Recent Advances in Metal-Organic Frameworks for Biomacromolecule Sensing
Abstract
:1. Introduction
2. Design and Fabrication of MOFs for Biomacromolecule Sensing
2.1. MOFs-Based Nano-Probes
2.1.1. MOF-Based Nanoprobes for Optical Sensing
2.1.2. MOF-Based Nanoprobes for Electrochemical Sensing
2.1.3. MOF-Based Nanoprobes for Photoelectrochemical (PEC) Sensing
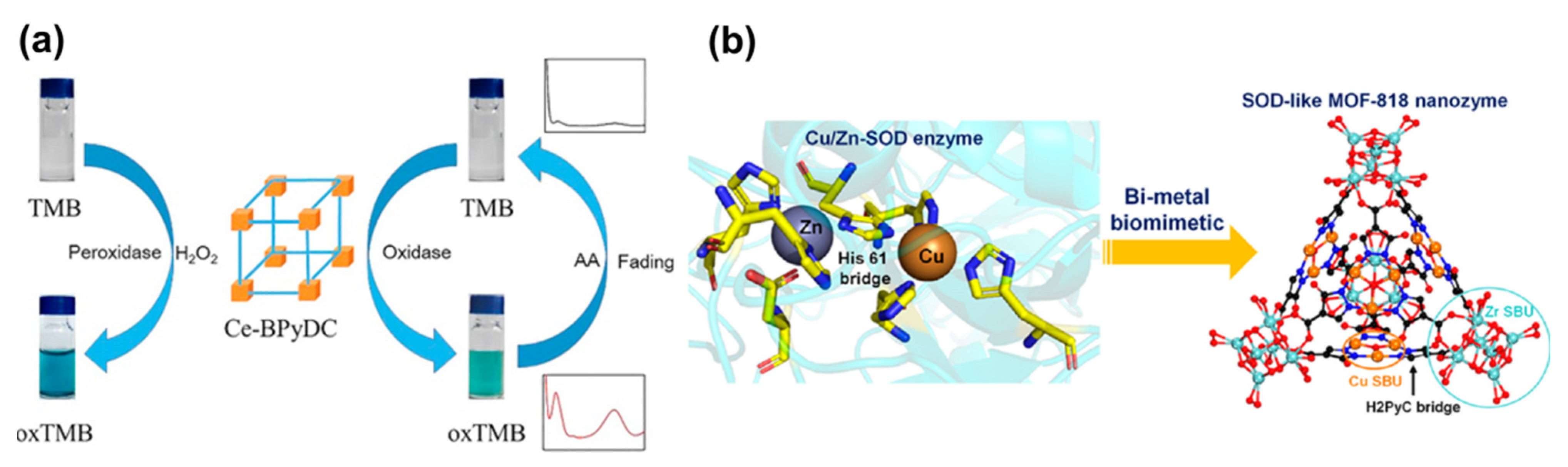
2.2. MOF-Based Nanoenzymes
2.3. MOF-Based Nanocarriers
3. Application of MOFs in Biomacromolecule Sensing
3.1. Application of MOFs in Proteins/Peptides Sensing
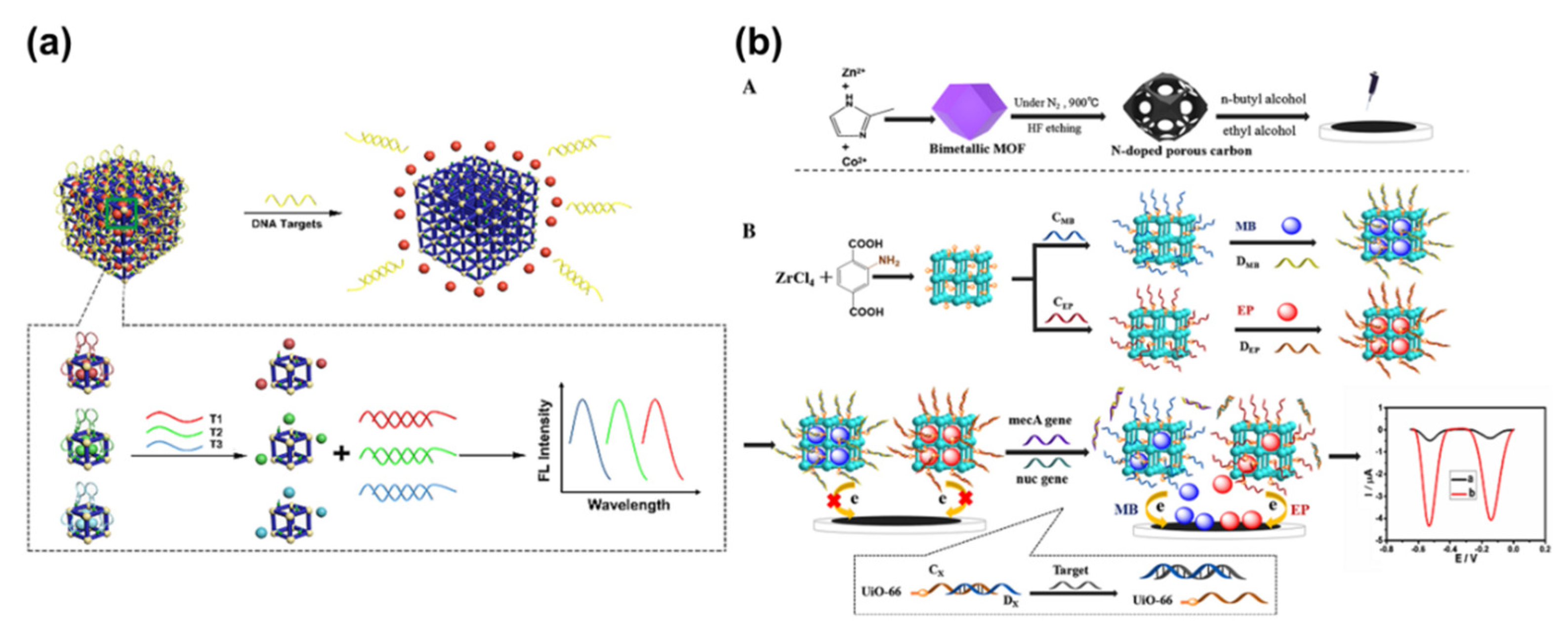
3.2. Application of MOFs in DNA Sensing
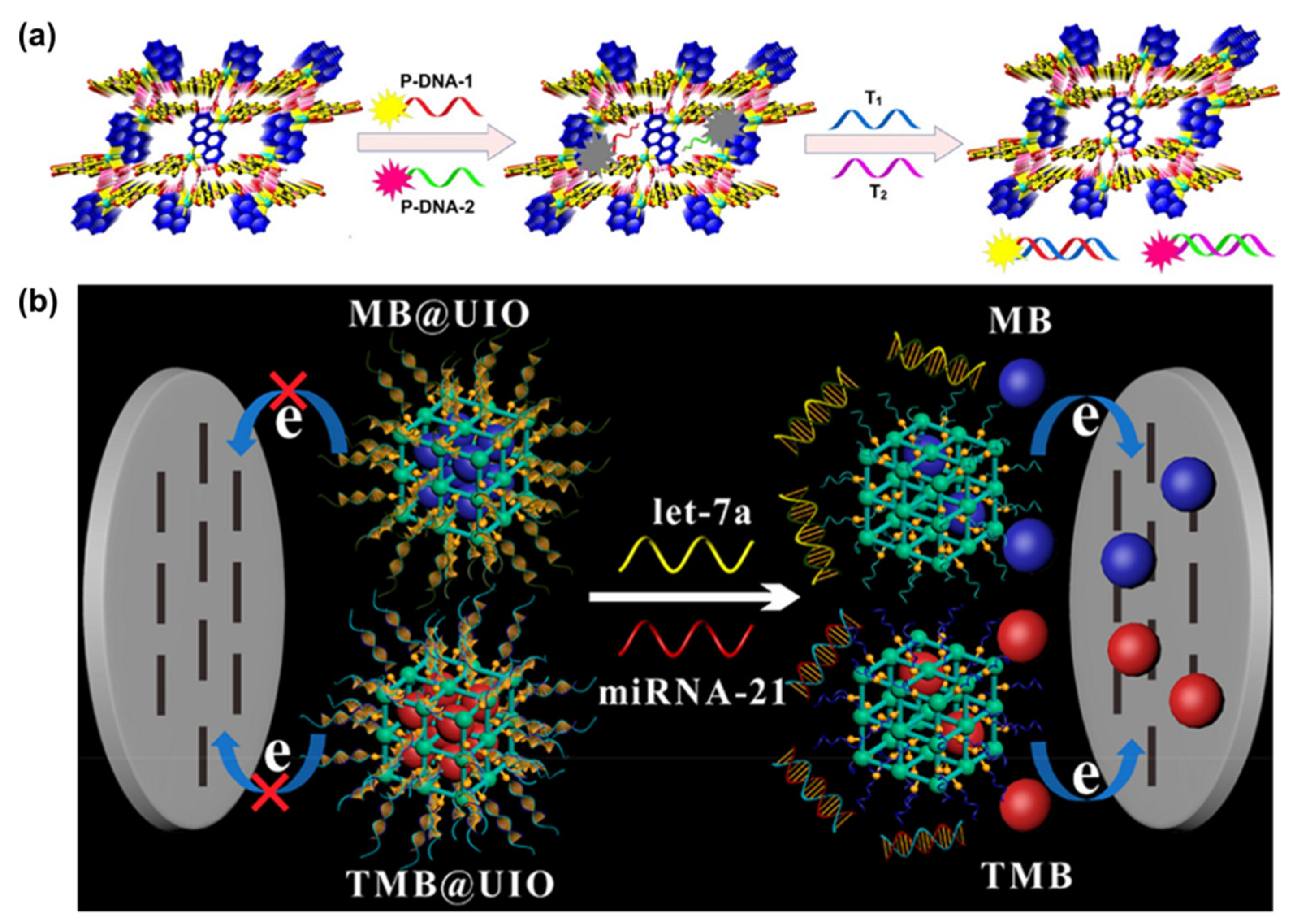
3.3. Application of MOFs in RNA Sensing
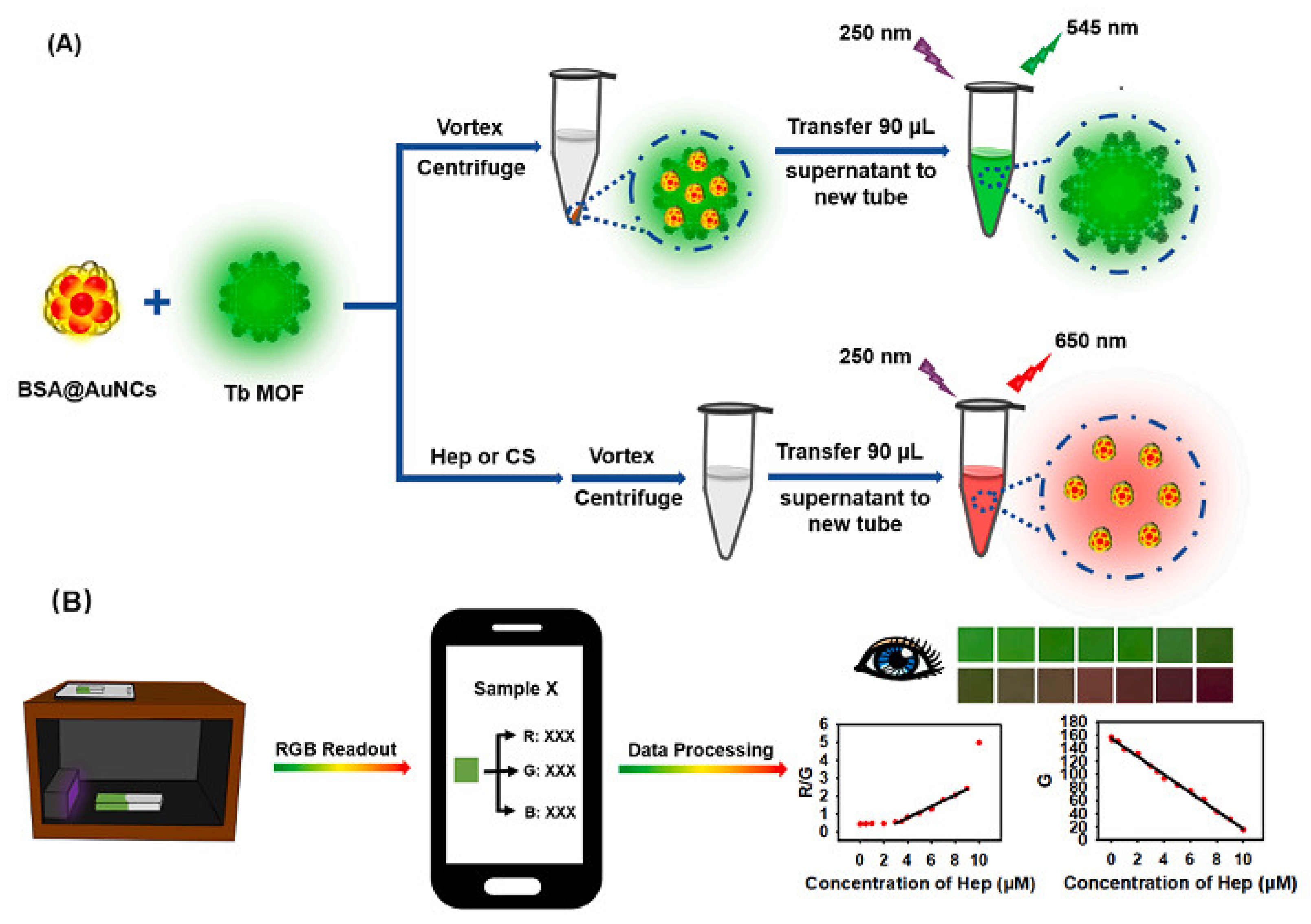
3.4. Application of MOFs in Polysaccharide Sensing
4. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasegawa, M. Biomarkers in systemic sclerosis: Their potential to predict clinical courses. J. Dermatol. 2016, 43, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Chedid, V. Actionable biomarkers: The key to resolving disorders of gastrointestinal function. Gut 2020, 69, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Guo, S.; Jin, S.; Chen, L.; Jung, Y.M. Biomarkers determination based on surface-enhanced Raman scattering. Chemosensors 2020, 8, 118. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Li, H.L.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev. 2014, 114, 1343–1370. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Lavenn, C.; Ginet, P.; Otake, K.-I.; Kitagawa, S. Upscale synthesis of a binary pillared layered MOF for hydrocarbon gas storage and separation. Green Chem. 2020, 22, 718–724. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Enery. 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Fan, W.D.; Wang, X.; Zhang, X.R.; Liu, X.P.; Wang, Y.T.; Kang, Z.X.; Dai, F.N.; Xu, B.; Wang, R.M.; Sun, D.F. Fine-Tuning the pore environment of the microporous Cu-MOF for high propylene storage and efficient separation of light hydrocarbons. ACS Cent. Sci. 2019, 5, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wu, A.P.; Yan, H.J.; Zhang, L.; Tian, C.G.; Wang, L.; Fu, H.G. A “MOFs plus MOFs” strategy toward Co-Mo2N tubes for efficient electrocatalytic overall water splitting. J. Mater. Chem. A 2018, 6, 20100–20109. [Google Scholar] [CrossRef]
- Meng, R.J.; Du, Q.J.; Zhong, N.; Zhou, X.; Liu, S.H.; Yin, S.F.; Liang, X. A tandem electrocatalysis of sulfur reduction by bimetal 2D MOFs. Adv. Energy Mater. 2021, 11, 2102819–2102829. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y. Detection of tyrosine catalyzed by a Tb-MOF luminescent nanozyme. Sens. Actuators B. Chem. 2022, 350, 130842–130848. [Google Scholar] [CrossRef]
- Suresh, K.; Matzger, A.J. Enhanced drug delivery by dissolution of amorphous drug encapsulatedina water unstable metal-organic framework (MOF). Angew. Chem. Int. Ed. 2019, 58, 16790–16794. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Loe, J.; Souza, B.E.; Missyul, A.; Giraldo, G.; Tan, J.-C.; Silvestre-Albero, J. MOF-based polymeric nanocomposite films as potential materials for drug delivery devices in ocular therapeutics. ACS Appl. Mater. Interfaces 2020, 12, 30189–30197. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.J.; Ma, X.X.; Duan, Y.F.; Yao, Y.W.; Cai, Q. Small titanium-based MOFs prepared with the introduction of tetraethyl orthosilicate and their potential for use in drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 13325–13332. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Mukherjee, S.; Hou, S.J.; Li, W.J.; Elsner, M.; Fischer, R.A. Porphyrinic MOF film for multifaceted electrochemical sensing. Angew. Chem. Int.Ed. 2021, 60, 20551–20557. [Google Scholar] [CrossRef] [PubMed]
- Portorreal-Bottier, A.; Gutiérrez-Tarriño, S.; Calvente, J.J.; Andreu, R.; Roldán, E.; Oña-Burgos, P.; Olloqui-Sariego, J.L. Enzyme-like activity of cobalt-MOF nanosheets for hydrogen peroxide electrochemical sensing. Sens. Actuators B. Chem. 2022, 368, 132129–132137. [Google Scholar] [CrossRef]
- Dong, S.Y.; Zhang, D.D.; Cui, H.; Huang, T.L. ZnO/porous carbon composite from a mixed-ligand MOF for ultrasensitive electrochemical immunosensing of C-reactive protein. Sens. Actuators B. Chem. 2019, 284, 354–361. [Google Scholar] [CrossRef]
- Liu, Z.B.; Liu, Y.F. Metal-organic frameworks as sensors of biomolecules. ACS Symp. Ser. Wash. Am. Chem. Soc. 2021, 1394, 1–31. [Google Scholar]
- Lin, Y.N.; Dai, Y.X.; Sun, Y.L.; Ding, C.F.; Sun, W.Y.; Zhu, X.D.; Liu, H.; Luo, C.N. A turn-on chemiluminescence biosensor for selective and sensitive detection of adenosine based on HKUST-1 and QDs-luminol-aptamer conjugates. Talanta 2018, 182, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Wang, X.Y.; Sun, Y.L.; Dai, Y.X.; Sun, W.Y.; Zhu, X.D.; Liu, H.; Han, R.; Gao, D.D.; Luo, C.N. A chemiluminescent biosensor for ultrasensitive detection of adenosine based on target-responsive DNA hydrogel with Au@HKUST-1 encapsulation. Sens. Actuators B-Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Chen, X.; Dong, J.J.; Chi, K.; Wang, L.J.; Xiao, F.; Wang, S.; Zhao, Y.; Liu, Y.Q. Electrically conductive metal-organic framework thin film-based on-chip micro-biosensor: A platform to unravel surface morphology-dependent biosensing. Adv. Funct. Mater. 2021, 31, 2102855–2102863. [Google Scholar] [CrossRef]
- Lin, Y.N.; Sun, Y.L.; Dai, Y.X.; Sun, W.Y.; Zhu, X.D.; Liu, H.; Han, R.; Gao, D.D.; Luo, C.N.; Wang, X.Y. A “signal-on” chemiluminescence biosensor for thrombin detection based on DNA functionalized magnetic sodium alginate hydrogel and metalloporphyrinic metal-organic framework nanosheets. Talanta 2020, 207, 120300–120308. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Luo, F.; Lin, C.Y.; Wang, J.; Qiu, B.; Lin, Z.Y. Electrochemiluminescence biosensor for thrombin detection based on metal organic framework with electrochemiluminescence indicator embedded in the framework. Biosens. Bioelectron. 2021, 189, 113374–113380. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.B.; Sun, D.P.; Tong, Y.L.; Zhong, Y.S.; Chen, Z.G. DNA nanotetrahedron linked dual-aptamer based voltammetric aptasensor for cardiac troponin I using a magnetic metal-organic framework as a label. Microchim. Acta 2019, 186, 374–383. [Google Scholar] [CrossRef]
- Li, C.C.; Li, Y.X.; Zhang, Y.; Zhao, G.H.; Wang, Y.G.; Wang, H.B.; Wang, H.; Xu, R.; Wei, Q. Signal-enhanced electrochemiluminescence strategy using iron-based metal-organic frameworks modified with carboxylated Ru(II) complexes for neuron-specific enolase detection. Biosens. Bioelectron. 2022, 215, 114605–114612. [Google Scholar] [CrossRef]
- Li, M.J.; Zhang, G.Y.; Boakye, A.; Chai, H.M.; Qu, L.J.; Zhang, X.J. Recent advances in metal-organic frameworks-based electrochemical biosensing applications. Front. Bioeng. Biotech. 2021, 9, 797067–797074. [Google Scholar]
- Milller, S.E.; Teplensky, M.H.; Moghadam, P.Z.; Fairen-Jimenez, D. Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 2016, 6, 20160027–20160040. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.-H.; Liu, X.-M.; He, T.; Li, J.-R. Metal-organic frameworks for the capture of trace aromatic volatile organic compounds. Chem. 2018, 4, 1911–1927. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.-H.; Xu, M.-M.; Liu, X.-M.; Zhao, M.-J.; Li, J.-R. Hydrophobic metal-organic frameworks: Assessment, construction, and diverse applications. Adv. Sci. 2020, 7, 1901758–1901801. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.H.; Peng, J.; Du, Q.Z.; He, H. Ratiometric fluorescence sensing of metal-organic frameworks: Tactics and perspectives. Coordin. Chem. Rev. 2020, 404, 213113–213140. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.S.; Behrens, K.; Matthes, P.R.; Schönfeld, F.; Nitsch, J.; Steffen, A.; Primus, P.-A.; Kumke, M.U.; Müller-Buschbaum, K.; Holdt, H.-J. White light emission of IFP-1 by in situ co-doping of the MOF pore system with Eu3+ and Tb3+. J. Mater. Chem. C 2015, 3, 4623–4631. [Google Scholar] [CrossRef] [Green Version]
- Abdelhameed, R.M.; Ananias, D.; Silva, A.M.S.; Rocha, J. Luminescent nanothermometers obtained by post-synthetic modification of metal-organic framework MIL-68. Eur. J. Inorg. Chem. 2019, 10, 1354–1359. [Google Scholar] [CrossRef]
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Ding, Y.R.; Lv, X.X.; Xia, L.; You, J.M.; Han, W.L. Emissions of terbium metal-organic frameworks modulated by dispersive/agglomerated gold nanoparticles for the construction of prostate-specific antigen biosensor. Anal. Bioanal. Chem. 2019, 411, 3979–3988. [Google Scholar] [CrossRef]
- Song, L.J.; Tian, F.L.; Liu, Z.L. Lanthanide doped metal-organic frameworks as a ratiometric fluorescence biosensor for visual and ultrasensitive detection of serotonin. J. Solid State Chem. 2022, 312, 12323–1123318. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Sun, L.B.; Du, J.F.; Liu, Y.C.; Shi, H.Z.; Liang, Z.Q.; Li, J.Y. Multifunctional zinc metal-organic framework based on designed H4TCPP ligand with aggregation-induced emission effect: CO2 adsorption, luminescence, and sensing property. Cryst. Growth Des. 2017, 17, 2090–2096. [Google Scholar] [CrossRef]
- Ying, X.D.; Chen, J.X.; Tu, D.Y.; Zhuang, Y.C.; Wu, D.; Shen, L. Tetraphenylpyrazine-based luminescent metal-organic framework for chemical sensing of carcinoids biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 6421–6429. [Google Scholar] [CrossRef]
- Kong, X.J.; Ji, X.T.; He, T.; Xie, L.H.; Zhang, Y.Z.; Lv, H.Y.; Ding, C.F.; Li, J.R. A Green-Emission Metal−Organic Framework-Based Nanoprobe for Imaging Dual Tumor Biomarkers in Living Cells. ACS Appl. Mater. Interfaces 2020, 12, 35375–35384. [Google Scholar] [CrossRef]
- Sun, Z.W.; Li, J.; Tong, Y.; Han, H.C.; Yang, Y.F.; Wang, C.X.; Li, H.; Du, L.T.; Jiang, Y.Y. A ratiometric fluorescent biosensor based on self-fluorescent MOF and target-triggered rolling circle amplification for sensitive detection of exosome-derived miRNA. Anal. Chim. Acta 2022, 122, 1340136–1340143. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bai, J.L.; Huo, Y.P.; Ning, B.A.; Peng, Y.; Li, S.; Han, D.P.; Kang, W.J.; Gao, Z.X. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol. Biosens. Bioelectron. 2020, 149, 111801–111808. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.Y.; Yang, F.; Hu, G.-B.; Yang, Y.; Huang, W.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Restriction of intramolecular motions (RIM) by metal-organic frameworks for electrochemiluminescence enhancement:2D Zr12-adb nanoplate as a novel ECL tag for the construction of biosensing platform. Biosens. Bioelectron. 2020, 155, 112099–112106. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.L.; Lin, H.; Lu, J.; Hu, Y.F.; Wang, S.; Huang, Y.J.; Guo, Z.Y. Potential-resolved Faraday cage-type electrochemiluminescence biosensor for simultaneous determination of miRNAs using functionalized g-C3N4 and metal organic framework nanosheets. Biosens. Bioelectron. 2018, 118, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.X.; Ye, J.; Zhu, Q.J.; Zhu, L.P.; Huang, J.S.; Yang, X.R. Ultrasensitive immunosensor for cardiac troponin I detection based on the electrochemiluminescence of 2D Ru-MOF nanosheets. Anal. Chem. 2019, 91, 10156–10163. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.Z.; Nie, H.; Tong, J.Q.; Yan, L.L.; Xu, B.; Sun, J.Z.; Tian, W.J.; Zhao, Z.J.; Qin, A.J.; et al. Tetraphenylpyrazine-based AIEgens: Facile preparation and tunable light emission. Chem. Sci. 2015, 6, 1932–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 23240. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-B.; Xiong, C.-Y.; Liang, W.-B.; Yang, Y.; Yao, L.-Y.; Huang, W.; Luo, W.; Yuan, R.; Xiao, D.-R. Highly stable Ru-complex-grafted 2D metal-organic layer with superior electrochemiluminescent efficiency as a sensing platform for simple and ultrasensitive detection of mucin 1. Biosens. Bioelectron. 2019, 135, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Karmakar, S.; Abraham, I.M.; Rambabu, D.; Dave, D.; Manjithaya, R.; Maji, T.K. Unraveling the effect on liminescent properties by postsynthetis covalent and noncovalent grafting of gfp chromophore analogues in nanoscale MOF-808. Inorg. Chem. 2020, 59, 8251–8258. [Google Scholar] [CrossRef]
- Shao, H.L.; Lu, J.; Zhang, Q.Q.; Hu, Y.F.; Wang, S.; Guo, Z.Y. Ruthenium-based metal organic framework (Ru-MOF)-derived novel Faraday-cage electrochemiluminescence biosensor for ultrasensitive detection of miRNA-141. Sens. Actuators B-Chem. 2018, 268, 39–46. [Google Scholar] [CrossRef]
- Mohan, B.; Kumar, S.; Kumar, V.; Jiao, T.H.; Sharma, H.K.; Chen, Q.S. Electrochemiluminescence metal-organic frameworks biosensing materials for detecting cancer biomarkers. Trend Anal. Chem. 2022, 157, 116735–116750. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, X.; Hu, C.Y.; Guo, W.; Wu, X.J.; Chen, G.X.; Dai, W.J.; Zhen, S.J.; Huang, C.Z.; Li, Y.F. Controlled synthesis of zinc-metal organic framework microflower with high efficiency electrochemiluminescence for miR-21 detection. Biosens. Bioelectron. 2022, 213, 114443–114450. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.-Q.; Peng, L.-Z.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhou, Y. Strong electrochemiluminescence from MOF accelerator enriched quantum dots for enhanced sensing of trace cTnI. Anal. Chem. 2018, 90, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.B.; Xiong, C.-Y.; Liang, W.-B.; Zeng, X.-S.; Xu, H.-L.; Yang, Y.; Yao, L.-Y.; Yuan, R.; Xiao, D.R. Highly stable mesoporous luminescence-functionalized MOF with excellent electrochemiluminescence property for ultrasensitive immunosensor construction. ACS Appl. Mater. Interfaces 2018, 10, 15913–15919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Shang, Y.H.; Zhu, Q.Y.; Zhang, X.X.; Zheng, J.B. A voltammetric immunoassay for the carcinoembryonic antigen using silver(I)-terephthalate metal-organic frameworks containing gold nanoparticles as a signal probe. Microchim. Acta 2019, 186, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Dong, P.F.; Huang, J.; Xu, H.; Lei, J.P.; Zhang, L. Direct electrochemistry of silver nanoparticles-decorated metal-organic frameworks for telomerase activity sensing via allosteric activation of an aptamer hairpin. Anal. Chim. Acta 2021, 1184, 339036–339045. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Feng, S.N.; Yan, M.X.; Huang, J.S.; Yang, X.R. A dual-amplification mode and Cu-based metal-organic frameworks mediated electrochemical biosensor for sensitive detection of microRNA. Biosens. Bioelectron. 2022, 202, 113992–113998. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. Enhanced electrocatalytic reduction activity of Fe-MOF/Pt nanoparticles as a sensitive sensor for ultra-trace determination of Tinidazole. Microchem. J. 2022, 172, 106976–106984. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, L.; Li, Z.Z.; Guo, C.P.; Wang, M.H.; Wang, C.B.; Du, M. CoNi bimetallic metal-organic framework as an efficient biosensing platform for miRNA 126 detection. Appl. Surf. Sci. 2021, 542, 148586–148596. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Fan, Q.L.; Chen, K.; Sun, D.K.; Hu, T.; Ni, Z.H. One-pot, rapid microwave-assisted synthesis of bimetallic metal-organic framework for efficient enzyme-free glucose detection. Microchem. J. 2022, 179, 107468–107475. [Google Scholar] [CrossRef]
- Guo, C.P.; Li, Z.Z.; Duan, F.H.; Zhang, Z.H.; Marchetti, F.; Du, M. Semiconducting CuxNi3-x(hexahydroxytriphenylene)2 framework for electrochemical aptasensing of C6 glioma cells and epidermal growth factor receptor. J. Mater. Chem. B 2020, 8, 9951–9960. [Google Scholar] [CrossRef]
- Ma, J.P.; Bai, W.S.; Liu, X.L.; Zheng, J.B. Electrochemical dopamine sensor based on bi-metallic o/Zn porphyrin metal-organic framework. Microchim. Acta 2022, 189, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jalal, N.R.; Madrakian, T.; Afkhami, A.; Ahmadi, M. Ni/Co bimetallic metal-organic frameworks on nitrogen-doped graphene oxide nanoribbons for electrochemical sensing of doxorubicin. ACS Appl. Nano Mater. 2022, 5, 11045–11058. [Google Scholar] [CrossRef]
- Kachouei, M.A.; Shahrokhian, S.; Ezzati, M. Bimetallic Co/Zn-MOFs easily derived from Co/Zn-LDHs, as a suitable platform in fabrication of a non-enzymatic electrochemical sensor for detecting glucose in human fluids. Sens. Actuators B-Chem. 2021, 344, 130254–130276. [Google Scholar] [CrossRef]
- Lv, S.Z.; Zhang, K.Y.; Zhu, L.; Tang, D.P.; Niessner, R.; Knopp, D. H2-based electrochemical biosensor with Pd nanowires@ZIF-67 molecular sieve bilayered sensing interface for immunoassay. Anal. Chem. 2019, 91, 12055–12062. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D.P. Recent Advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Lv, S.Z.; Zhang, K.Y.; Zhu, L.; Tang, D.P. ZIF-8-assisted NaYF4:Yb, Tm@ZnO converter with exonuclease III-powered DNA walker for near-infrared light responsive biosensor. Anal. Chem. 2020, 92, 1470–1476. [Google Scholar] [CrossRef]
- Chen, K.Y.; Xue, J.Y.; Zhou, Q.; Zhang, Y.; Zhang, M.M.; Zhang, Y.J.; Zhang, H.; Shen, Y.F. Coupling metal-organic framework nanosphere and nanobody for boosted photoelectrochemical immunoassay of Human Epididymis Protein 4. Anal. Chim. Acta 2020, 1107, 145–154. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhao, Y.C.; Li, F. Nucleic acid-functionalized metal-organic framework for ultrasensitive immobilization-free photoelectrochemical biosensing. Biosens. Bioelectron. 2021, 173, 112832–112838. [Google Scholar] [CrossRef]
- Rani, R.; Deep, A.; Mizaikoff, B.; Singh, S. Zirconium metal organic framework based opto-electrochemical sensor for nitrofurazone detection. Electroanal. Chem. 2022, 909, 116124–116133. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.-G.; Cheng, P. Silica-protection-assisted encapsulation of Cu2O nanocubes into a metal-organic framework (ZIF-8) to provide a composite catalyst. Angew. Chem. Int. Ed. 2018, 57, 6834–6837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, X.F.; Luan, D.Y.; Lou, X.W. Fabrication of heterostructured Fe2TiO5–TiO2 nanocages with enhanced photoelectrochemical performance for solar energy conversion. Angew. Chem. Int. Ed. 2020, 59, 8128–8132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Yan, W.Y.; Wang, X.M.; Yu, L.G.; Zhang, J.H.; Bai, B.Q.; Guo, C.X.; Fan, S.H. Development of a molecularly imprinted photoelectrochemical sensing platform based on NH2-MIL-125(Ti)-TiO2 composite for the sensitive and selective determination of oxtetracycline. Biosens. Bioelectron. 2021, 177, 113000–113008. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.X.; Wang, C.; Zhou, X.; Wu, T.; Wang, Y.Y.; Li, C.Y.; Yang, N.J. Ionic liquid and spatially confined gold nanoparticles enhanced photoelectrochemical response of zinc-metal organic frameworks and immunosensing squamous cell carcinoma antigen. Biosens. Bioelectron. 2019, 142, 111540–111548. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Wu, W.Y.; He, G.H.; Deng, W.F.; Tan, Y.M.; Xie, Q.J. Controllable sensitization of Zr-MOFs by using CdS and its application for photoelectrochemical detection of alkaline phosphatase. Chem. Commun. 2022, 58, 7960–7963. [Google Scholar] [CrossRef]
- Hang, T.X.; Li, C.P.; Liang, D.D.; Li, S.R.; Zhou, H.; Ge, P.; Zhu, X.D.; Liu, T.F. Metal-organic frameworks-based hierarchical heterojunction coupling with plasmonic nanoshells for self-powered photoelectrochemical immunoassay. Chem. Eng. J. 2022, 431, 133465–133472. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.N.; Wang, C.Y.; Hu, X.Y.; Liu, Y.L.; Wang, G.X. Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Qi, W.Z.; Zheng, L.Y.; Wang, S.H.; Huang, F.C.; Liu, Y.J.; Jiang, H.Y.; Lin, J.H. A microfluidic biosensor for rapid and automatic detection of Salmonella using metal-organic framework and Raspberry Pi. Biosens. Bioelectron. 2021, 178, 113020–113027. [Google Scholar] [CrossRef]
- Sha, M.; Xu, W.Q.; Wu, Y.; Jiao, L.; Chen, Y.F.; Huang, J.J.; Tang, Y.J.; Gu, W.L.; Zhu, C.Z. Histidine-engineered metal-organic frameworks with enhanced peroxidase-like activity for sensitive detection of metallothioneins. Sens. Actuators B-Chem. 2022, 366, 131927. [Google Scholar] [CrossRef]
- Mao, X.X.; He, F.N.; Qiu, D.H.; Wei, S.J.; Luo, R.G.; Chen, Y.; Zhang, X.B.; Lei, J.P.; Monchaud, D.; Mergny, J.-L.; et al. Efficient biocatalytic system for biosensing by combining metal-organic framework (MOF)-based nanozymes and G-quadruplex(G4)-DNAzymes. Anal. Chem. 2022, 94, 7295–7302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Li, S.Q.; Chen, Y.; Lv, W.Q.; Zhang, J.H.; Zhang, L.B.; Zhang, Z.; Lu, X.Q. Synergistic effect enhances the peroxidase-like activity in platinum nanoparticle-supported metal-organic framework hybrid nanozymes for ultrasensitive detection of glucose. Nano Res. 2021, 14, 4689–4695. [Google Scholar] [CrossRef]
- Zhou, R.L.; Zhuang, X.H.; Wu, Q.L.; Jin, M.; Zheng, C.H.; Jiang, Y.Y.; Luo, Y.L.; Zheng, L.B. Cu-MOF@Pt 3D nanocomposites prepared by one-step wrapping method with peroxidase-like activity for colorimetric detection of glucose. Colloid Surface B 2022, 216, 112601–112607. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, J.Y.; Liu, Y.Z.; Sun, W.Y.; Su, X.G. Constructing bifunctional metaleorganic framework based nanozymes with fluorescence and oxidase activity for the dual-channel detection of butyrylcholinesterase. Anal. Chim. Acta. 2022, 1205, 339717–339725. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Shen, Y.; Gao, S.B.; Chen, Y.J.; Cui, Y.S.; Ning, D.X.; Ji, X.B.; Liu, Z.W.; Wang, L.G. The Mn-modified porphyrin metal-organic framework with enhanced oxidase-like activity for sensitively colorimetric detection of glutathione. Biosens. Bioelectron. 2022, 213, 114446–114453. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.P.; Huang, L.J.; Liu, X.N.; Zhang, W.T.; Yao, X.L.; Dou, L.N.; Zhang, X.; Nian, Y.; Sun, J.; Wang, J.L. Mixed-valence Ce-BPyDC metal-organic framework with dual enzyme-like activities for colorimetric biosensing. Inorg. Chem. 2019, 58, 11382–11388. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shi, H.M.; Feng, B.; Ding, C.F.; Yu, S.N. A versatile biomimetic multienzyme cascade nanoplatform based on boronic acid-modified metal-organic framework for colorimetric biosensing. J. Mater. Chem. B 2022, 10, 3444–3451. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, S.M.; Yang, H.S.; Ye, N.R.; Tong, L.J.; Chen, G.S.; Zhou, Q.; Ouyang, G.F. Bimetal Biomimetic Engineering Utilizing Metal-Organic Frameworks for Superoxide Dismutase Mimic. ACS Mater. Lett. 2022, 4, 751–757. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, Z.Z.; Cao, F.F.; Sang, Y.J.; Dong, K.; Pu, F.; Ren, J.S.; Qu, X.G. Constructing metal-organic framework nanodots as bio-inspired artificial superoxide dismutase for alleviating endotoxemia. Mater. Horiz. 2019, 6, 1682–1687. [Google Scholar] [CrossRef]
- Sun, Z.W.; Wang, L.; Wu, S.; Pan, Y.H.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.M.; Li, G.X. An electrochemical biosensor designed by using Zr-based metal-organic frameworks for the detection of glioblastoma-derived exosomes with practical application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef]
- Yang, X.; Cui, A.P.; Zhang, Y.J.; Li, S.J.; Li, Y. Electrogenerated chemiluminescence biosensor for microRNA detection incorporating enzyme-free dual DNA cyclic amplification and Ru(bpy)32+-functionalized metal-organic framework. Talanta 2022, 245, 123458–123465. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Wang, F.; Deng, P.Y.; Wang, Y.; Cai, K.; Chen, Y.H.; Wang, Z.H.; Liu, Y. Sensitive electrogenerated chemiluminescence biosensors for protein kinase activity analysis based on bimetallic catalysis signal amplification and recognition of Au and Pt loaded metal-organic frameworks nanocomposites. Biosens. Bioelectron. 2018, 109, 132–138. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.F.; Tan, Y.M.; Xie, Q.J.; Yao, S.Z. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.X.; Zhang, M.Z.; Lu, L.D.; Lou, X.P.; Dong, M.L.; Zhu, L.Q. Metal-organic framework/enzyme coated optical fibers as waveguide-based biosensors. Sens. Actuators B-Chem. 2019, 288, 12–19. [Google Scholar] [CrossRef]
- Kong, L.Y.; Lv, S.Z.; Qiao, Z.J.; Yan, Y.C.; Zhang, J.; Bi, S. Metal-organic framework nanoreactor-based electrochemical biosensor coupled with three-dimensional DNA walker for label-free detection of microRNA. Biosens. Bioelectron. 2022, 207, 114188–114195. [Google Scholar] [CrossRef]
- Wei, Y.-P.; Zhang, Y.-W.; Mao, C.-J. A silver nanoparticle-assisted signal amplification electrochemiluminescence biosensor for highly sensitive detection of mucin 1. J.Mater. Chem. B 2020, 8, 2471–2475. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal-organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157:H7. Analyst 2018, 143, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, S.M.; Dorraji, P.S.; Fotouhi, L.; Hosseini, M.; Khatami, F.; Moazami, H.R.; Omidfar, K. Enhanced electrochemiluminescence biosensing of gene-specific methylation in thyroid cancer patients’ plasma based integrated graphitic carbon nitride-encapsulated metal-organic framework nanozyme optimized by central composite design. Sens. Actuators B-Chem. 2022, 364, 131895–131906. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, Y.X.; Zhuang, X.M.; Tian, C.Y.; Fu, X.L.; Luan, F. Ru(bpy)32+ encapsulated cyclodextrin based metal organic framework with improved biocompatibility for sensitive electrochemiluminescence detection of CYFRA21-1 in cell. Biosens. Bioelectron. 2021, 190, 113371–113377. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhao, G.H.; Chi, H.; Yang, S.H.; Niu, Q.F.; Wu, D.; Cao, W.; Li, T.D.; Ma, H.M.; Wei, Q. Self-Luminescent lanthanide metal-organic frameworks as signal probes in electrochemiluminescence immunoassay. J. Am. Chem. Soc. 2021, 143, 504–512. [Google Scholar] [CrossRef]
- Wang, D.D.; Wu, H.H.; Zhou, J.J.; Xu, P.P.; Wang, C.L.; Shi, R.H.; Wang, H.B.; Wang, H.; Guo, Z.; Chen, Q.W. In situ one-pot synthesis of MOF-polydopamine hybrid nanogels with enhanced photothermal effect for targeted cancer therapy. Adv. Sci. 2018, 5, 1800287–1800294. [Google Scholar] [CrossRef]
- Fan, G.; Dundas, C.M.; Zhang, C.; Lynd, N.A.; Keitz, B.K. Sequence-dependent peptide surface functionalization of metal-organic frameworks. ACS Appl. Mater. Interfaces 2018, 10, 18601–18609. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-K.; Wang, X.-Y.; Liu, X.; Yang, T.; Chen, M.-L.; Wang, J.-H. Ensuring high selectivity for preconcentration and detection of ultra-trace cadmium using a phage- functionalized metal-organic framework. Analyst 2020, 145, 5280–5288. [Google Scholar] [CrossRef] [PubMed]
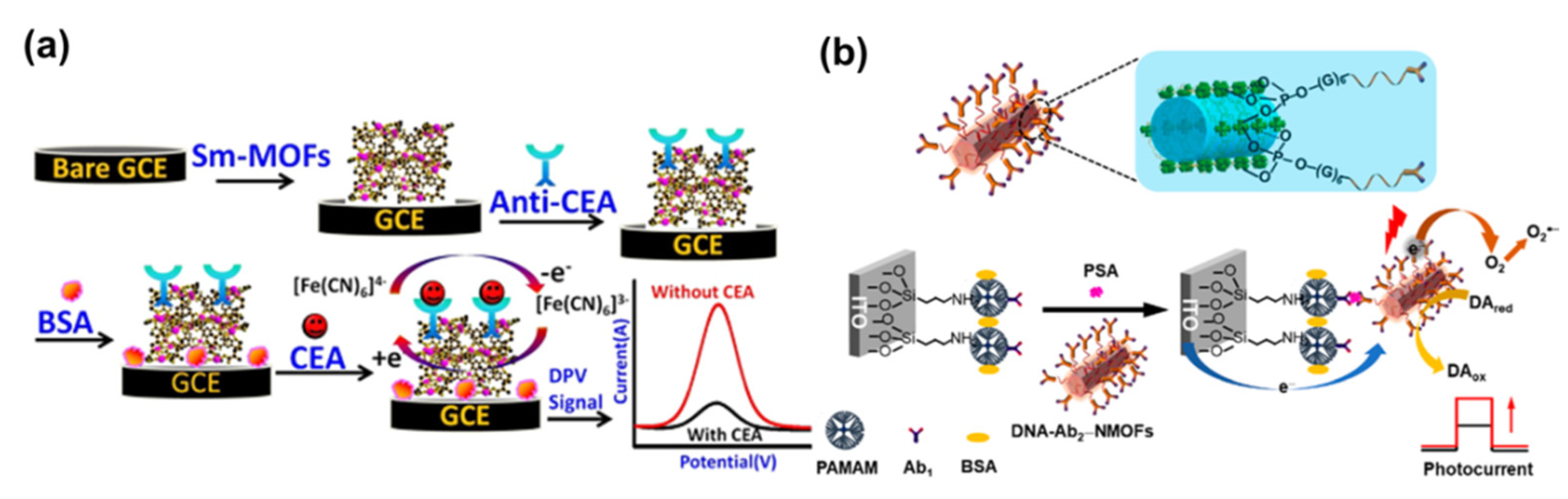
- Biswas, S.; Lan, Q.C.; Li, C.R.; Xia, X.-H. Morphologically flex Sm-MOF based electrochemical immunosensor for ultrasensitive detection of a colon cancer biomarker. Anal. Chem. 2022, 94, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Shan, D.; Dong, H.F.; Cosnier, S.; Al-Ghanim, K.A.; Ahmad, Z.; Mahboob, S.; Zhang, X.J. DNA-mediated nanoscale metal-organic frameworks for ultrasensitive photoelectrochemical enzyme-free immunoassay. Anal. Chem. 2018, 90, 12284–12291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.Y.; Cheng, Y.; Xie, Q.J.; Liu, R.S.; Yang, X.P. Immunosensing of NT-proBNP via Cu2+-based MOFs biolabeling and in situ microliter-droplet anodic stripping voltammetry. Electroanalysis 2020, 32, 1754–1762. [Google Scholar] [CrossRef]
- Wu, S.; Li, C.; Shi, H.; Huang, Y.; Li, G.X. Design of metal-organic framework-based nanoprobes for multicolor detection of DNA targets with improved sensitivity. Anal. Chem. 2018, 90, 9929–9935. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Wang, A.-J.; Yuan, P.-X.; Feng, J.-J. Flower-like metal-organic framework microsphere as a novel enhanced ECL luminophore to construct the coreactant-free biosensor for ultrasensitive detection of breast cancer 1 gene. Sens. Actuators B-Chem. 2020, 320, 128395–128402. [Google Scholar] [CrossRef]
- Kouzegaran, V.J.; Farhadi, K.; Forough, M.; Bahram, M.; Çetinkol, O.P. Highly-sensitive and fast detection of human telomeric G-Quadruplex DNA based on a hemin-conjugated fluorescent metal-organic framework platform. Biosens. Bioelectron. 2021, 178, 112999–113007. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Liu, W.-S.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Jin, B.-K. Metal-organic gel and metal-organic framework based switchable electrochemiluminescence RNA sensing platform for Zika virus. Sens. Actuators B-Chem. 2020, 321, 128456–128462. [Google Scholar] [CrossRef]
- Kong, X.-J.; Tian, J.-X.; Fang, Y.-Z.; Chen, T.-L.; Yu, R.; He, J.-Y.; Zhang, Z.-Y.; Xiao, Q. Terbium metal-organic framework/bovine serum albumin capped gold nanoclusters-based dual-emission reverse change ratio fluorescence nanoplatform for fluorimetric and colorimetric sensing of heparin and chondroitin sulfate. Sens. Actuators B-Chem. 2022, 356, 131331–131340. [Google Scholar] [CrossRef]
- Duan, Y.Y.; Wang, N.; Huang, Z.X.; Dai, H.X.; Xu, L.; Sun, S.J.; Ma, H.Y.; Lin, M. Electrochemical endotoxin aptasensor based on a metal-organic framework labeled analytical platform. Mat. Sci. Eng. C 2020, 108, 110501–110507. [Google Scholar] [CrossRef] [PubMed]
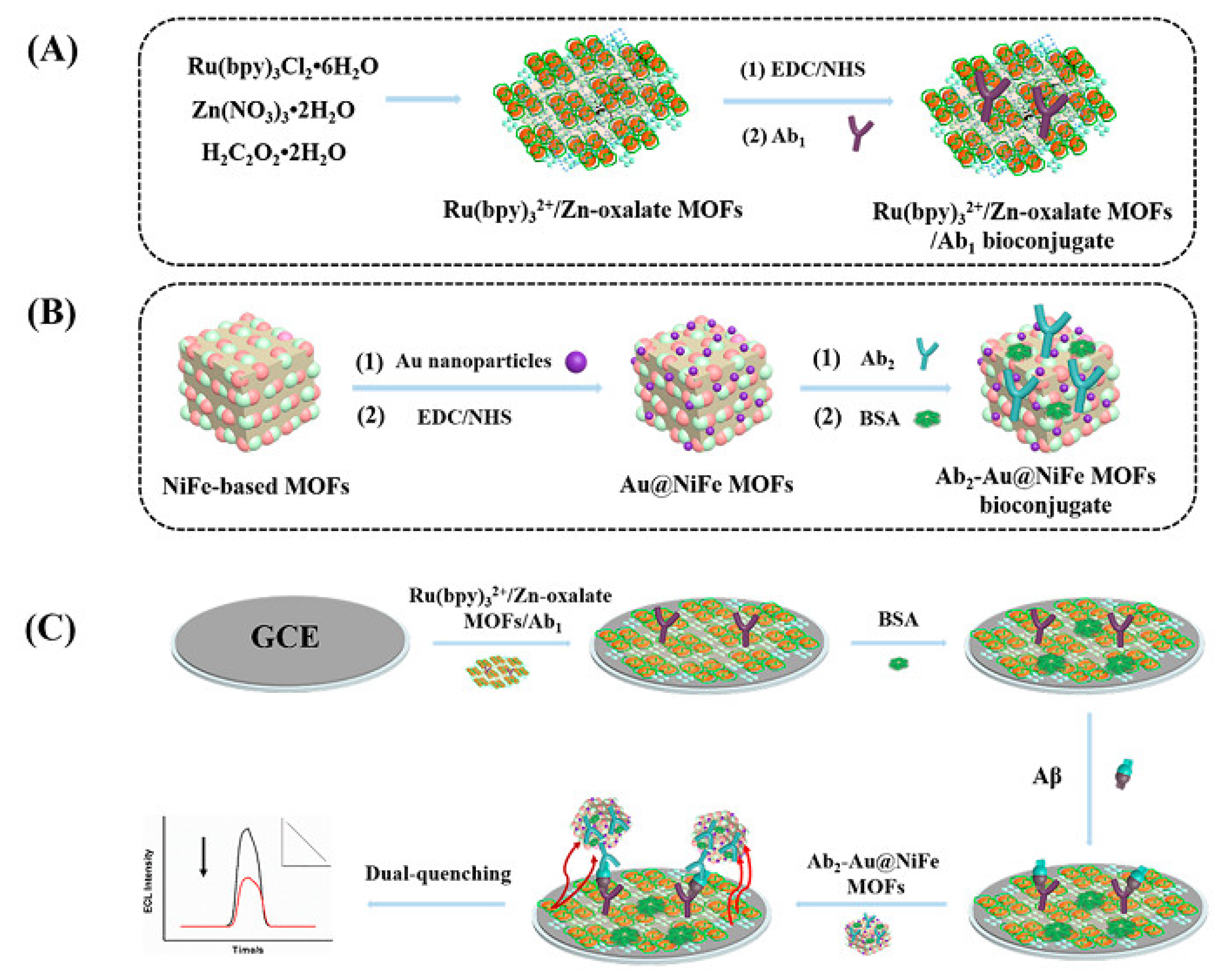
- Zhao, G.H.; Wang, Y.G.; Li, X.J.; Yue, Q.; Dong, X.; Du, B.; Cao, W.; Wei, Q. Dual-quenching electrochemiluminescence strategy based on three-dimensional metal-organic frameworks for ultrasensitive detection of amyloid-β. Anal. Chem. 2019, 91, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Gao, J.; Tan, H.L. Integrated antibody with catalytic metal-organic framework for colorimetric immunoassay. ACS Appl. Mater. Interfaces 2018, 10, 25113–25120. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.F.; Yan, B. Dye-functionalized metal-organic frameworks with the uniform dispersion of MnO2 nanosheets for visualized fluorescence detection of alanine aminotransferase. Nanoscale 2021, 13, 20205–20212. [Google Scholar] [CrossRef]
- Li, S.Q.; Hu, X.; Chen, Q.M.; Zhang, X.D.; Chai, H.X.; Huang, Y.M. Introducing bifunctional metal-organic frameworks to the construction of a novel ratiometric fluorescence sensor for screening acid phosphatase activity. Biosens. Bioelectron. 2019, 137, 133–139. [Google Scholar] [CrossRef]
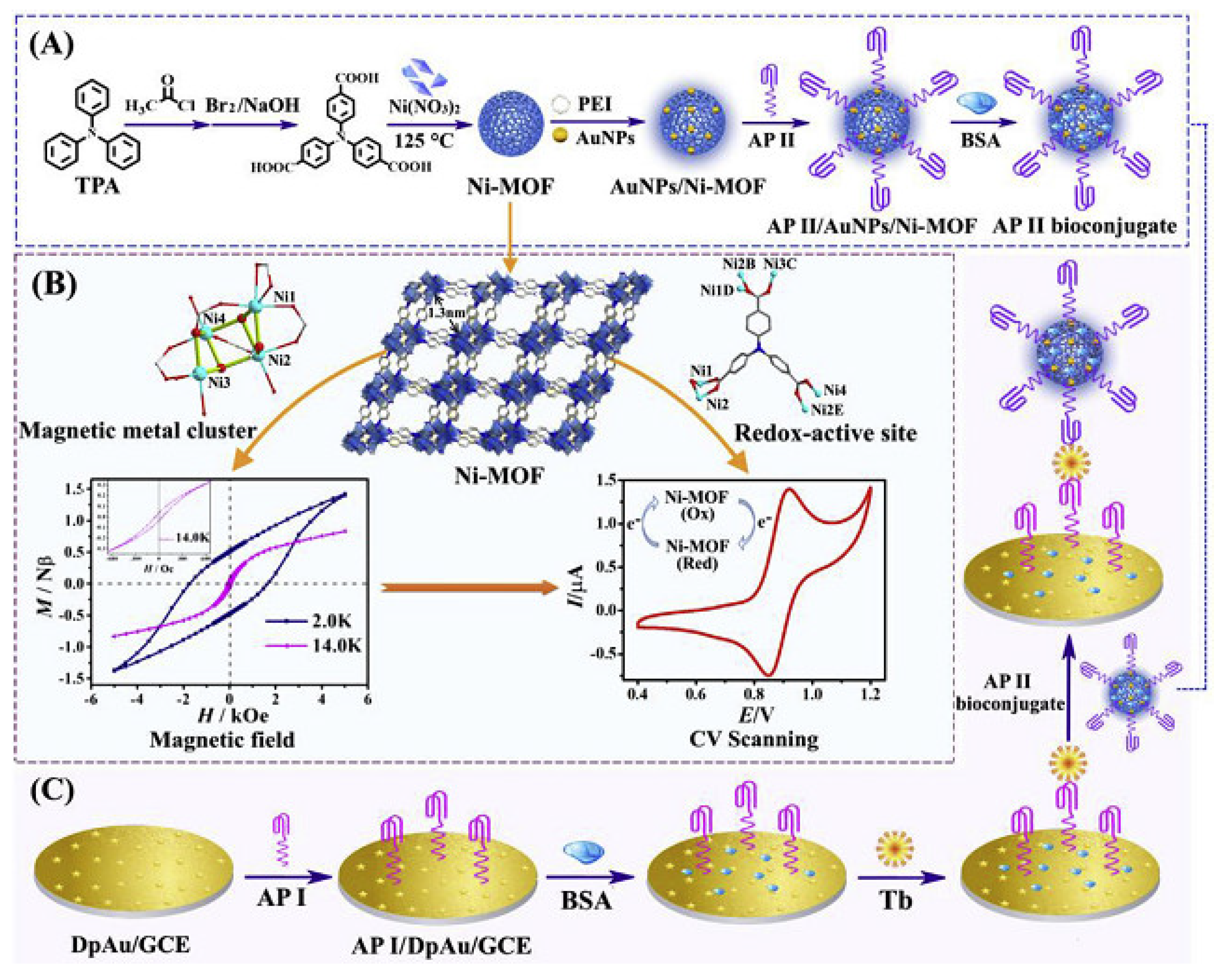
- Wu, H.P.; Li, M.; Wang, Z.; Yu, H.; Han, J.; Xie, G.; Chen, S.P. Highly stable Ni-MOF comprising triphenylamine moieties as a high-performance redox indicator for sensitive aptasensor construction. Anal. Chim. Acta 2019, 1049, 74–81. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Wang, T.J. Unexpectedly low natriuretic peptide levels in patients with heart failure. JACC-Heart Fail 2021, 9, 192–200. [Google Scholar] [CrossRef]
- Kim, H.K.; Jang, H.J.; Moon, J.; Byun, J.H.; Jeong, J.Y.; Jung, J.Y.; Lim, E.-K.; Kang, T.J. Metal-organic framework coating for the preservation of silver nanowire surface-enhanced Raman scattering platform. Adv. Mater. Interfaces 2019, 6, 1900427–1900435. [Google Scholar] [CrossRef]
- Dai, G.; Li, Z.; Luo, F.F.; Lu, Y.Q.; Chu, Z.H.; Zhang, J.W.; Zhang, F.; Wang, Q.J.; He, P.G. Simultaneous electrochemical determination of nuc and mecA genes for identification of methicillin-resistant Staphylococcus aureus using N-doped porous carbon and DNA-modified MOF. Microchim. Acta 2021, 188, 39–47. [Google Scholar] [CrossRef]
- Liang, L.S.; Chen, M.; Tong, Y.L.; Tan, W.G.; Chen, Z.G. Detection of Mycobacterium Tuberculosis IS6110 gene fragment by fluorescent biosensor based on FRET between two-dimensional metal-organic framework and quantum dots-labeled DNA probe. Anal. Chim. Acta 2021, 1186, 339090–339097. [Google Scholar] [CrossRef]
- Liu, S.; Huo, Y.P.; Fan, L.X.; Ning, B.A.; Sun, T.Q.; Gao, Z.X. Rapid and ultrasensitive detection of DNA and microRNA-21 using a zirconium porphyrin metal-organic framework-based switch fluorescence biosensor. Anal. Chim. Acta 2022, 1192, 339340–339348. [Google Scholar] [CrossRef]
- Qiu, G.H.; Weng, Z.-H.; Hu, P.-P.; Duan, W.-J.; Xie, B.-P.; Sun, B.; Tang, X.-Y.; Chen, J.-X. Synchronous detection of ebolavirus conserved RNA sequences and ebolavirus-encoded miRNA-like fragment based on a zwitterionic copper (II) metal-organic framework. Talanta 2018, 180, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Wang, X.; Wang, J.; Li, H.Y.; Li, F. Nucleic acid-functionalized metal-organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.; Zhang, D.D.; Zhang, R.; Tang, J.P.; Xu, K.Y.; Shao, M.Z.; Li, Y.Y.; Du, P.Y.; Zhang, R.Z.; Yang, D.Y.; et al. NA-functionalized metal-organic framework ratiometric nanoprobe for MicroRNA detection and imaging in live cells. Sens. Actuators B-Chem. 2022, 361, 131676–131683. [Google Scholar] [CrossRef]
- Bromfield, S.M.; Barnard, A.; Posocco, P.; Fermeglia, M.; Pricl, S.; Smith, D.K. Mallard Blue: A high-affinity selective heparin sensor that operates in highly competitive media. J. Am. Chem. Soc. 2013, 135, 2911–2914. [Google Scholar] [CrossRef]
- Kalita, M.; Balivada, S.; Swarup, V.P.; Mencio, C.; Raman, K.; Desai, U.R.; Troyer, D.; Kuberan, B. A nanosensor for ultrasensitive detection of oversulfated chondroitin sulfate contaminant in heparin. J. Am. Chem. Soc. 2014, 136, 554–557. [Google Scholar] [CrossRef]
- Capila, I.; Linhardt, R.J. Heparin -Protein Interactions. Angew. Chem. Int. Ed. 2002, 41, 390–412. [Google Scholar] [CrossRef]
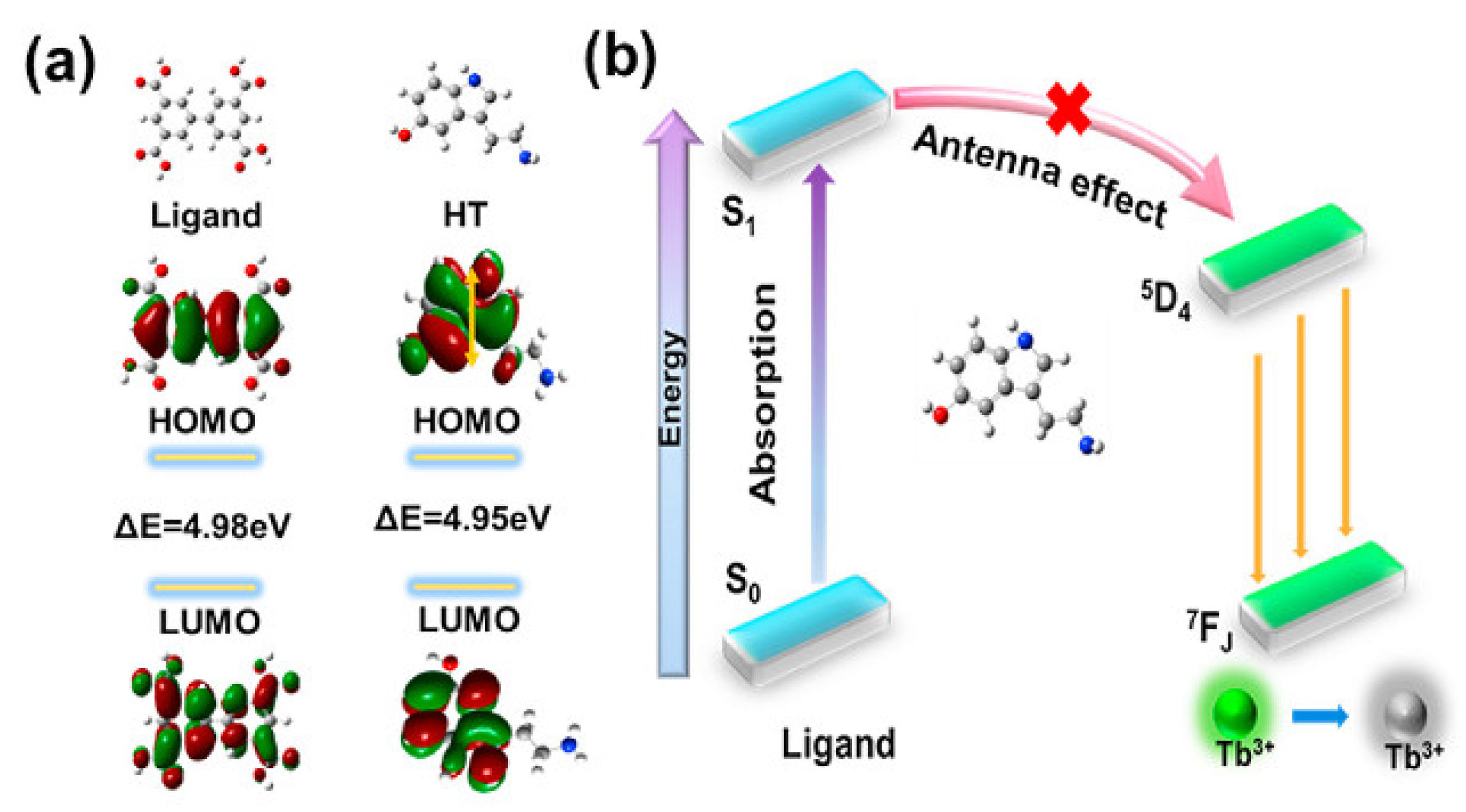

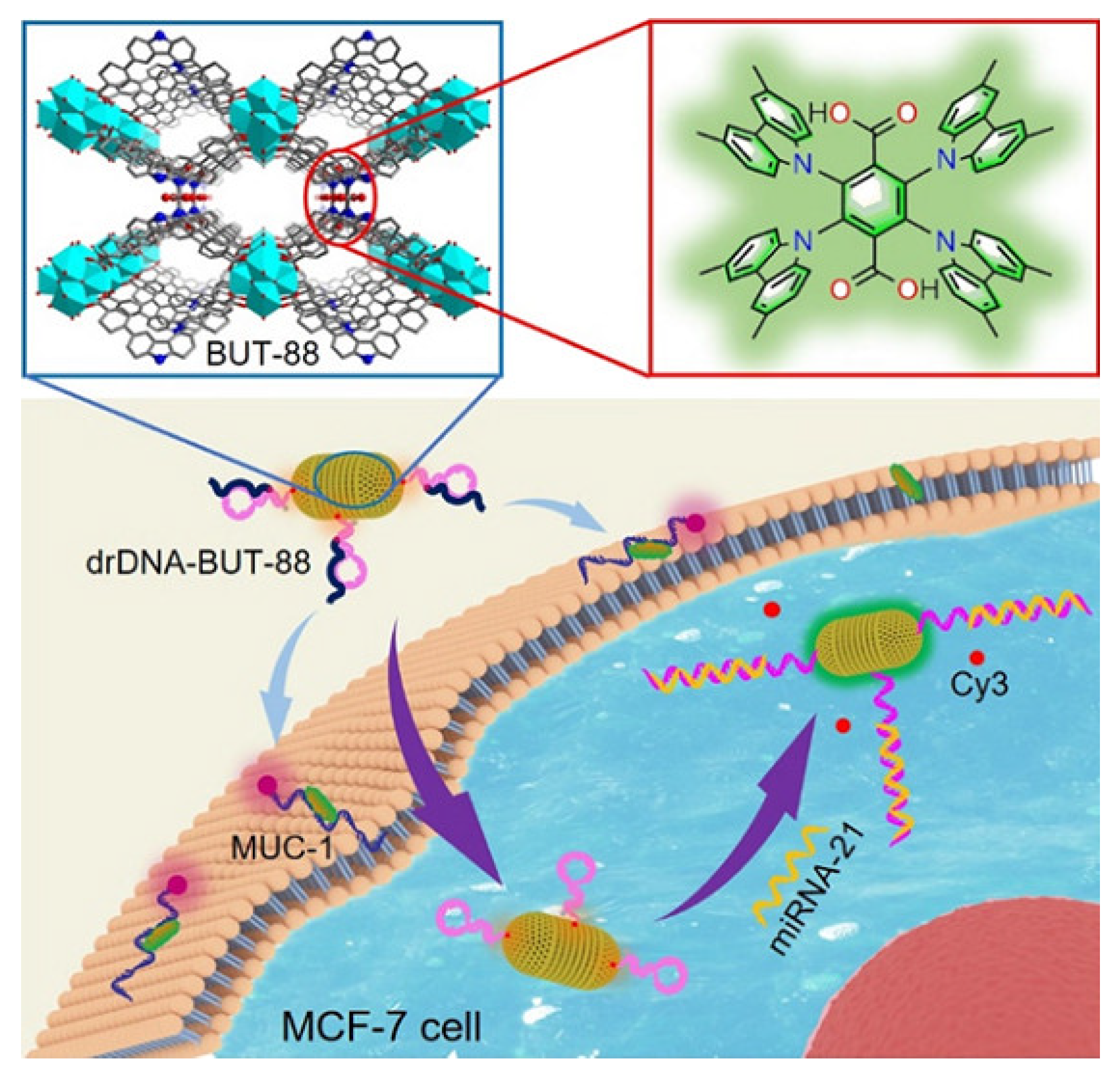
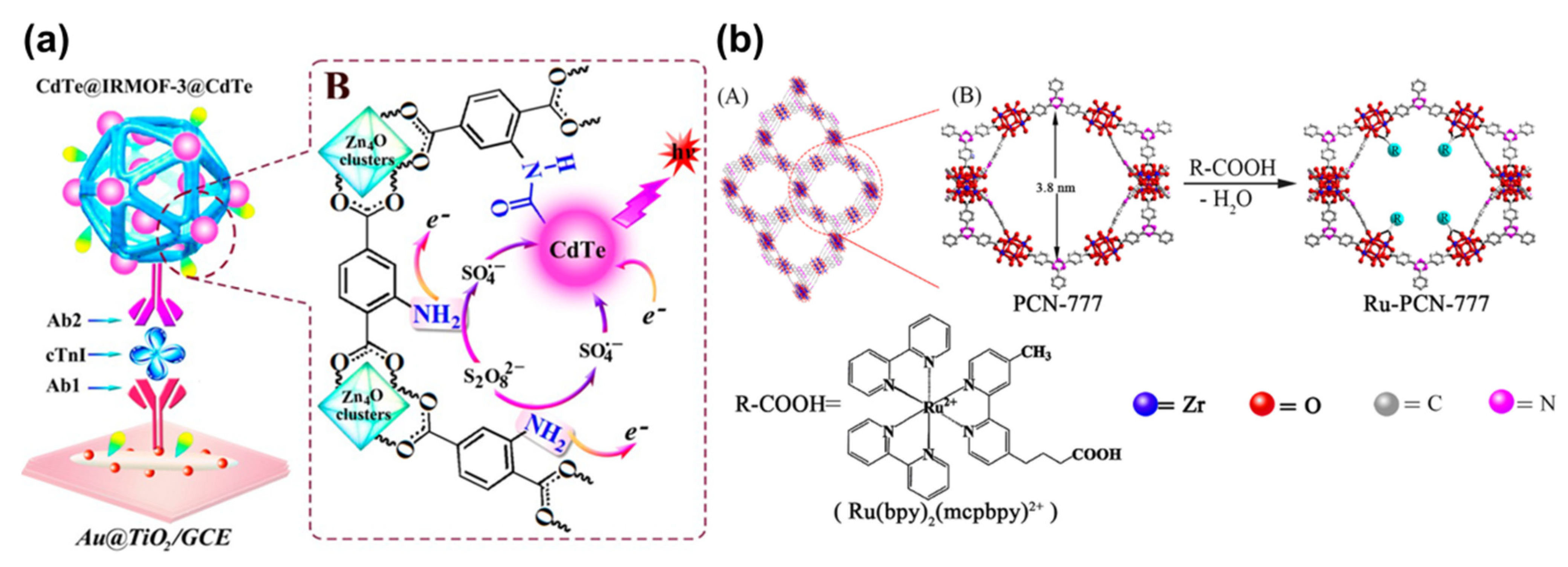

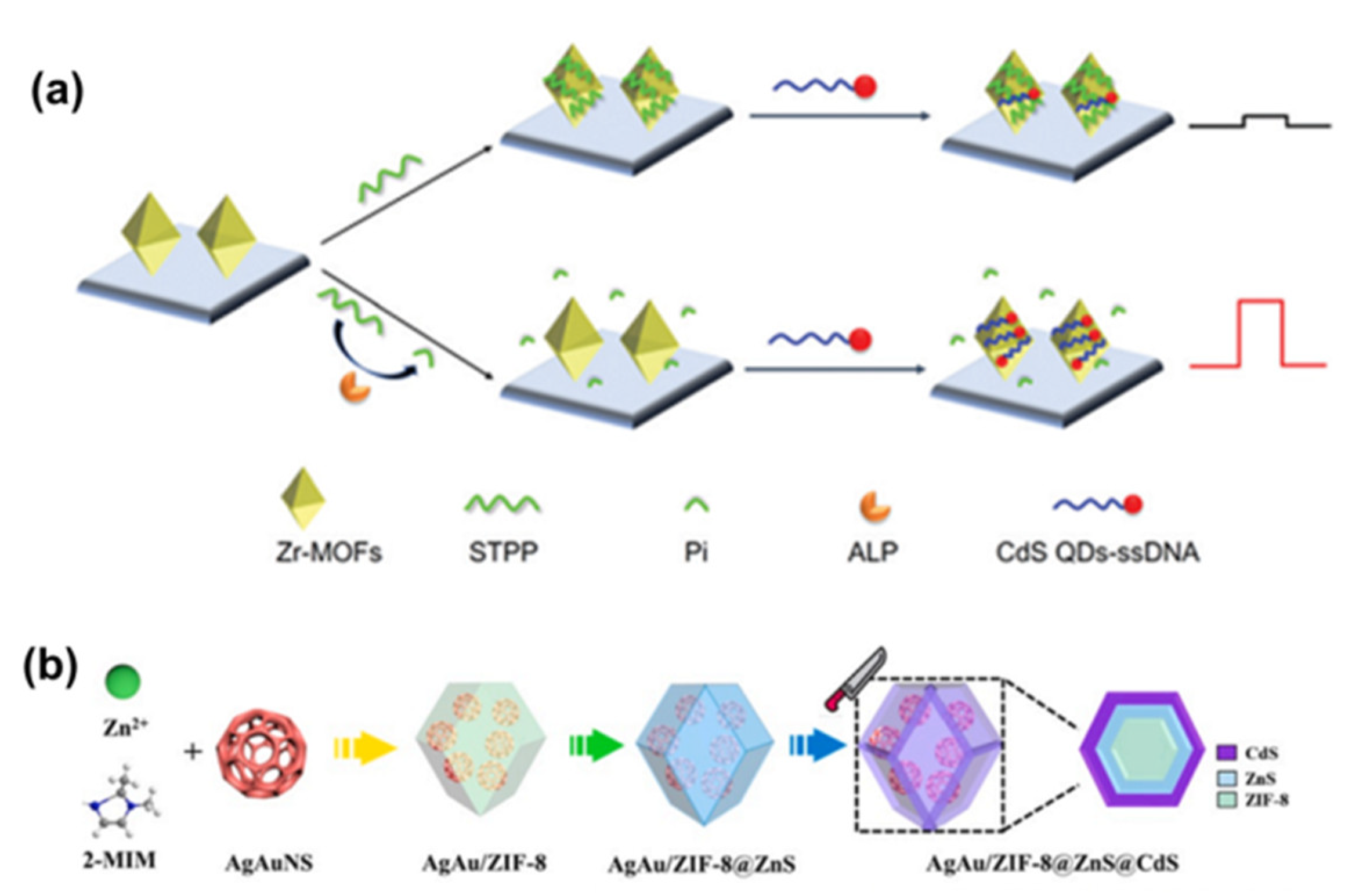
| Linkers | Metal Ions | LMOF | Ref. |
|---|---|---|---|
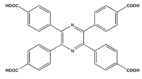 | Zn2+ |  Zn-TCPP | [38] |
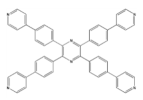 | Zn2+ | 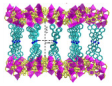 Zn3(TPyTPP)0.5(BDC)3 | [39] |
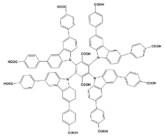 | Zr4+ |  BUT-88 | [40] |
 | Zr4+ |  MOF-525 | [41] |
 | Zr4+ |  PCN-222 | [42] |
 | Zr4+ |  Zr12-adb | [43] |
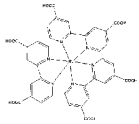 | Zn2+ |  Ru-MOF | [44] |
 | Zn2+ | 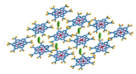 Ru-MOF | [45] |
| MOFs | Analytes | Methods | Limit of Detection | Samples | Ref. |
|---|---|---|---|---|---|
| Sm-MOF | Carcinoembryonic antigen | Electrochemistry | 0.001 U mL−1 | Human serum | [103] |
| Zr-MOF | Prostate specific antigen | Photoelectrochemistry | 0.2 pg mL−1 | Human serum | [104] |
| CD-MOF@ Ru(bpy)32+ | Cytokeratin 19 fragment antigen 21-1 | Electrochemiluminescence | 0.006 ng mL−1 | A549 lung cancer cells | [98] |
| His-MIL-101 | Metallothioneins | Colorimetry | 10.49 nM | Human serum | [79] |
| Zr12-adb MOF | Mucin 1 | Electrochemiluminescence | 0.25 fg mL−1 | Human serum | [43] |
| RuMOFNSs | Cardiac troponin I | Electrochemiluminescence | 0.48 fg mL−1 | Human serum | [45] |
| MIL-53(Fe) | Alkaline phosphatase | Colorimetry | 0.02 U L−1 | human plasma | [80] |
| Ru-PEI-L-lys-ZIF-8 | thrombin | Electrochemiluminescence | 0.02 aM | Serum | [26] |
| NH2-MIL-88 (Fe) | Neuron-specific enolase | Electrochemiluminescence | 31.6 fg mL−1 | Human serum | [28] |
| MOF-818 | Phosphopeptide-S | Colorimetry | 20 ng mL−1 | - | [87] |
| Cu-MOF | Amino-terminal pro-B-type natriuretic peptide | Electrochemistry | 0.33 fg mL−1 | Serum | [105] |
| Uio-66-NH2 | DNA | Fluorescence | 20 fM | - | [106] |
| ZnMOF(Ru) | Breast cancer 1 gene | Electrochemiluminescence | 0.71 fM | Human serum | [107] |
| Ce-MOF | Human telomeric DNA | Fluorescence | 665 pM | - | [108] |
| MOF-525 | miR-92a-3p | Fluorescence | 0.03 pM | Human serum | [41] |
| Ru-MOF | miRNA-141 | Electrochemiluminescence | 0.3 fM | Human serum | [50] |
| CoNi-MOF | miRNA-126 | Electrochemistry | 0.14 fM | Cancer cells | [59] |
| Ru@MIL-101(Al)–NH2 | miRNA-21 | Electrochemiluminescence | 4 fM | MCF-7 cells | [90] |
| Fe-MIL-88 | Zika virus RNA | Electrochemiluminescence | 0.1 nM | Zika virus | [109] |
| Tb MOF | Heparin and chondroitin sulfate | Fluorescence and colorimetry | 0.018 μM, 0.167 μg mL−1 and 0.089 μM, 3.966 μg mL−1 | Human serum | [110] |
| Cu-MOF | Lipopolysaccharide | Electrochemistry | 0.29 pg mL−1 | Tap water | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Huang, Y.; Chen, X. Recent Advances in Metal-Organic Frameworks for Biomacromolecule Sensing. Chemosensors 2022, 10, 412. https://doi.org/10.3390/chemosensors10100412
Lin Y, Huang Y, Chen X. Recent Advances in Metal-Organic Frameworks for Biomacromolecule Sensing. Chemosensors. 2022; 10(10):412. https://doi.org/10.3390/chemosensors10100412
Chicago/Turabian StyleLin, Yanna, Yong Huang, and Xuwei Chen. 2022. "Recent Advances in Metal-Organic Frameworks for Biomacromolecule Sensing" Chemosensors 10, no. 10: 412. https://doi.org/10.3390/chemosensors10100412
APA StyleLin, Y., Huang, Y., & Chen, X. (2022). Recent Advances in Metal-Organic Frameworks for Biomacromolecule Sensing. Chemosensors, 10(10), 412. https://doi.org/10.3390/chemosensors10100412







