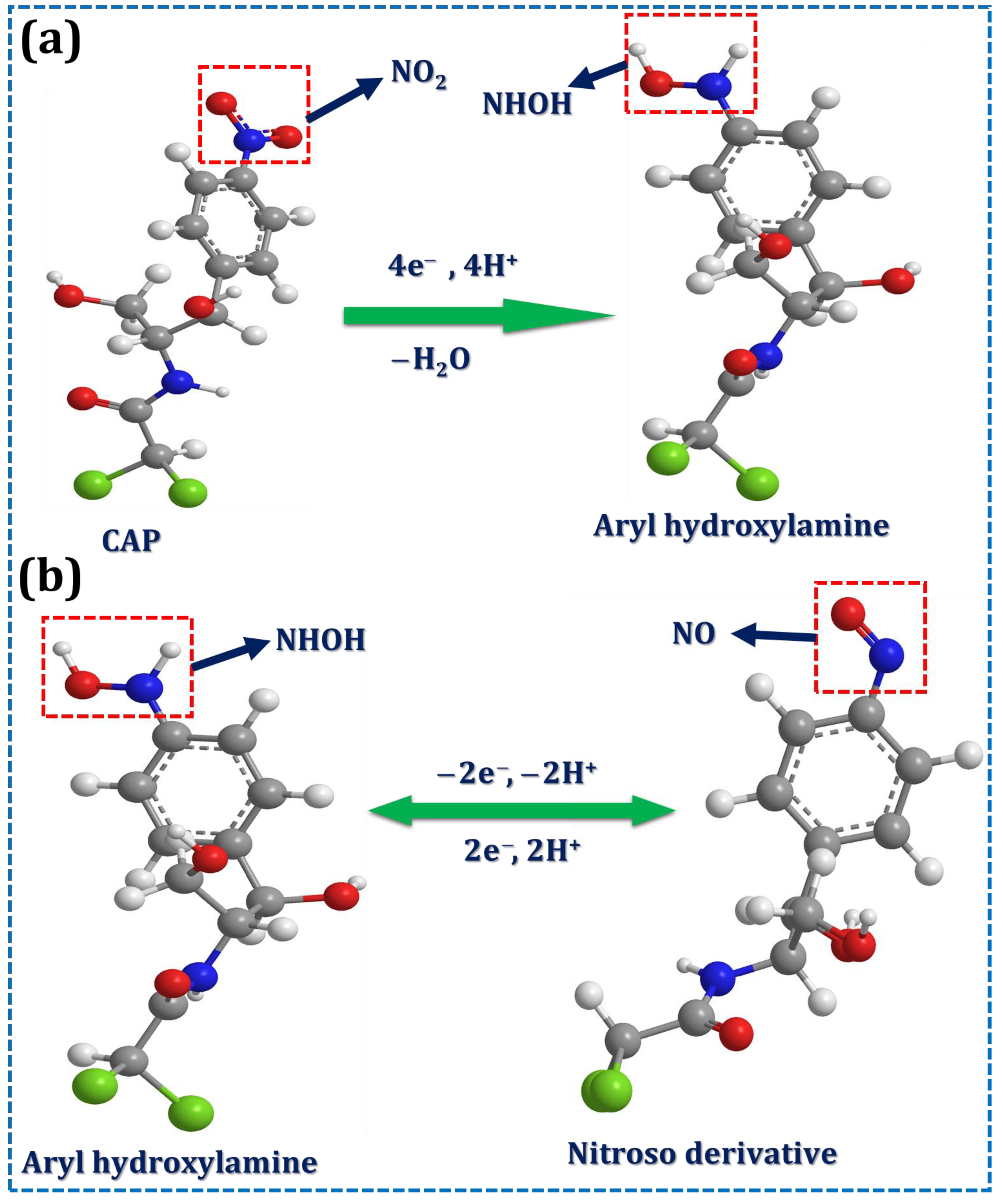
3.2. Electrochemical Performance of the Sr@Se/PGCN Composite with CAP
3.3. pH and CAP Concentrations
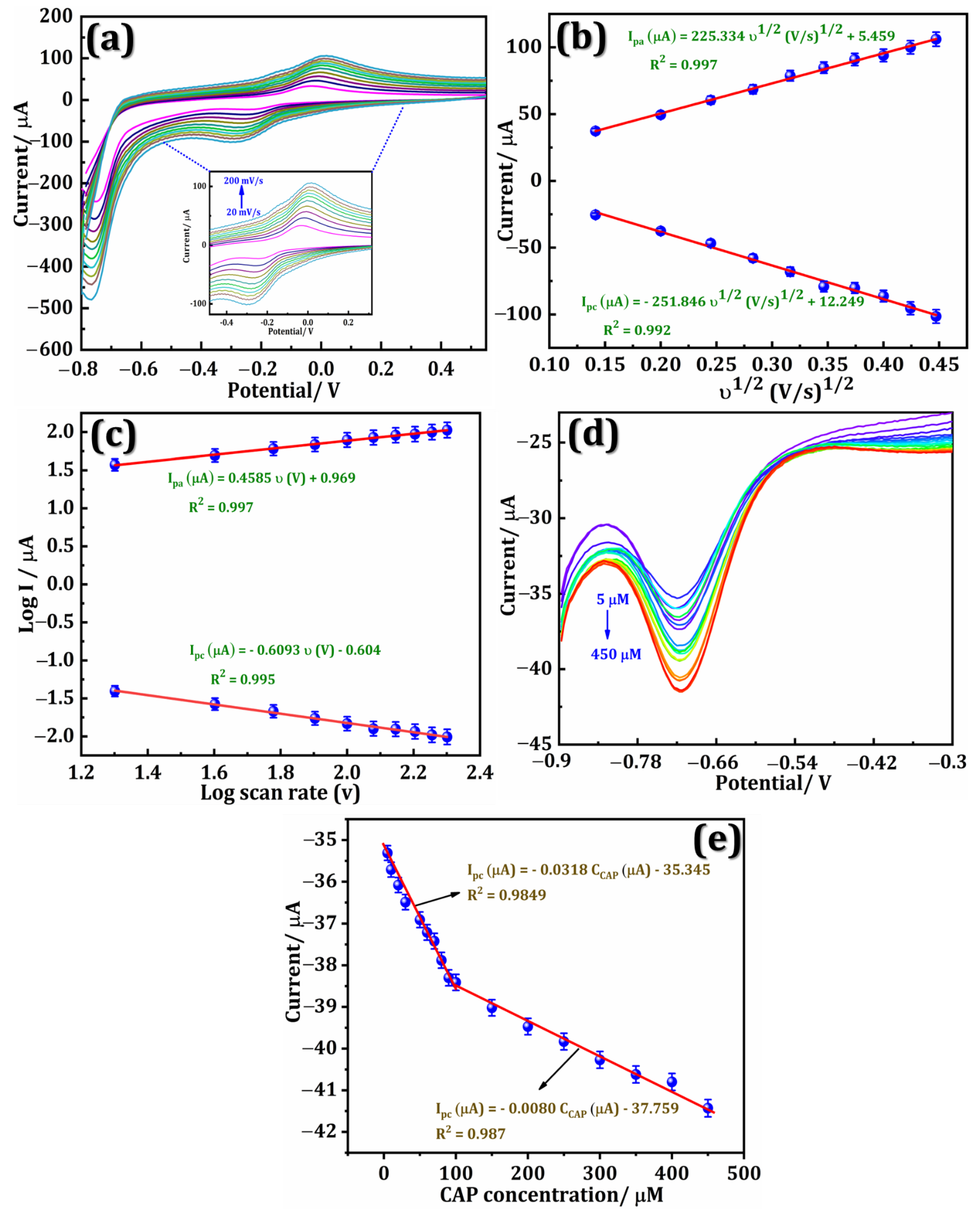
3.4. Scan Rate
3.5. Quantification of CAP
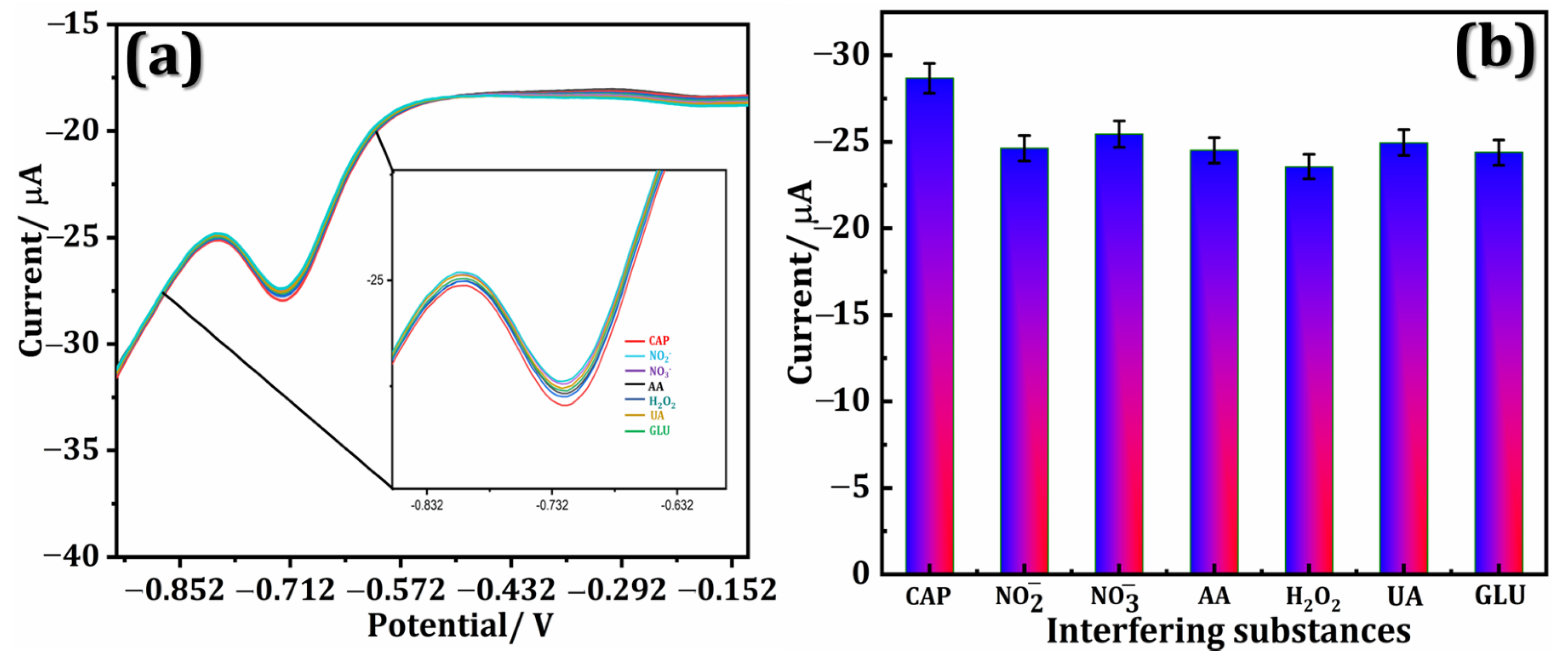
3.6. Interference Studies
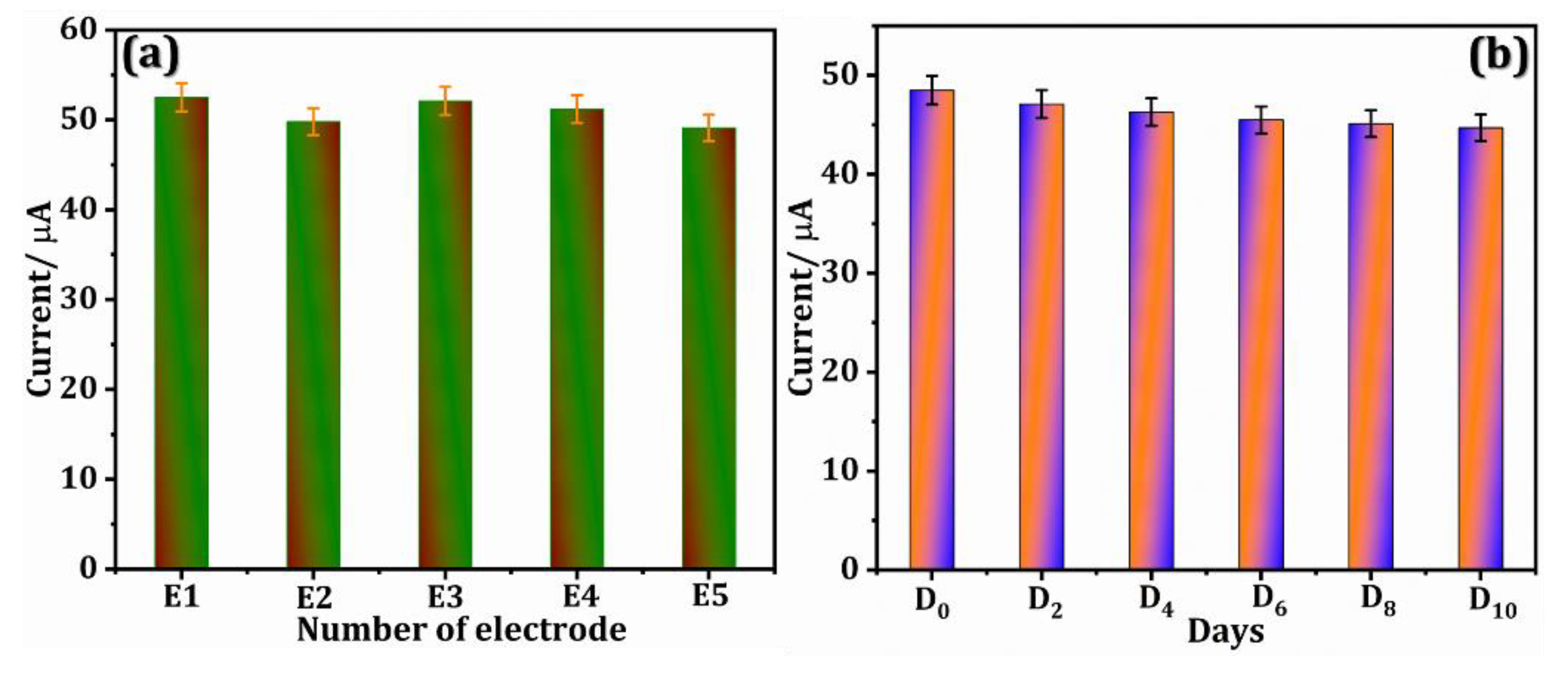
3.7. Reproducibility and Stability
3.8. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Zhao, F. Electrochemical Sensor for Chloramphenicol Based on Novel Multiwalled Carbon Nanotubes@molecularly Imprinted Polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.C.; Zhang, J.W. A Highly Selective Electrochemical Sensor for Chloramphenicol Based on Three-Dimensional Reduced Graphene Oxide Architectures. Talanta 2016, 161, 567–573. [Google Scholar] [CrossRef]
- Wang, K.P.; Zhang, Y.C.; Zhang, X.; Shen, L. Green Preparation of Chlorine-Doped Graphene and Its Application in Electrochemical Sensor for Chloramphenicol Detection. SN Appl. Sci. 2019, 1, 157. [Google Scholar] [CrossRef]
- Rajaji, U.; Manavalan, S.; Chen, S.M.; Govindasamy, M.; Chen, T.W.; Maiyalagan, T. Microwave-Assisted Synthesis of Europium(III) Oxide Decorated Reduced Graphene Oxide Nanocomposite for Detection of Chloramphenicol in Food Samples. Compos. Part B Eng. 2019, 161, 29–36. [Google Scholar] [CrossRef]
- Sniegocki, T.; Posyniak, A.; Gbylik-Sikorska, M.; Zmudzki, J. Determination of Chloramphenicol in Milk Using a QuEChERS-Based on Liquid Chromatography Tandem Mass Spectrometry Method. Anal. Lett. 2014, 47, 568–578. [Google Scholar] [CrossRef]
- Kawano, S.I.; Hao, H.Y.; Hashi, Y.; Lin, J.M. Analysis of Chloramphenicol in Honey by On-Line Pretreatment Liquid Chromatography–Tandem Mass Spectrometry. Chinese Chem. Lett. 2015, 26, 36–38. [Google Scholar] [CrossRef]
- Ji, W.; Yao, W. Rapid Surface Enhanced Raman Scattering Detection Method for Chloramphenicol Residues. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Mamani, M.C.V.; Amaya-Farfan, J.; Reyes, F.G.R.; da Silva, J.A.F.; Rath, S. Use of Experimental Design and Effective Mobility Calculations to Develop a Method for the Determination of Antimicrobials by Capillary Electrophoresis. Talanta 2008, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Malekinejad, H.; Razavi-Rouhani, S.M.; Pajouhi, M.R.; Mahmoudi, R.; Haghnazari, A. Chloramphenicol Residues in Chicken Liver, Kidney and Muscle: A Comparison among the Antibacterial Residues Monitoring Methods of Four Plate Test, ELISA and HPLC. Food Chem. Toxicol. 2010, 48, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Sagi, S.; Wang, Y.H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Corrigendum to “Characterization and Screening of Pyrrolizidine Alkaloids and N-Oxides from Botanicals and Dietary Supplements Using UHPLC-High Resolution Mass Spectrometry” [Food Chem. 178 (2015) 136–148]. Food Chem. 2018, 248, 361–363. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Zhai, H.; Shen, Z.; Han, J.; Yu, Y.; Fang, H.; Li, F.; Sun, X.; Guo, Y. Molecularly Imprinted Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes for Specific Recognition and Determination of Chloramphenicol in Milk. Microchem. J. 2022, 182, 107887. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular Biosynthesis and Transformation of Selenium Nanoparticles and Application in H2O2 Biosensor. Colloids Surfaces B Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant Properties of Aqueous Selenium Nanoparticles (ASeNPs) and Its Catalysts Activity for 1, 1-Diphenyl-2-Picrylhydrazyl (DPPH) Reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ji, Y.; Ma, X.; Xu, J.; Que, D. Selenium Nanotubes Synthesized by a Novel Solution Phase Approach. J. Phys. Chem. B 2004, 108, 1179–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, S.; Yuan, R.; Liu, H. Defective Se-Doped In2S3 Nanomaterial-Based Photoelectrochemical Biosensor for the Ultrasensitive Detection of Chloramphenicol. Sens. Actuators B Chem. 2022, 373, 132705. [Google Scholar] [CrossRef]
- Feng, H.; Li, J.; Liu, Y.; Xu, Z.; Cui, Y.; Liu, M.; Liu, X.; He, L.; Jiang, J.; Qian, D. Cubic MnSe2 Nanoparticles Dispersed on Multi-Walled Carbon Nanotubes: A Robust Electrochemical Sensing Platform for Chloramphenicol. J. Electroanal. Chem. 2022, 922, 116755. [Google Scholar] [CrossRef]
- Prasad, K.S.; Vaghasiya, J.V.; Soni, S.S.; Patel, J.; Patel, R.; Kumari, M.; Jasmani, F.; Selvaraj, K. Microbial Selenium Nanoparticles (SeNPs) and Their Application as a Sensitive Hydrogen Peroxide Biosensor. Appl. Biochem. Biotechnol. 2015, 177, 1386–1393. [Google Scholar] [CrossRef]
- Praharaj, R.; Mishra, S.; Rautray, T.R. The Structural and Bioactive Behaviour of Strontium-Doped Titanium Dioxide Nanorods. J. Korean Ceram. Soc. 2020, 57, 271–280. [Google Scholar] [CrossRef]
- Taha, K.K.; Mustafa, M.M.; Ahmed, H.A.M.; Talab, S. Selenium Zinc Oxide (Se/ZnO) Nanoparticles: Synthesis, Characterization, and Photocatalytic Activity. Z. Naturforsch. A 2019, 74, 1043–1056. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Ganguly, A.; Santhan, A.; Sharma, T.S.K. Construction of Three-Dimensional/One-Dimensional Heterostructure of Flower-like Sr Nanoflowers on Se Microrods Decorated on Reduced Graphene Oxide: An Efficient Electrocatalyst for Oxidation of Promethazine Hydrochloride. Mater. Today Chem. 2022, 23, 100654. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Ashiq, M.N.; Razaq, A.; Saleem, M.; Parveen, B.; Hassan, M.U. Excellent Electrochemical Performance of Graphene Oxide Based Strontium Sulfide Nanorods for Supercapacitor Applications. Electrochim. Acta 2018, 273, 136–144. [Google Scholar] [CrossRef]
- Fethi, A. Novel Materials for Electrochemical Sensing Platforms. Sens. Int. 2020, 1, 100035. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic Carbon Nitride (g–C3N4)–Based Metal-Free Photocatalysts for Water Splitting: A Review. Carbon N. Y. 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Lou, W.; Ali, A.; Shen, P.K. Recent Development of Au Arched Pt Nanomaterials as Promising Electrocatalysts for Methanol Oxidation Reaction. Nano Res. 2022, 15, 18–37. [Google Scholar] [CrossRef]
- Chan, M.-H.; Liu, R.-S.; Hsiao, M. Graphitic Carbon Nitride-Based Nanocomposites and Their Biological Applications: A Review. Nanoscale 2019, 11, 14993–15003. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Jia, F.; Xie, J.; Li, W. Three-Dimensional Phosphorus-Doped Graphitic-C3N4 Self-Assembly with NH2-Functionalized Carbon Composite Materials for Enhanced Oxygen Reduction Reaction. Langmuir 2016, 32, 12569–12578. [Google Scholar] [CrossRef]
- Bian, J.; Huang, C.; Zhang, R.-Q. Graphitic Carbon Nitride Film: An Emerging Star for Catalytic and Optoelectronic Applications. ChemSusChem 2016, 9, 2723–2735. [Google Scholar] [CrossRef]
- Ran, J.; Ma, T.Y.; Gao, G.; Du, X.-W.; Qiao, S.Z. Porous P-Doped Graphitic Carbon Nitride Nanosheets for Synergistically Enhanced Visible-Light Photocatalytic H 2 Production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; He, B.B.; Wang, H.W.; Wang, R.; Gong, Y.S. In-Situ Construction of Coral-like Porous P-Doped g-C3N4 Tubes with Hybrid 1D/2D Architecture and High Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 241, 159–166. [Google Scholar] [CrossRef]
- Chai, B.; Yan, J.; Wang, C.; Ren, Z.; Zhu, Y. Enhanced Visible Light Photocatalytic Degradation of Rhodamine B over Phosphorus Doped Graphitic Carbon Nitride. Appl. Surf. Sci. 2017, 391, 376–383. [Google Scholar] [CrossRef]
- Veerakumar, P.; Rajkumar, C.; Chen, S.-M.; Thirumalraj, B.; Lin, K.-C. Ultrathin 2D Graphitic Carbon Nitride Nanosheets Decorated with Silver Nanoparticles for Electrochemical Sensing of Quercetin. J. Electroanal. Chem. 2018, 826, 207–216. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. Fabrication of G-C3N4/NiO Heterostructured Nanocomposite Modified Glassy Carbon Electrode for Quercetin Biosensor. Ultrason. Sonochem. 2018, 41, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Puziy, A.; Poddubnaya, O.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-Containing Carbons: Preparation, Properties and Utilization. Carbon N. Y. 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Wang, J.; Liao, T.; Wei, Z.; Sun, J.; Guo, J.; Sun, Z. Heteroatom-Doping of Non-Noble Metal-Based Catalysts for Electrocatalytic Hydrogen Evolution: An Electronic Structure Tuning Strategy. Small Methods 2021, 5, 2000988. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon Quantum Dots Supported AgI /ZnO/Phosphorus Doped Graphitic Carbon Nitride as Z-Scheme Photocatalyst for Efficient Photodegradation of 2, 4-Dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Márquez-Herrera, A.; Ovando-Medina, V.M.; Castillo-Reyes, B.E.; Zapata-Torres, M.; Meléndez-Lira, M.; González-Castañeda, J. Facile Synthesis of SrCO3-Sr(OH)2/PPy Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Materials 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Nawaz, Q.; Rehman, M.A.U.; Cresswell, M.; Jackson, P.; Hurle, K.; Detsch, R.; Goldmann, W.H.; Shah, A.T.; Boccaccini, A.R. Synthesis, Characterization, Antibacterial Properties, and In Vitro Studies of Selenium and Strontium Co-Substituted Hydroxyapatite. Int. J. Mol. Sci. 2021, 22, 4246. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Ghosh, D.; Kaley, N.M.; Pati, S.K. Photocatalytic Activity of G-C3N4 Quantum Dots in Visible Light: Effect of Physicochemical Modifications. J. Phys. Chem. C 2017, 121, 1982–1989. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, L.; Hu, C. Chemical-Bond Conjugated BiO(OH)xI1−x-AgI Heterojunction with High Visible Light Activity and Stability in Degradation of Pollutants. Appl. Catal. B Environ. 2017, 218, 443–451. [Google Scholar] [CrossRef]
- Jiang, D.; Cao, L.; Liu, W.; Su, G.; Qu, H.; Sun, Y.; Dong, B. Synthesis and Luminescence Properties of Core/Shell ZnS:Mn/ZnO Nanoparticles. Nanoscale Res. Lett. 2009, 4, 78–83. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Alizadeh, M.; Karaman, O.; Vasseghian, Y.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo Dyes Using an Impressive Electrochemical Sensor Based on Carbon Paste Electrode Modified with ZIF-8/g-C3N4/Co and Ionic Liquid in Mouthwash and Toothpaste as Real Samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar] [PubMed]
- Sanjay, B.P.; Sandeep, S.; Santhosh, A.S.; Karthik, C.S.; Varun, D.N.; Kumara Swamy, N.; Mallu, P.; Nithin, K.S.; Rajabathar, J.R.; Muthusamy, K. Unprecedented 2D GNR-CoB Nanocomposite for Detection and Degradation of Malachite Green—A Computational Prediction of Degradation Pathway and Toxicity. Chemosphere 2022, 287, 132153. [Google Scholar] [CrossRef] [PubMed]
- Krzyczmonik, P.; Socha, E.; Skrzypek, S.; Soliwoda, K.; Celichowski, G.; Grobelny, J. Honeycomb-Structured Porous Poly(3,4-Ethylenedioxythiophene) Composite Layers on a Gold Electrode. Thin Solid Films 2014, 565, 54–61. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, J.; Liu, G.; Jia, D.; Ma, Z.; Zhang, L.; Peng, Z.; Zhu, X. High-Performance, Flexible, Binder-Free Silicon–Carbon Anode for Lithium Storage Applications. Electrochem. Commun. 2022, 137, 107257. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous Determination of Salbutamol and Propranolol in Biological Fluid Samples Using an Electrochemical Sensor Based on Functionalized-Graphene, Ionic Liquid and Silver Nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A Graphene Oxide-Based Electrochemical Sensor for Sensitive Determination of 4-Nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Mani, V.; Lou, B.-S.; Devasenathipathy, R.; Hou, Y.-S.; Elangovan, A. Green Synthesized Gold Nanoparticles Decorated Graphene Oxide for Sensitive Determination of Chloramphenicol in Milk, Powdered Milk, Honey and Eye Drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef]
- Karthik, R.; Vinoth Kumar, J.; Chen, S.M.; Karuppiah, C.; Cheng, Y.H.; Muthuraj, V. A Study of Electrocatalytic and Photocatalytic Activity of Cerium Molybdate Nanocubes Decorated Graphene Oxide for the Sensing and Degradation of Antibiotic Drug Chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 6547–6559. [Google Scholar] [CrossRef]
- Bhuvaneswari, C.; Ganesh Babu, S. Nanoarchitecture and Surface Engineering Strategy for the Construction of 3D Hierarchical CuS-RGO/g-C3N4 Nanostructure: An Ultrasensitive and Highly Selective Electrochemical Sensor for the Detection of Furazolidone Drug. J. Electroanal. Chem. 2022, 907, 116080. [Google Scholar] [CrossRef]
- Zaveri, N.; Sun, R.; Zufelt, N.; Zhou, A.; Chen, Y.Q. Evaluation of Microbially Influenced Corrosion with Electrochemical Noise Analysis and Signal Processing. Electrochim. Acta 2007, 52, 5795–5807. [Google Scholar] [CrossRef]
- Yang, B.; Shao, M.; Xu, Y.; Du, Y.; Yang, H.; Bin, D.; Liu, B.; Lu, H. Core–Shell ZIF-8@ZIF-67-Derived Cobalt Nanoparticles In Situ Grown on N-doped Carbon Nanotube Polyhedra for Ultrasensitive Electrochemical Detection of Chloramphenicol. ChemElectroChem 2022, 9, e202200438. [Google Scholar] [CrossRef]
- Chang, C.; Wang, Q.; Xue, Q.; Liu, F.; Hou, L.; Pu, S. Highly Efficient Detection of Chloramphenicol in Water Using Ag and TiO2 Nanoparticles Modified Laser-Induced Graphene Electrode. Microchem. J. 2022, 173, 107037. [Google Scholar] [CrossRef]
- Gopi, P.K.; Srinithi, S.; Chen, S.-M.; Hunsur Ravikumar, C. Simple Construction of GdBiVO 4 Assembled on Reduced Graphene Oxide for Selective and Sensitive Electrochemical Detection of Chloramphenicol in Food Samples. New J. Chem. 2022, 46, 1577–1587. [Google Scholar] [CrossRef]
- Umesh, N.; Sathiyan, A.; Wang, S.F.; Elanthamilan, E.; Merlin, J.P.; Jesila, J.A. A Simple Chemical Approach for Synthesis of Sr2Co2O5 Nanoparticles and Its Application in the Detection of Chloramphenicol and in Energy Storage Systems. J. Electroanal. Chem. 2021, 880, 114911. [Google Scholar] [CrossRef]
| Electrocatalyst | Detection Technique | pH Values | Linear Range (µM) | LOD (nM) | References |
|---|---|---|---|---|---|
| Fe3O4/Au/GCE | DPV | 5 | 1.0–12 | 144 | [51] |
| Co@NCNP/GCE | DPV | 7 | 5–268.83 | 500 | [52] |
| Ag/CMC@TiO2/LIG | DPV | 7 | 0.01–100 | 7 | [53] |
| GdBiVO4@rGO/SPCE | DPV | 7 | 0.05–2336.55 | 22.9 | [54] |
| Sr2Co2O5/GCE | DPV | 7 | 0.01–931.1 | 2.3 | [55] |
| Sr@Se/PGCN/GCE | DPV | 9 | 5–450 | 2.7 | Present work |
| Samples | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
 Milk | 50 | 49.97 | 99.40 | 2.8 |
| 100 | 99.9 | 99.9 | 2.3 | |
 Honey | 50 | 49.5 | 90.0 | 3.6 |
| 100 | 99.95 | 99.77 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasanna, S.B.; Kumar, G.K.S.; Shadakshari, S.; Shivamurthy, S.A.; Shanthakumar, K.C.; Nagaraja, B.M.; Chung, R.-J. Advanced Sensing of Antibiotics with Sr@Se Flower-Like Structure on Phosphorus-Doped g-C3N4 Composite: Application towards Detection of Chloramphenicol in Food Samples. Chemosensors 2022, 10, 425. https://doi.org/10.3390/chemosensors10100425
Prasanna SB, Kumar GKS, Shadakshari S, Shivamurthy SA, Shanthakumar KC, Nagaraja BM, Chung R-J. Advanced Sensing of Antibiotics with Sr@Se Flower-Like Structure on Phosphorus-Doped g-C3N4 Composite: Application towards Detection of Chloramphenicol in Food Samples. Chemosensors. 2022; 10(10):425. https://doi.org/10.3390/chemosensors10100425
Chicago/Turabian StylePrasanna, Sanjay Ballur, Gagan Kumar Sakaleshpur Kumar, Sandeep Shadakshari, Santhosh Arehalli Shivamurthy, Karthik Chimatahalli Shanthakumar, Bhari Mallanna Nagaraja, and Ren-Jei Chung. 2022. "Advanced Sensing of Antibiotics with Sr@Se Flower-Like Structure on Phosphorus-Doped g-C3N4 Composite: Application towards Detection of Chloramphenicol in Food Samples" Chemosensors 10, no. 10: 425. https://doi.org/10.3390/chemosensors10100425
APA StylePrasanna, S. B., Kumar, G. K. S., Shadakshari, S., Shivamurthy, S. A., Shanthakumar, K. C., Nagaraja, B. M., & Chung, R.-J. (2022). Advanced Sensing of Antibiotics with Sr@Se Flower-Like Structure on Phosphorus-Doped g-C3N4 Composite: Application towards Detection of Chloramphenicol in Food Samples. Chemosensors, 10(10), 425. https://doi.org/10.3390/chemosensors10100425






