Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.1.1. Materials
2.1.2. Instruments and Data Analysis
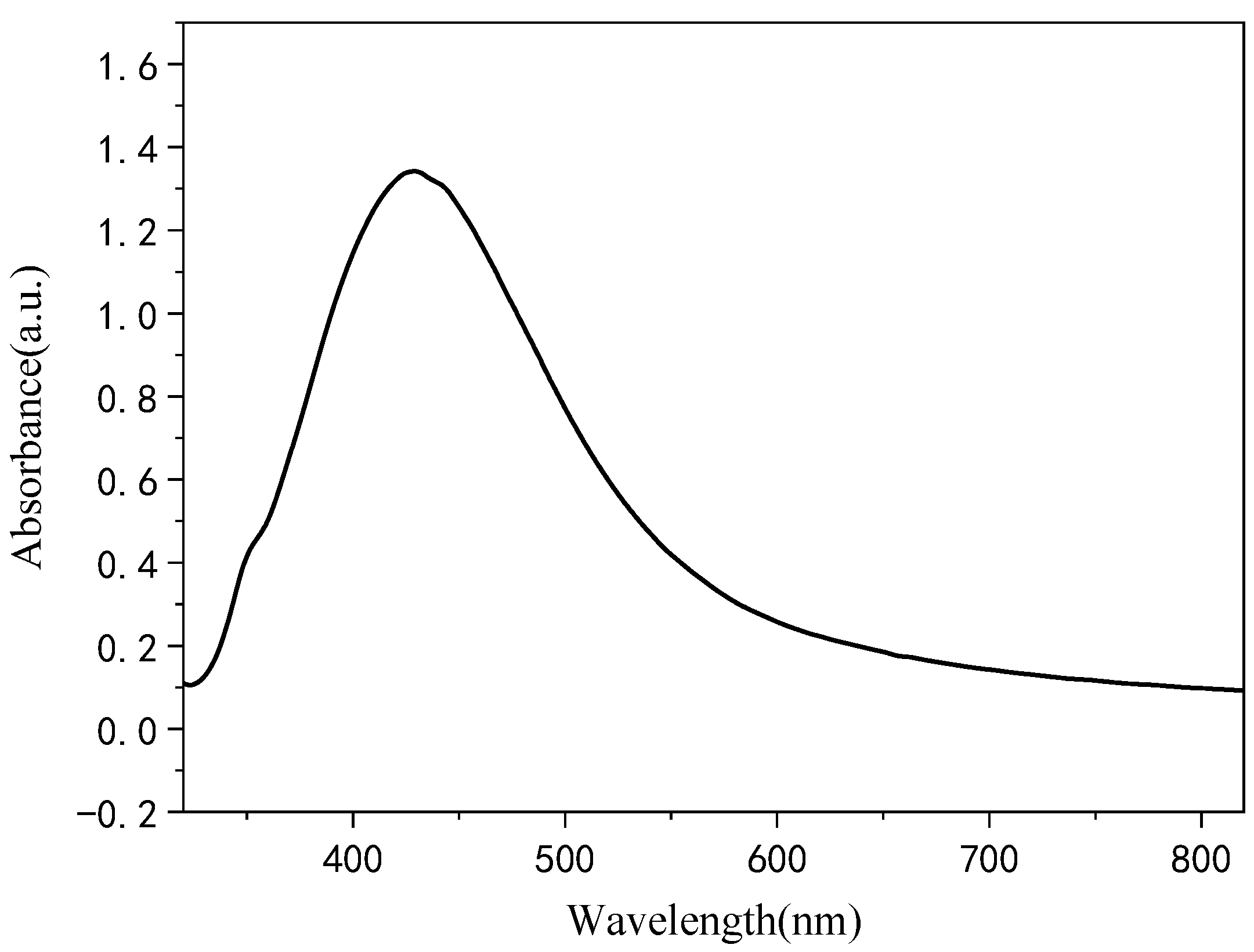
2.2. Preparation of Ag NPs
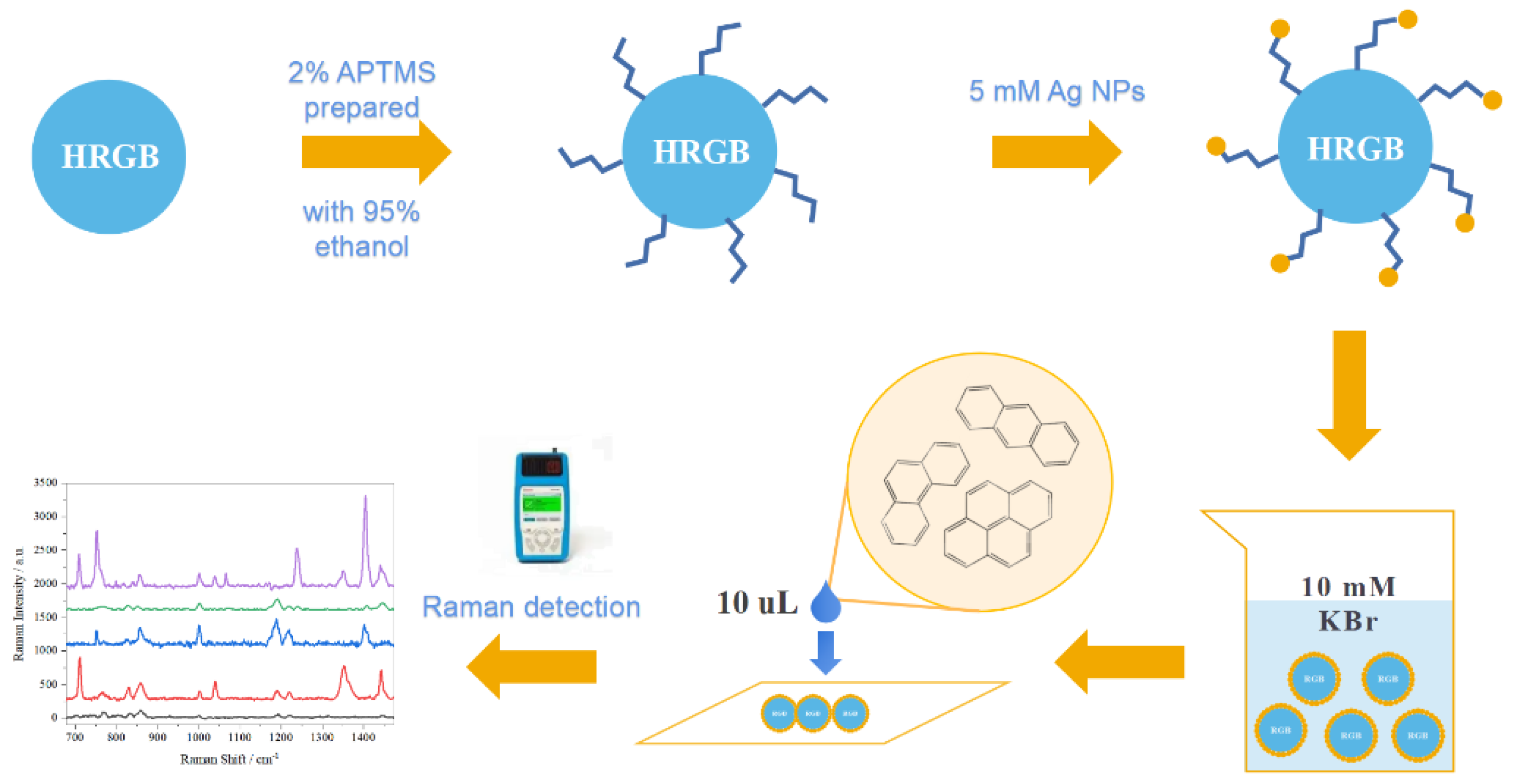
2.3. Preparation of HRGB-SERS Substrate
2.3.1. Preparation of APTMS Sol–Gel Solution
2.3.2. Deposition of Ag NPs
2.4. Modification of HRGB-SERS Substrate
2.5. Stability of HRGB-SERS Substrate
2.6. SERS Detection of Water Samples
3. Results and Discussion
3.1. Improvement of HRGB-SERS Substrate Preparation
3.2. Modification of HRGB-SERS Substrate
3.3. Stability of HRGB-SERS Substrate
3.4. Application of RGB-SERS Substrate to Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jesus, F.; Pereira, J.L.; Campos, I.; Santos, M.; Re, A.; Keizer, J.; Nogueira, A.; Goncalves, F.J.M.; Abrantes, N.; Serpa, D. A review on polycyclic aromatic hydrocarbons distribution in freshwater ecosystems and their toxicity to benthic fauna. Sci. Total Environ. 2022, 820, 153282. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, M.; Rykowska, I.; Wolski, R.; Andrzejewski, P. Polycyclic Aromatic Hydrocarbons (PAHs) and their Derivatives (O-PAHs, N-PAHs, OH-PAHs): Determination in Suspended Particulate Matter (SPM)—A Review. Environ. Process. 2021, 9, 2. [Google Scholar] [CrossRef]
- Dat, N.D.; Chang, M.B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Zhou, Q.; Zhao, X.; Sun, Z. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard Mater. 2018, 343, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Y.; Han, C.; Fang, H.; Weng, J.; Shu, X.; Pan, Y.; Ma, L. Polycyclic aromatic hydrocarbons in surface waters from the seven main river basins of China: Spatial distribution, source apportionment, and potential risk assessment. Sci. Total Environ. 2021, 752, 141764. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A review on occurrence and risk of polycyclic aromatic hydrocarbons (PAHs) in lakes of China. Sci. Total Environ. 2019, 651, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.M.; Melymuk, L.; Bharat, G.K.; Pribylova, P.; Sanka, O.; Klanova, J.; Nizzetto, L. Spatial gradients of polycyclic aromatic hydrocarbons (PAHs) in air, atmospheric deposition, and surface water of the Ganges River basin. Sci. Total Environ. 2018, 627, 1495–1504. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, D.; Bharti, S.K.; Kumar, A.; Pandey, A.K.; Ana, G.R.; Verma, A.; Khan, A.H.; Patel, D.K.; Mudiam, M.K.; Jain, S.K.; et al. Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int. J Hyg. Environ. Health 2013, 216, 553–565. [Google Scholar] [CrossRef]
- Groner, A.R.M.; Myrick, M.L. Identification of Major Water-soluble Fluorescent Components of Some Petrochemicals. Pergamon 2001, 42, 935–941. [Google Scholar] [CrossRef]
- Valcarcel, S.C.; Gallego, M. Sample screening systems in analytical chemistry. Trends Anal. Chem. 1999, 18, 685–694. [Google Scholar] [CrossRef]
- Chuang, J.C.; Van Emon, J.M.; Chou, Y.-L.; Junod, N.; Finegold, J.K.; Wilson, N.K. Comparison of immunoassay and gas chromatography–mass spectrometry for measurement of polycyclic aromatic hydrocarbons in contaminated soil. Anal. Chim. Acta 2003, 486, 31–39. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, D.L.; Zou, Z.X.; Li, Y.Q. A novel approach for simultaneous determination of polycyclic aromatic hydrocarbons by Shpol’skii non-linear variable-angle synchronous fluorescence spectrometry. Talanta 2007, 71, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Yang, Y.; Korenaga, T. Determination of Polynuclear Aromatic Hydrocarbons (PAHs) in Environmental Samples by Capillary Electrophoresis. Anal. Sci. 1997, 13, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Tommasini, M.; Lucotti, A.; Alfe, M.; Ciajolo, A.; Zerbi, G. Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Fähnrich, K.A.; Pravda, M.; Guilbault, G.G. Immunochemical Detection of Polycyclic Aromatic Hydrocarbons (PAHs). Anal. Lett. 2002, 35, 1269–1300. [Google Scholar] [CrossRef]
- Kang, R.H.; Wang, Y.S.; Yang, H.M.; Li, G.R.; Tan, X.; Xue, J.H.; Zhang, J.Q.; Yuan, Y.K.; Shi, L.F.; Xiao, X.L. Rapid simultaneous analysis of 1-hydroxypyrene, 2-hydroxyfluorene, 9-hydroxyphenanthrene, 1- and 2-naphthol in urine by first derivative synchronous fluorescence spectrometry using Tween-20 as a sensitizer. Anal. Chim. Acta 2010, 658, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Marlow, M.; Hurtubise, R.J. Liquid–liquid–liquid microextraction for the enrichment of polycyclic aromatic hydrocarbon metabolites investigated with fluorescence spectroscopy and capillary electrophoresis. Anal. Chim. Acta 2004, 526, 41–49. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta. 2020, 1097, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Y.; Wang, L.; Li, X.; Liu, M.; Li, G. Facile Detection and Quantification of Acetamiprid Using a Portable Raman Spectrometer Combined with Self-Assembled Gold Nanoparticle Array. Chemosensors 2021, 9, 327. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-enhanced Raman spectroscopy for on-site analysis: A review of recent developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kuppe, C.; Valev, V.K.; Fu, H.; Zhang, L.; Chen, J. Surface-Enhanced Raman Spectroscopy: A Facile and Rapid Method for the Chemical Component Study of Individual Atmospheric Aerosol. Environ. Sci. Technol. 2017, 51, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.V.; Liu, Y.; Huang, Y.; Zhao, Y. A high sensitive fiber SERS probe based on silver nanorod arrays. Opt. Express 2007, 15, 12230–12239. [Google Scholar] [CrossRef]
- Li, D.; Qu, L.; Zhai, W.; Xue, J.; Fossey, J.S.; Long, Y. Facile on-site detection of substituted aromatic pollutants in water using thin layer chromatography combined with surface-enhanced Raman spectroscopy. Environ. Sci. Technol. 2011, 45, 4046–4052. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Jones, R.R.; Li, K.; Xu, G.; Cheng, H.; Feng, Y.; Valev, V.K.; Zhang, L. Sensing pH of individual microdroplet by combining SERS and indicator paper. Sens. Actuators B Chem. 2021, 346, 130521. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, P.; Liu, X.; Sun, X.; Li, H.; Lin, M. Detection of Pesticides in Fruits by Surface-Enhanced Raman Spectroscopy Coupled with Gold Nanostructures. Food Bioprocess Technol. 2012, 6, 710–718. [Google Scholar] [CrossRef]
- He, S.; Xie, W.; Zhang, W.; Zhang, L.; Wang, Y.; Liu, X.; Liu, Y.; Du, C. Multivariate qualitative analysis of banned additives in food safety using surface enhanced Raman scattering spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1092–1099. [Google Scholar] [CrossRef]
- Chou, A.; Jaatinen, E.; Buividas, R.; Seniutinas, G.; Juodkazis, S.; Izake, E.L.; Fredericks, P.M. SERS substrate for detection of explosives. Nanoscale 2012, 4, 7419–7424. [Google Scholar] [CrossRef]
- Emamian, S.; Eshkeiti, A.; Narakathu, B.B.; Avuthu, S.G.R.; Atashbar, M.Z. Gravure printed flexible surface enhanced Raman spectroscopy (SERS) substrate for detection of 2,4-dinitrotoluene (DNT) vapor. Sens. Actuators B Chem. 2015, 217, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Gillibert, R.; Huang, J.Q.; Zhang, Y.; Fu, W.L.; Lamy de la Chapelle, M. Explosive detection by Surface Enhanced Raman Scattering. Trends Anal. Chem. 2018, 105, 166–172. [Google Scholar] [CrossRef]
- Liyanage, T.; Rael, A.; Shaffer, S.; Zaidi, S.; Goodpaster, J.V.; Sardar, R. Fabrication of a self-assembled and flexible SERS nanosensor for explosive detection at parts-per-quadrillion levels from fingerprints. Analyst 2018, 143, 2012–2022. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Puebla, R.A.; Liz-Marzan, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Feuillie, C. Raman spectroscopy in biomedicine: New advances in SERRS cancer imaging. Ann. Transl. Med. 2015, 3, 347. [Google Scholar] [CrossRef]
- Shumskaya, A.; Kozhina, E.; Bedin, S.; Andreev, S.; Kulesh, E.; Rogachev, A.; Yarmolenko, M.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; et al. Detection of Polynitro Compounds at Low Concentrations by SERS Using Ni@Au Nanotubes. Chemosensors 2022, 10, 306. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Etchegoin. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Zhou, S.-N.; Wang, D.-M.; Gong, Z.-J.; Fan, M.-K. Free-Standing Membrane Liquid-State Platform for SERS-Based Determination of Norfloxacin in Environmental Samples. J. Anal. Test. 2021, 5, 217–224. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Zhao, H.; Sun, J.; He, Z.; Shan, W.; Li, G.; Liu, J.; Jiang, G. Ligand-Sharing-Mediated Synthesis of Intermetallic FeM Clusters Embedded in Ultrathin γ-Fe2O3 Nanosheets. Adv. Funct. Mater. 2019, 30, 1906995. [Google Scholar] [CrossRef]
- Cai, L.J.; Wang, M.; Hu, Y.; Qian, D.J.; Chen, M. Synthesis and mechanistic study of stable water-soluble noble metal nanostructures. Nanotechnology 2011, 22, 285601. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.Y.; Tian, Z.; Shang, Y.; Xing, J.; Jiang, Y.; Zeng, J. Ag Nanoparticles with Ultrathin Au Shell-Based Lateral Flow Immunoassay for Colorimetric and SERS Dual-Mode Detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Huang, Z.; Xu, F.; Sun, D.W. Two-dimensional self-assembled Au-Ag core-shell nanorods nanoarray for sensitive detection of thiram in apple using surface-enhanced Raman spectroscopy. Food Chem. 2021, 343, 128548. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, T.; Pu, H.; Sun, D.-W. Functionalization techniques for improving SERS substrates and their applications in food safety evaluation: A review of recent research trends. Trends Food Sci. Technol. 2018, 72, 162–174. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Guo, S.; Jin, S.; Park, E.; Chen, L.; Jung, Y.M. Charge transfer study for semiconductor and semiconductor/metal composites based on surface-enhanced Raman scattering. Bull. Korean Chem. Soc. 2021, 42, 1411–1418. [Google Scholar] [CrossRef]
- Song, G.; Gong, W.; Cong, S.; Zhao, Z. Ultrathin Two-Dimensional Nanostructures: Surface Defects for Morphology-Driven Enhanced Semiconductor SERS. Angew Chem. Int. Ed. Engl. 2021, 60, 5505–5511. [Google Scholar] [CrossRef]
- Eshkeiti, A.; Narakathu, B.B.; Reddy, A.S.G.; Moorthi, A.; Atashbar, M.Z.; Rebrosova, E.; Rebros, M.; Joyce, M. Detection of heavy metal compounds using a novel inkjet printed surface enhanced Raman spectroscopy (SERS) substrate. Sens. Actuators B Chem. 2012, 171-172, 705–711. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, Z.; Hu, J.; Cheng, F.; Wang, C.; Tang, C.; Lin, J.; Brolo, A.G.; Zhan, H. Ag decorated sandpaper as flexible SERS substrate for direct swabbing sampling. Mater. Lett. 2014, 133, 57–59. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Hui, B.; Gong, Z.; Fan, M. Halogen ion-modified silver nanoparticles for ultrasensitive surface-enhanced Raman spectroscopy detection of polycyclic aromatic hydrocarbons. Luminescence 2022, 37, 1541–1546. [Google Scholar] [CrossRef]
- Gong, X.; Liao, X.; Li, Y.; Cao, H.; Zhao, Y.; Li, H.; Cassidy, D.P. Sensitive detection of polycyclic aromatic hydrocarbons with gold colloid coupled chloride ion SERS sensor. Analyst 2019, 144, 6698–6705. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Preparation of Thiol Modified Fe3O4@Ag Magnetic SERS Probe for PAHs Detection and Identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.T.; Gu, H.X.; Xue, P.F.; Qin, L.X.; Han, S. A wearable screen-printed SERS array sensor on fire-retardant fibre gloves for on-site environmental emergency monitoring. Anal. Methods 2022, 14, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, X.; Zhang, Q.; Ren, X.; Jiang, L.; Li, D.; Deng, W.; Liu, H. Facile in situ synthesis of core–shell MOF@Ag nanoparticle composites on screen-printed electrodes for ultrasensitive SERS detection of polycyclic aromatic hydrocarbons. J. Mater. Chem. A 2019, 7, 14108–14117. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Y.; Jiao, A.; Ma, H.; Wang, C.; Zheng, L.; Li, S.; Chen, M. Synergistic double laser beam-boosted liquid-NIR-SERS for ultralow detection of non-adsorptive polycyclic aromatic hydrocarbons in lake water. Nanophotonics 2022, 11, 2875–2889. [Google Scholar] [CrossRef]
- Yuan, J.; Emura, K.; Farnham, C. Geometrical-optics analysis of reflective glass beads applied to building coatings. Sol. Energy 2015, 122, 997–1010. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Gong, Z.; Wu, W.; Fan, M.; Wang, D.; Brolo, A.G. Detection of Buried Explosives Using a Surface-Enhanced Raman Scattering (SERS) Substrate Tailored for Miniaturized Spectrometers. ACS Sens. 2020, 5, 2933–2939. [Google Scholar] [CrossRef]
- Szlag, V.M.; Rodriguez, R.S.; He, J.; Hudson-Smith, N.; Kang, H.; Le, N.; Reineke, T.M.; Haynes, C.L. Molecular Affinity Agents for Intrinsic Surface-Enhanced Raman Scattering (SERS) Sensors. ACS Appl. Mater. Interfaces 2018, 10, 31825–31844. [Google Scholar] [CrossRef] [PubMed]
- Sadrara, M.; Khorrami, M.K.; Darian, J.T.; Garmarudi, A.B. Fabrication of highly mesoporous ZSM-48 zeolite by anionic surfactant-organosilane system for catalytic conversion of methanol to gasoline. Solid State Sci. 2022, 128, 106888. [Google Scholar] [CrossRef]
- Andrade, G.F.; Fan, M.; Brolo, A.G. Multilayer silver nanoparticles-modified optical fiber tip for high performance SERS remote sensing. Biosens. Bioelectron. 2010, 25, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, W.; Wang, D.; Gong, Z.; Fan, M. Boosting bacteria differentiation efficiency with multidimensional surface-enhanced Raman scattering: The example of Bacillus cereus. Luminescence 2022, 37, 1145–1151. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Wang, J.; Zou, Y.; Liu, T.; Zhang, Y.; Liu, G.; Tian, Z. Trace detection of polycyclic aromatic hydrocarbons in environmental waters by SERS. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 234, 118250. [Google Scholar] [CrossRef]
- Lu, J.; Cai, Z.; Zou, Y.; Wu, D.; Wang, A.; Chang, J.; Wang, F.; Tian, Z.; Liu, G. Silver Nanoparticle-Based Surface-Enhanced Raman Spectroscopy for the Rapid and Selective Detection of Trace Tropane Alkaloids in Food. ACS Appl. Nano Mater. 2019, 2, 6592–6601. [Google Scholar] [CrossRef]
- Fan, W.; Yang, S.; Zhang, Y.; Huang, B.; Gong, Z.; Wang, D.; Fan, M. Multifunctional Flexible SERS Sensor on a Fixate Gel Pad: Capturing, Derivation, and Selective Picogram Indirect Detection of Explosive 2,2’,4,4’,6,6’-Hexanitrostilbene. ACS Sens. 2020, 5, 3599–3606. [Google Scholar] [CrossRef] [PubMed]
- Hasi, W.-L.-J.; Lin, X.; Lou, X.-T.; Lin, S.; Yang, F.; Lin, D.-Y.; Lu, Z.-W. Chloride ion-assisted self-assembly of silver nanoparticles on filter paper as SERS substrate. Appl. Phys. A 2014, 118, 799–807. [Google Scholar] [CrossRef]
- Jones, C.L.; Bantz, K.C.; Haynes, C.L. Partition layer-modified substrates for reversible surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2009, 394, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.L.; Li, Y.T.; Li, D.W.; Xue, J.Q.; Fossey, J.S.; Long, Y.T. Humic acids-based one-step fabrication of SERS substrates for detection of polycyclic aromatic hydrocarbons. Analyst 2013, 138, 1523–1528. [Google Scholar] [CrossRef] [PubMed]

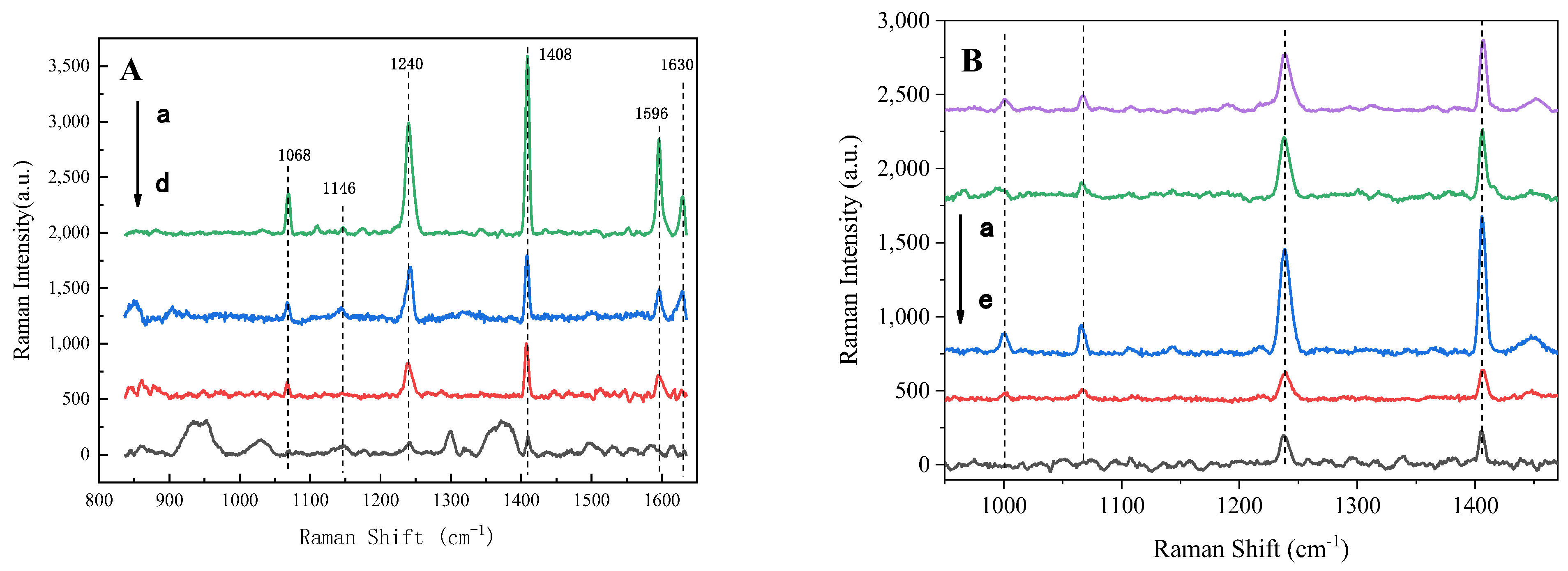
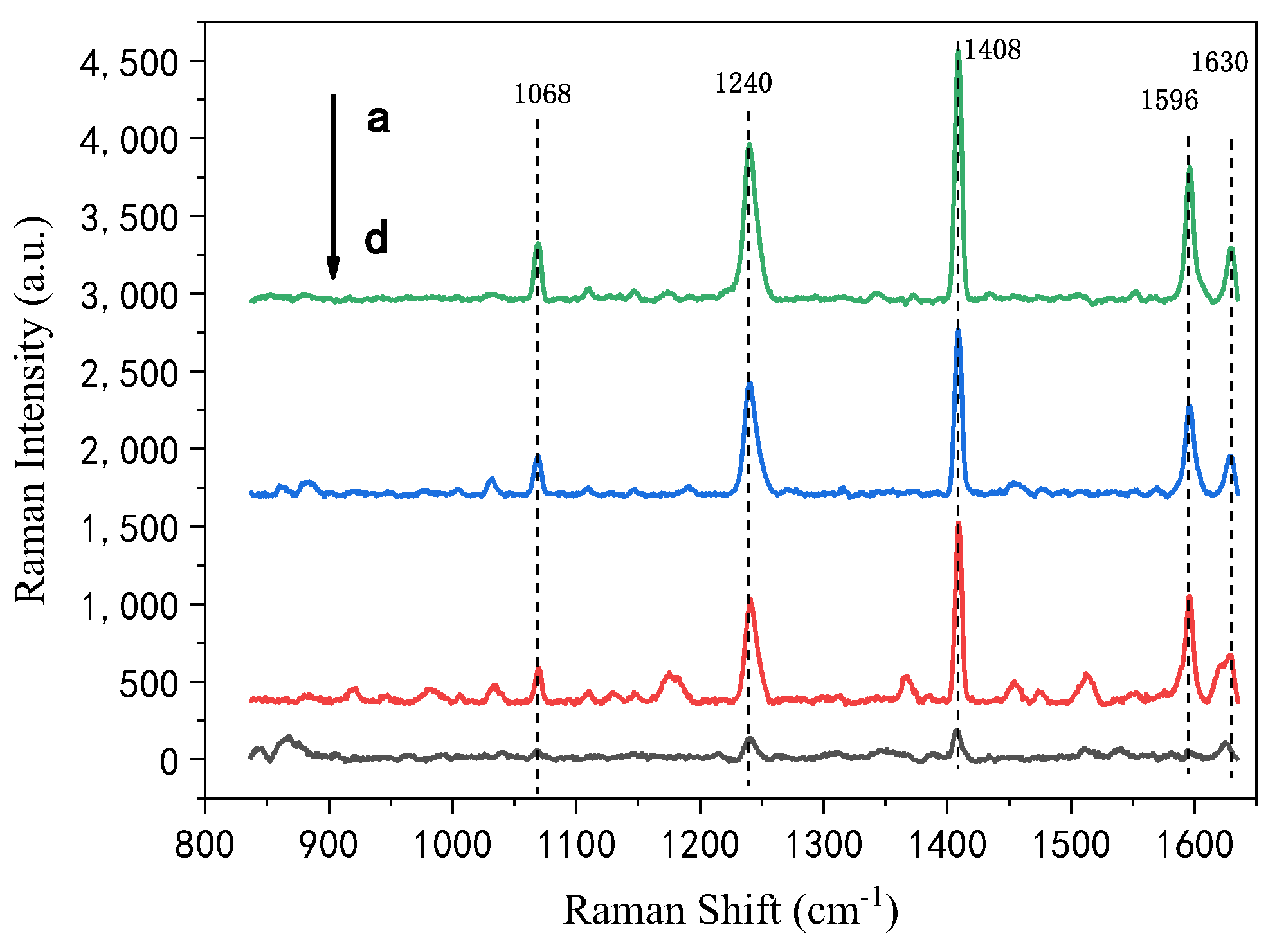
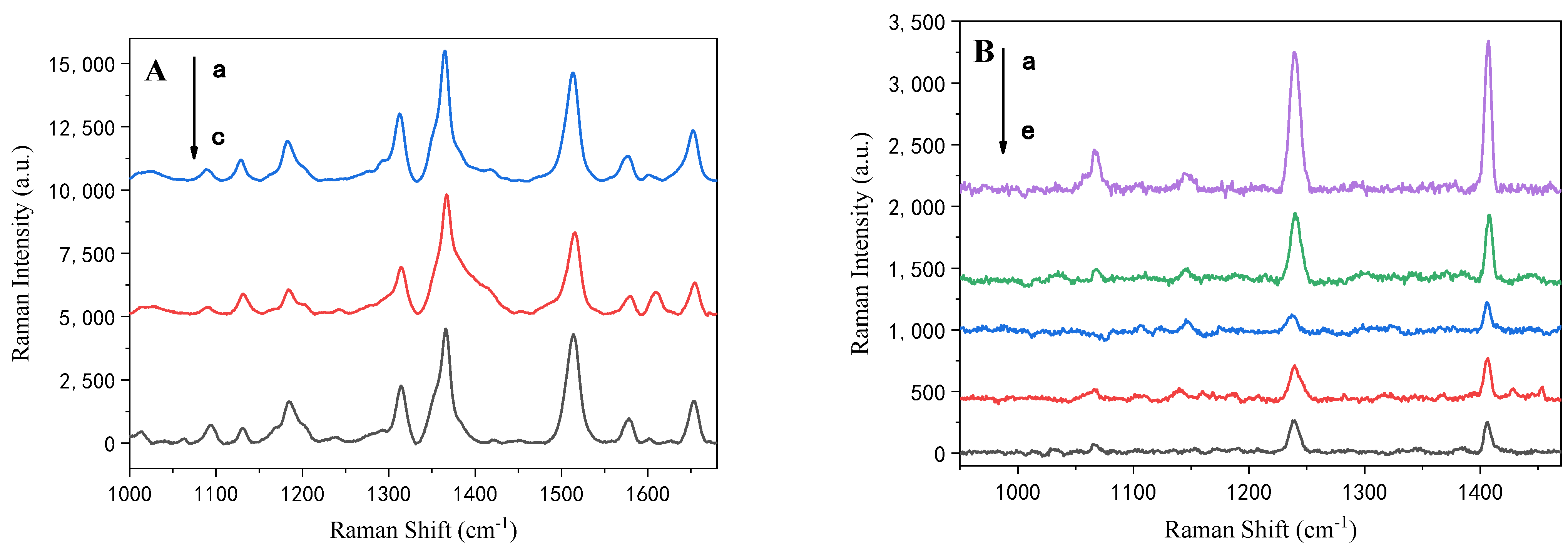
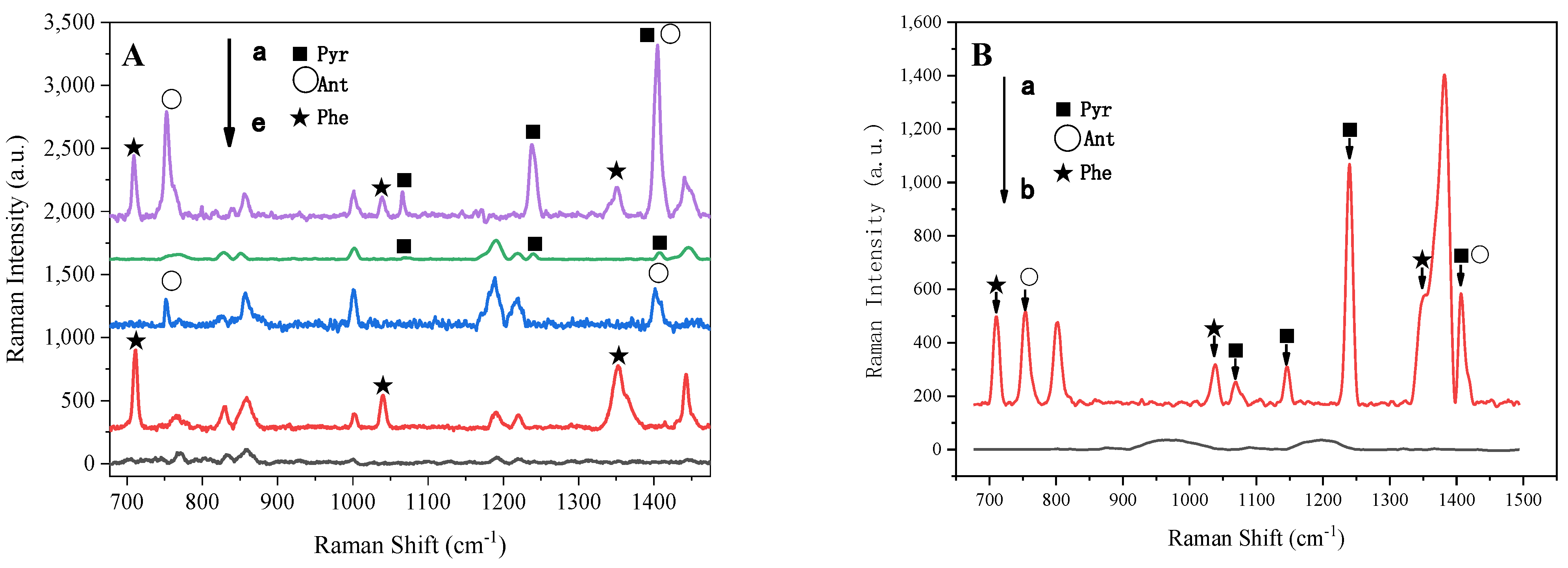
| PAHs | Band (cm−1) | Stretching Modes [65] |
|---|---|---|
| Phenanthrene | 711 | Skeletal deformation |
| 1037 | C–C stretching | |
| 1353 | H–C–C bending | |
| Anthracene | 752 | Para-disubstituted benzenes |
| 1042 | Ring stretching | |
| Pyrene | 1069 | C–H rocking |
| 1240 | C–H in-plane bending | |
| 1408 | C–C stretching |
| Temperature/°C | pH | Turbidity | Chrominance | Electrical Conductivity | COD/mg·L−1 |
|---|---|---|---|---|---|
| 20 | 8.50 | 27.0 | 22 | 135.5 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Hui, B.; Zhang, X.; Zhu, J.; Gong, Z.; Fan, M. Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water. Chemosensors 2022, 10, 406. https://doi.org/10.3390/chemosensors10100406
Wang D, Hui B, Zhang X, Zhu J, Gong Z, Fan M. Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water. Chemosensors. 2022; 10(10):406. https://doi.org/10.3390/chemosensors10100406
Chicago/Turabian StyleWang, Dongmei, Binyu Hui, Xueqi Zhang, Jingyi Zhu, Zhengjun Gong, and Meikun Fan. 2022. "Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water" Chemosensors 10, no. 10: 406. https://doi.org/10.3390/chemosensors10100406
APA StyleWang, D., Hui, B., Zhang, X., Zhu, J., Gong, Z., & Fan, M. (2022). Facile Preparation of Ag-NP-Deposited HRGB-SERS Substrate for Detection of Polycyclic Aromatic Hydrocarbons in Water. Chemosensors, 10(10), 406. https://doi.org/10.3390/chemosensors10100406






