Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration
Abstract
:1. Introduction
2. Evanescent Waveguide-Based Biosensors
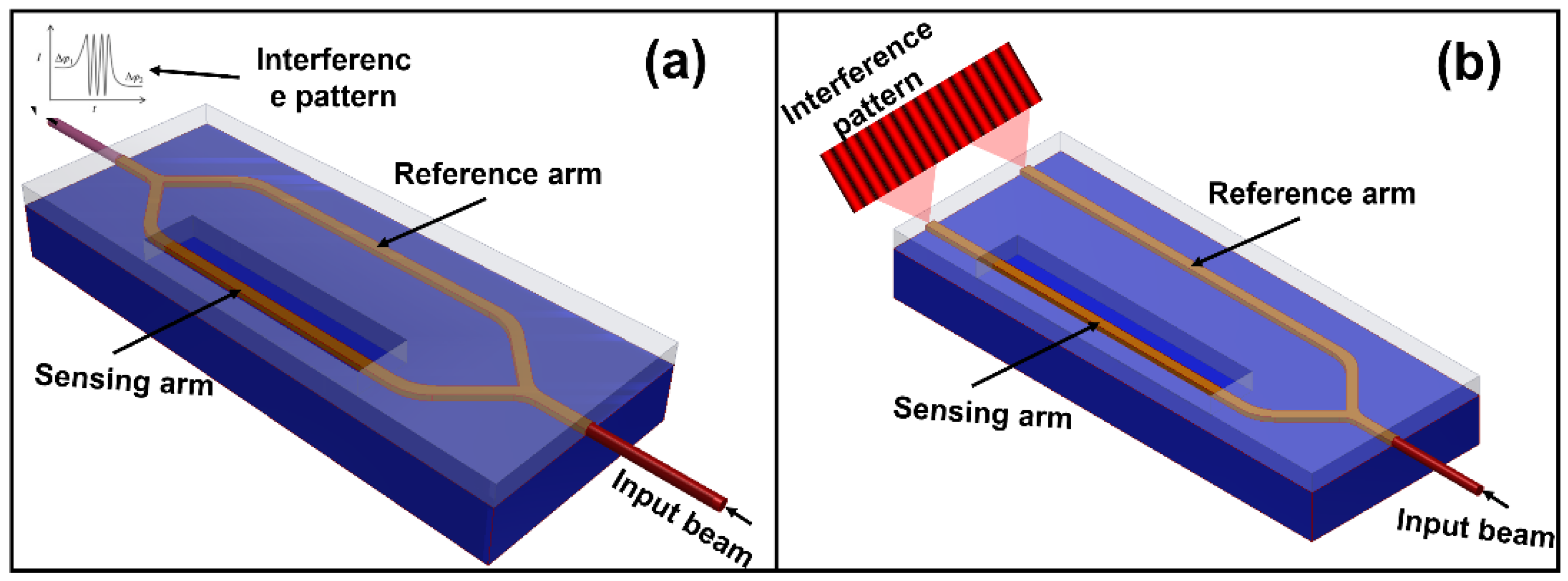
2.1. Interferometric Biosensors
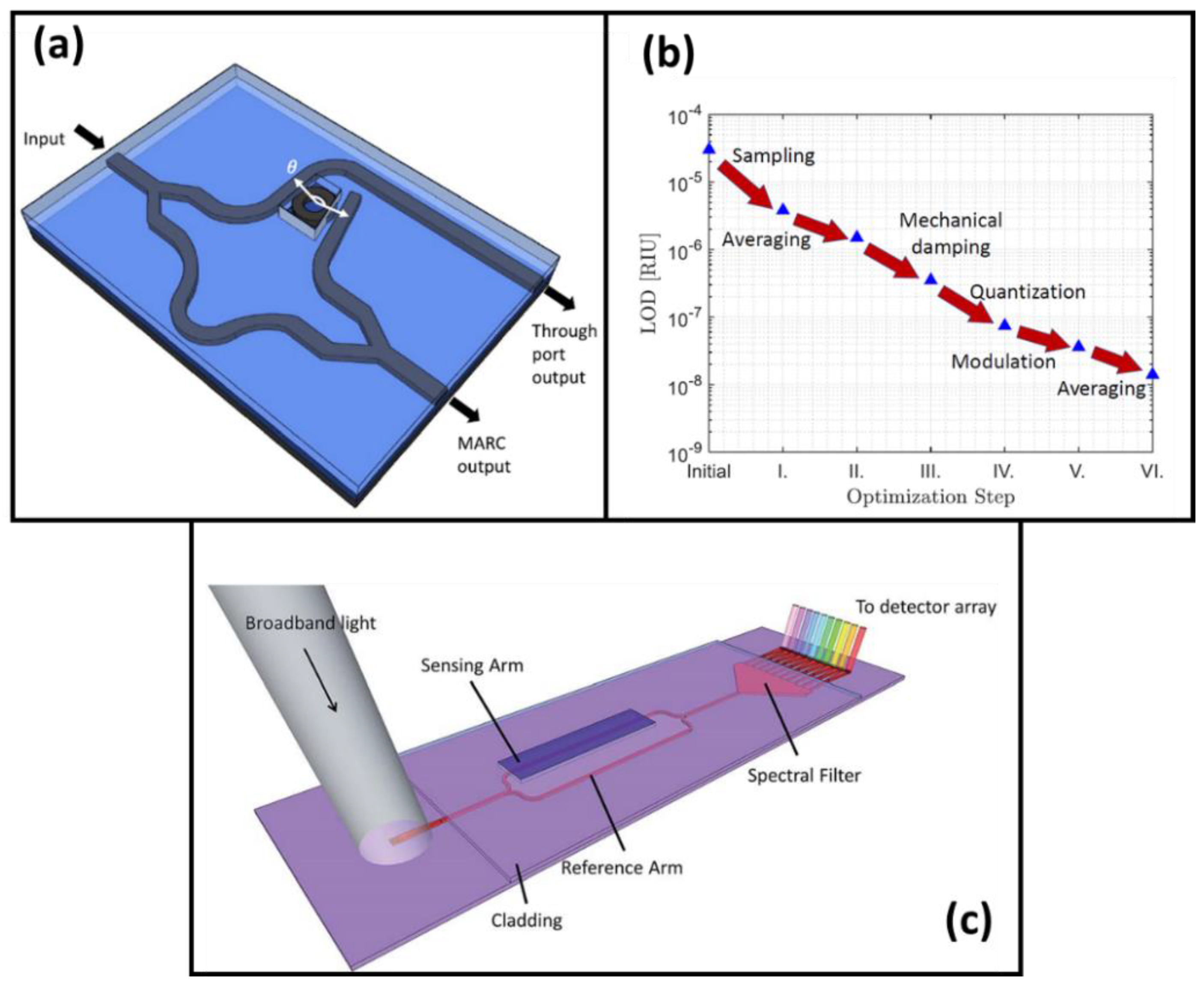
2.2. Ring Resonator Biosensors
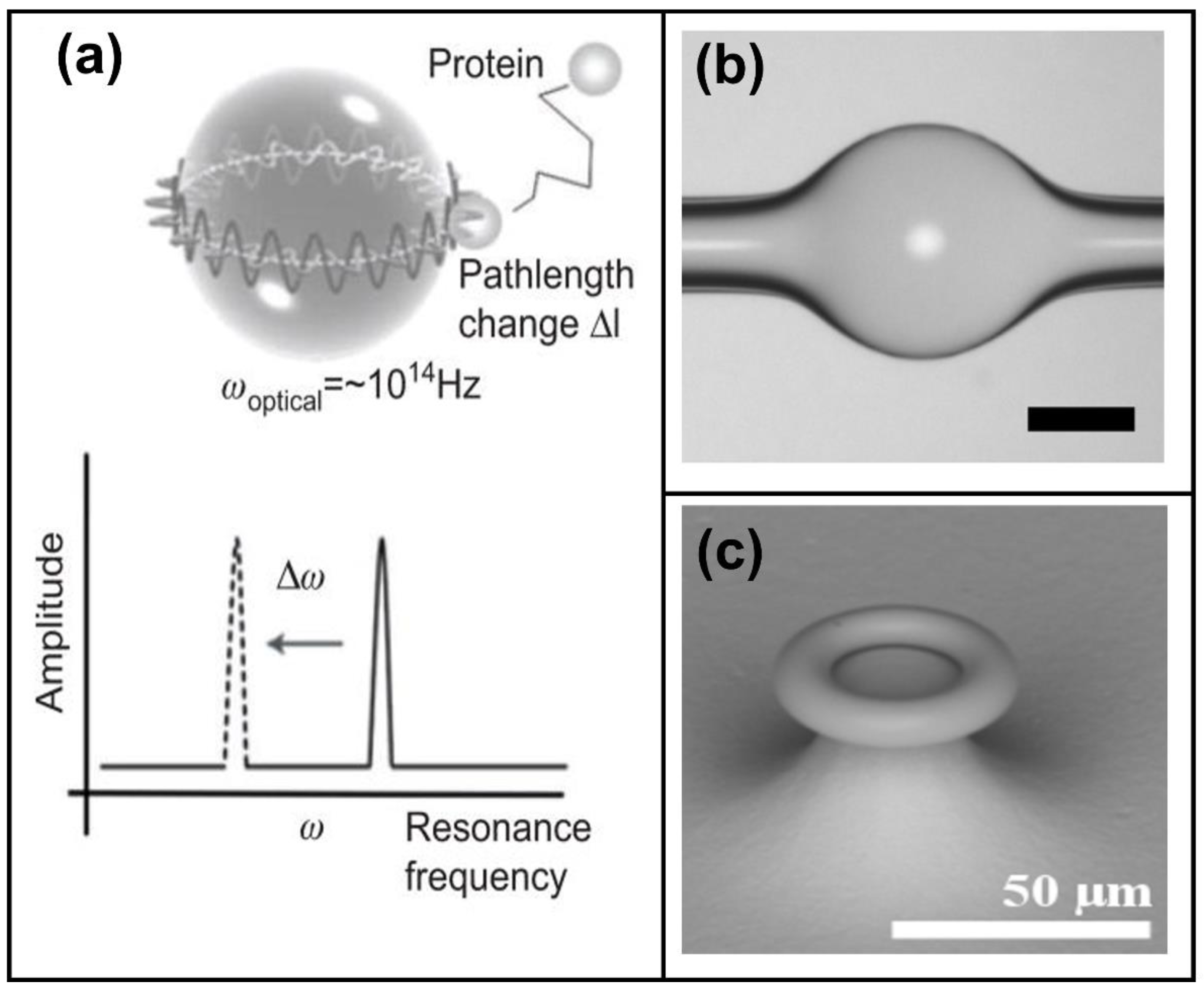
2.3. Photonic Crystal Biosensors
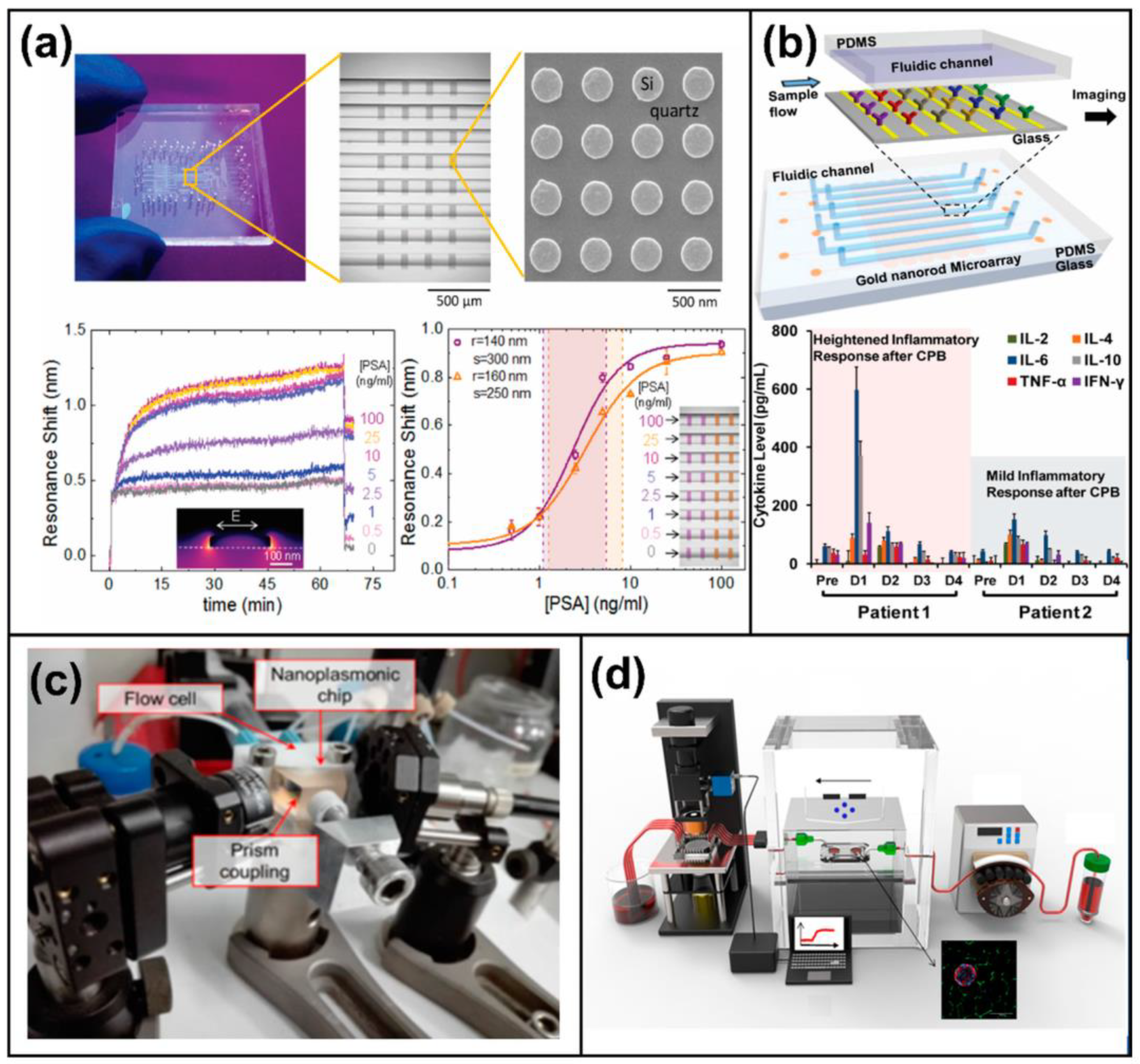
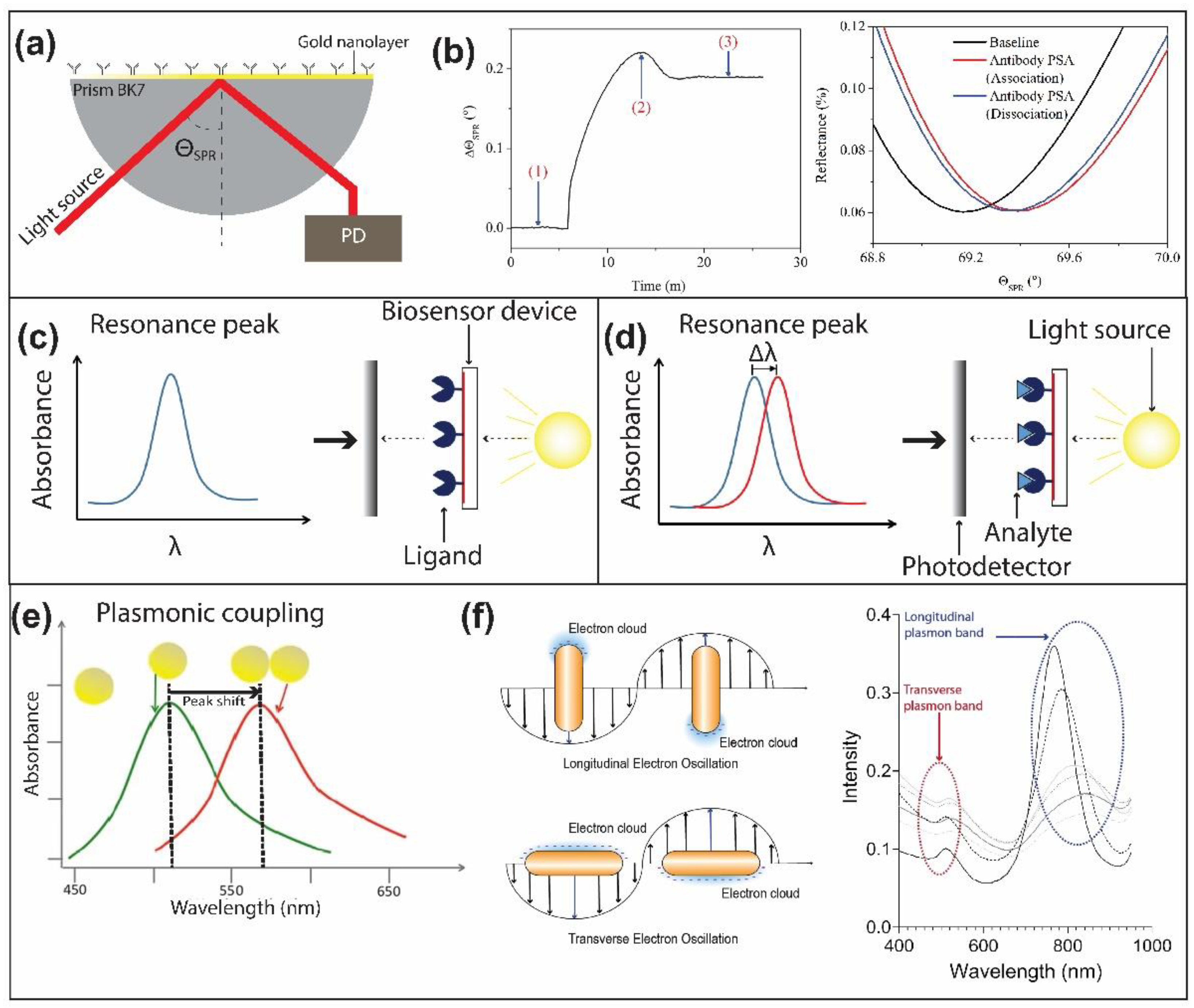
3. Plasmonic-Based Biosensors
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barceló, D. Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef]
- Rivet, C.; Lee, H.; Hirsch, A.; Hamilton, S.; Lu, H. Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 2011, 66, 1490–1507. [Google Scholar] [CrossRef]
- Zamani, M.; Dupaty, J.; Baer, R.C.; Kuzmanovic, U.; Fan, A.; Grinstaff, M.W.; Galagan, J.E.; Klapperich, C.M. Paper-Based Progesterone Sensor Using an Allosteric Transcription Factor. ACS Omega 2022, 7, 5804–5808. [Google Scholar] [CrossRef]
- Amín, N.; Torralba, A.S.; Álvarez-Diduk, R.; Afkhami, A.; Merkoçi, A. Lab in a Tube: Point-of-Care Detection of Escherichia coli. Anal. Chem. 2020, 92, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Temiz, Y.; Lovchik, R.D.; Kaigala, G.v.; Delamarche, E. Lab-on-a-chip devices: How to close and plug the lab? Microelectron. Eng. 2015, 132, 156–175. [Google Scholar] [CrossRef]
- De la Paz, E.; Barfidokht, A.; Rios, S.; Brown, C.; Chao, E.; Wang, J. Extended Noninvasive Glucose Monitoring in the Interstitial Fluid Using an Epidermal Biosensing Patch. Anal. Chem. 2021, 93, 12767–12775. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Lin, B.; Qiu, J.; Li, P.; Pepper, J.; Hugh, B. A plastic colorimetric resonant optical biosensor for multiparallel detection of label-free biochemical interactions. Sens. Actuators B Chem. 2002, 85, 219–226. [Google Scholar] [CrossRef]
- Qian, Y.; Zeng, X.; Gao, Y.; Li, H.; Kumar, S.; Gan, Q.; Cheng, X.; Bartoli, F.J. Intensity-modulated nanoplasmonic interferometric sensor for MMP-9 detection. Lab Chip 2019, 19, 1267–1276. [Google Scholar] [CrossRef]
- Liu, Q.; Shin, Y.; Kee, J.S.; Kim, K.W.; Mohamed Rafei, S.R.; Perera, A.P.; Tu, X.; Lo, G.-Q.; Ricci, E.; Colombel, M.; et al. Mach–Zehnder interferometer (MZI) point-of-care system for rapid multiplexed detection of microRNAs in human urine specimens. Biosens. Bioelectron. 2015, 71, 365–372. [Google Scholar] [CrossRef]
- Hsiao, Y.-P.; Mukundan, A.; Chen, W.-C.; Wu, M.-T.; Hsieh, S.-C.; Wang, H.-C. Design of a Lab-On-Chip for Cancer Cell Detection through Impedance and Photoelectrochemical Response Analysis. Biosensors 2022, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Osmond, J.; Dante, S.; Dominguez, C.; Lechuga, L.M. Grating couplers integrated on Mach-Zehnder interferometric biosensors operating in the visible range. IEEE Photonics J. 2013, 5, 3700108. [Google Scholar] [CrossRef]
- Xu, D.-X.; Vachon, M.; Densmore, A.; Ma, R.; Delâge, A.; Janz, S.; Lapointe, J.; Li, Y.; Lopinski, G.; Zhang, D.; et al. Label-free biosensor array based on silicon-on-insulator ring resonators addressed using a WDM approach. Opt. Lett. 2010, 35, 2771–2773. [Google Scholar] [CrossRef] [PubMed]
- Jahns, S.; Bräu, M.; Meyer, B.-O.; Karrock, T.; Gutekunst, S.B.; Blohm, L.; Selhuber-Unkel, C.; Buhmann, R.; Nazirizadeh, Y.; Gerken, M. Handheld imaging photonic crystal biosensor for multiplexed, label-free protein detection. Biomed. Opt. Express 2015, 6, 3724. [Google Scholar] [CrossRef]
- Rosman, C.; Prasad, J.; Neiser, A.; Henkel, A.; Edgar, J.; Sönnichsen, C. Multiplexed Plasmon Sensor for Rapid Label-Free Analyte Detection. Nano Lett. 2013, 13, 3243–3247. [Google Scholar] [CrossRef]
- Marn, A.M.; Needham, J.; Chiodi, E.; Ünlü, M.S. Multiplexed, high-sensitivity measurements of antibody affinity using interferometric reflectance imaging sensor. Biosensors 2021, 11, 483. [Google Scholar] [CrossRef]
- Holgado, M.; Maigler, M.; Santamaría, B.; Hernandez, A.; Lavín, A.; Laguna, M.; Sanza, F.; Granados, D.; Casquel, R.; Portilla, J.; et al. Towards reliable optical label-free point-of-care (PoC) biosensing devices. Sens. Actuators B Chem. 2016, 236, 765–772. [Google Scholar] [CrossRef]
- Murillo, A.M.M.; Tomé-Amat, J.; Ramírez, Y.; Garrido-Arandia, M.; Valle, L.G.; Hernández-Ramírez, G.; Tramarin, L.; Herreros, P.; Santamaría, B.; Díaz-Perales, A.; et al. Developing an Optical Interferometric Detection Method based biosensor for detecting specific SARS-CoV-2 immunoglobulins in Serum and Saliva, and their corresponding ELISA correlation. Sens. Actuators B Chem. 2021, 345, 130394. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, Z.; Huang, F.; He, Y.; Ye, Z.; Zhang, H.; Guo, C. A Self-Reference Interference Sensor Based on Coherence Multiplexing. Front. Chem. 2022, 10, 880081. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon photonic biosensors using label-free detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [PubMed]
- Gavela, A.F.; García, D.G.; Ramirez, J.C.; Lechuga, L.M. Last advances in silicon-based optical biosensors. Sensors 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanchez, M.M.; Yin, Y.; Herzer, R.; Ma, L.; Schmidt, O.G. Silicon-Based Integrated Label-Free Optofluidic Biosensors: Latest Advances and Roadmap. Adv. Mater. Technol. 2020, 5, 1901138. [Google Scholar] [CrossRef]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Development of an optical waveguide interferometric immunosensor. Sens. Actuators B Chem. 1991, 4, 297–299. [Google Scholar] [CrossRef]
- Brandenburg, A.; Henninger, R. Integrated optical Young interferometer. Appl. Opt. 1994, 33, 5941. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, A.; Krauter, R.; Künzel, C.; Stefan, M.; Schulte, H. Interferometric sensor for detection of surface-bound bioreactions. Appl. Opt. 2000, 39, 6396. [Google Scholar] [CrossRef]
- Schipper, E.F.; Brugman, A.M.; Dominguez, C.; Lechuga, L.M.; Kooyman, R.P.H.; Greve, J. The realization of an integrated Mach-Zehnder waveguide immunosensor in silicon technology. Sens. Actuators B Chem. 1997, 40, 147–153. [Google Scholar] [CrossRef]
- Lechuga, L.M.; Lenferink, A.T.M.; Kooyman, R.P.H.; Greve, J. Feasibility of evanescent wave interferometer immunosensors for pesticide detection: Chemical aspects. Sens. Actuators B Chem. 1995, 25, 762–765. [Google Scholar] [CrossRef]
- Prieto, F.; Lveda, B.S.; Calle, A.; Llobera, A.; Nguez, C.D.; Abad, A.; Montoya, A.; Lechuga, L. An integrated optical interferometric nanodevice based on silicon technology for biosensor applications. Nanotechnology 2003, 14, 907–912. [Google Scholar] [CrossRef]
- Densmore, A.; Vachon, M.; Xu, D.-X.; Janz, S.; Ma, R.; Li, Y.-H.; Lopinski, G.; Delâge, A.; Lapointe, J.; Luebbert, C.C.; et al. Silicon photonic wire biosensor array for multiplexed real-time and label-free molecular detection. Opt. Lett. 2009, 34, 3598–3600. [Google Scholar] [CrossRef] [Green Version]
- Crespi, A.; Gu, Y.; Ngamsom, B.; Hoekstra, H.J.W.M.; Dongre, C.; Pollnau, M.; Ramponi, R.; van den Vlekkert, H.H.; Watts, P.; Cerulloa, G.; et al. Three-dimensional Mach-Zehnder interferometer in a microfluidic chip for spatially-resolved label-free detection. Lab Chip 2010, 10, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ishihara, S.; Arakawa, T.; Kokubun, Y. Highly sensitive optical biosensor based on silicon-microring-resonator-loaded Mach-Zehnder interferometer. Jpn. J. Appl. Phys. 2017, 56, 04CH08. [Google Scholar] [CrossRef]
- Yadav, M.; Aksnes, A. Multiplexed Mach-Zehnder interferometer assisted ring resonator sensor. Opt. Express 2022, 30, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, L.; Liu, L. Ultrahigh sensitivity mach−zehnder interferometer sensor based on a weak one-dimensional field confinement silica waveguide. Sensors 2021, 21, 6600. [Google Scholar] [CrossRef]
- Leuermann, J.; Fernández-Gavela, A.; Torres-Cubillo, A.; Postigo, S.; Sánchez-Postigo, A.; Lechuga, L.M.; Halir, R.; Molina-Fernández, Í. Optimizing the limit of detection of waveguide-based interferometric biosensor devices. Sensors 2019, 19, 3671. [Google Scholar] [CrossRef]
- Dante, S.; Duval, D.; Sepúlveda, B.; González-Guerrero, A.B.; Sendra, J.R.; Lechuga, L.M. All-optical phase modulation for integrated interferometric biosensors. Opt. Express 2012, 20, 7195–7205. [Google Scholar] [CrossRef]
- Halir, R.; Vivien, L.; le Roux, X.; Xu, D.-X.; Cheben, P. Direct and Sensitive Phase Readout for Integrated Waveguide Sensors. IEEE Photonics J. 2013, 5, 6800906. [Google Scholar] [CrossRef]
- Molina-Fernández, Í.; Leuermann, J.; Ortega-Moñux, A.; Wangüemert-Pérez, J.G.; Halir, R. Fundamental limit of detection of photonic biosensors with coherent phase read-out. Opt. Express 2019, 27, 12616. [Google Scholar] [CrossRef]
- Leuermann, J.; Stamenkovic, V.; Ramirez-Priego, P.; Sánchez-Postigo, A.; Fernández-Gavela, A.; Chapman, C.A.; Bailey, R.C.; Lechuga, L.M.; Perez-Inestrosa, E.; Collado, D.; et al. Coherent silicon photonic interferometric biosensor with an inexpensive laser source for sensitive label-free immunoassays. Opt. Lett. 2020, 45, 6595. [Google Scholar] [CrossRef]
- Martens, D.; Ramirez-Priego, P.; Murib, M.S.; Elamin, A.A.; Gonzalez-Guerrero, A.B.; Stehr, M.; Jonas, F.; Anton, B.; Hlawatsch, N.; Soetaert, P.; et al. A low-cost integrated biosensing platform based on SiN nanophotonics for biomarker detection in urine. Anal. Methods 2018, 10, 3066–3073. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Priego, P.; Martens, D.; Elamin, A.A.; Soetaert, P.; Van Roy, W.; Vos, R.; Anton, B.; Bockstaele, R.; Becker, H.; Singh, M.; et al. Label-Free and Real-Time Detection of Tuberculosis in Human Urine Samples Using a Nanophotonic Point-of-Care Platform. ACS Sens. 2018, 3, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Zinoviev, K.E.; Gonzalez-Guerrero, A.B.; Dominguez, C.; Lechuga, L.M. Integrated Bimodal Waveguide Interferometric Biosensor for Label-Free Analysis. J. Light. Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- González-Guerrero, A.B.; Maldonado, J.; Dante, S.; Grajales, D.; Lechuga, L.M. Direct and label-free detection of the human growth hormone in urine by an ultrasensitive bimodal waveguide biosensor. J. Biophotonics 2017, 10, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dante, S.; Duval, D.; Fariña, D.; González-Guerrero, A.B.; Lechuga, L.M. Linear readout of integrated interferometric biosensors using a periodic wavelength modulation. Laser Photonics Rev. 2015, 9, 248–255. [Google Scholar] [CrossRef]
- Bassols-Cornudella, B.; Ramirez-Priego, P.; Soler, M.; Estevez, M.-C.; Luis-Ravelo, H.J.D.; Cardenosa-Rubio, M.; Lechuga, L.M. Novel Sensing Algorithm for Linear Read-Out of Bimodal Waveguide Interferometric Biosensors. J. Light. Technol. 2022, 40, 237–244. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, K.W.; Gu, Z.; Kee, J.S.; Park, M.K. Single-channel Mach-Zehnder interferometric biochemical sensor based on two-lateral-mode spiral waveguide. Opt. Express 2014, 22, 27910–27920. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Gabrielli, L.H.; Lechuga, L.M.; Hernandez-Figueroa, H.E. Trimodal waveguide demonstration and its implementation as a high order mode interferometer for sensing application. Sensors 2019, 19, 2821. [Google Scholar] [CrossRef]
- Isayama, Y.H.; Hernández-Figueroa, H.E. High-order multimode waveguide interferometer for optical biosensing applications. Sensors 2021, 21, 3254. [Google Scholar] [CrossRef]
- Torrijos-Morán, L.; Lisboa, B.D.; Soler, M.; Lechuga, L.M.; García-Rupérez, J. Integrated optical bimodal waveguide biosensors: Principles and applications. Results Opt. 2022, 9, 100285. [Google Scholar] [CrossRef]
- Petrou, P.; Makarona, E.; Raptis, I.; Kakabakos, S.; Misiakos, K. Monolithically Integrated Label-Free Optical Immunosensors. Eng. Proc. 2022, 16, 11. [Google Scholar]
- Vogelbacher, F.; Kothe, T.; Muellner, P.; Melnik, E.; Sagmeister, M.; Kraft, J.; Hainberger, R. Waveguide Mach-Zehnder biosensor with laser diode pumped integrated single-mode silicon nitride organic hybrid solid-state laser. Biosens. Bioelectron. 2022, 197, 113816. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Makarona, E.; Salapatas, A.; Misiakos, K.; Synolaki, E.; Ioannidis, A.; Chatzipanagiotou, S.; Ritvos, M.A.; Pasternack, A.; Ritvos, O.; et al. Directly immersible silicon photonic probes: Application to rapid SARS-CoV-2 serological testing. Biosens. Bioelectron. 2022, 215, 114570. [Google Scholar] [CrossRef]
- Fernández-Gavela, A.; Herranz, S.; Chocarro, B.; Falke, F.; Schreuder, E.; Leeuwis, H.; Heideman, R.G.; Lechuga, L.M. Full integration of photonic nanoimmunosensors in portable platforms for on-line monitoring of ocean pollutants. Sens. Actuators B Chem. 2019, 297, 126758. [Google Scholar] [CrossRef]
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 detection in milk by fab’ functionalized Si3N4 asymmetric mach-Zehnder interferometric biosensors. Toxins 2019, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetrou, M.; Gounaridis, L.; Tsekenis, G.; Dimadi, M.; Vestering-Stenger, R.; Schreuder, E.F.; Trilling, A.; Besselink, G.; Scheres, L.; van der Meer, A.; et al. A miniature bio-photonics companion diagnostics platform for reliable cancer treatment monitoring in blood fluids. Sensors 2021, 21, 2230. [Google Scholar] [CrossRef] [PubMed]
- Besselink, G.; Schütz-Trilling, A.; Veerbeek, J.; Verbruggen, M.; van der Meer, A.; Schonenberg, R.; Dam, H.; Evers, K.; Lindhout, E.; Garritsen, A.; et al. Asymmetric Mach–Zehnder Interferometric Biosensing for Quantitative and Sensitive Multiplex Detection of Anti-SARS-CoV-2 Antibodies in Human Plasma. Biosensors 2022, 12, 553. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Besselink, G.A.J.; Falke, F.; Everhardt, A.S.; Cornelissen, J.J.L.M.; Huskens, J. Highly Sensitive Protein Detection by Asymmetric Mach-Zehnder Interferometry for Biosensing Applications. ACS Appl. Bio. Mater. 2020, 3, 4566–4572. [Google Scholar] [CrossRef]
- Niu, H.; Yu, P.; Zhu, Y.; Jing, Z.; Li, P.; Wang, B.; Ma, C.; Wang, J.; Wu, J.; O Govorov, A.; et al. Mach-Zehnder interferometer based integrated-photonic acetone sensor approaching the sub-ppm level detection limit. Opt. Express 2022, 30, 29665–29679. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.; Misiakos, K.; Raptis, I.; Kakabakos, S. Simultaneous Detection of Salmonella typhimurium and Escherichia coli O157:H7 in Drinking Water and Milk with Mach–Zehnder Interferometers Monolithically Integrated on Silicon Chips. Biosensors 2022, 12, 507. [Google Scholar] [CrossRef]
- Chocarro-Ruiz, B.; Herranz, S.; Fernandez-Gavela, A.; Sanchís, J.; Farre, M.; Marco, M.P.; Lechuga, L.M. Interferometric nanoimmunosensor for label-free and real-time monitoring of Irgarol 1051 in seawater. Biosens. Bioelectron. 2018, 117, 47–52. [Google Scholar] [CrossRef]
- Ramirez-Priego, P.; Estévez, M.C.; Díaz-Luisravelo, H.J.; Manclús, J.J.; Montoya, Á.; Lechuga, L.M. Real-time monitoring of fenitrothion in water samples using a silicon nanophotonic biosensor. Anal. Chim. Acta 2021, 1152, 338276. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Estévez, M.C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef]
- Maldonado, J.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Label-free bimodal waveguide immunosensor for rapid diagnosis of bacterial infections in cirrhotic patients. Biosens. Bioelectron. 2016, 85, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Huertas, C.S.; Fariña, D.; Lechuga, L.M. Direct and Label-Free Quantification of Micro-RNA-181a at Attomolar Level in Complex Media Using a Nanophotonic Biosensor. ACS Sens. 2016, 1, 748–756. [Google Scholar] [CrossRef]
- Mudumba, S.; de Alba, S.; Romero, R.; Cherwien, C.; Wu, A.; Wang, J.; Gleeson, M.A.; Iqbal, M.; Burlingame, R.W. Photonic Ring Resonance Is a Versatile Platform for Performing Multiplex Immunoassays in Real Time. J. Immunol. Methods 2017, 448, 34–43. [Google Scholar] [CrossRef]
- Vollmer, F.; Yang, L.; Fainman, S. Label-Free Detection with High-Q Microcavities: A Review of Biosensing Mechanisms for Integrated Devices. Nanophotonics 2012, 1, 267–291. [Google Scholar] [CrossRef]
- Claes, T.; Molera, J.G.; de Vos, K.; Schacht, E.; Baets, R.; Bienstman, P. Label-Free Biosensing with a Slot-Waveguide-Based Ring Resonator in Silicon on Insulator. IEEE Photonics J. 2009, 1, 197–204. [Google Scholar] [CrossRef]
- Voronin, K.; Stebunov, Y.; Voronov, A.A.; Arsenin, A.; Volkov, V.S. Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications. Sensors 2020, 20, 203. [Google Scholar] [CrossRef]
- Flueckiger, J.; Schmidt, S.; Donzella, V.; Sherwali, A.; Ratner, D.M.; Chrostowski, L.; Cheung, K.C. Sub-Wavelength Grating for Enhanced Ring Resonator Biosensor. Opt. Express 2016, 24, 15672. [Google Scholar] [CrossRef]
- Kwon, S.H. Plasmonicwaveguide Coupled Ring Cavity for a Non-Resonant Type Refractive Index Sensor. Sensors 2017, 17, 2526. [Google Scholar] [CrossRef]
- Steglich, P.; Hülsemann, M.; Dietzel, B.; Mai, A. Optical Biosensors Based on Silicon-on-Insulator Ring Resonators: A Review. Molecules 2019, 24, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perotto, S.; Biagini, C.; Hubarevich, A.; Tantussi, F.; de Angelis, F. Toward All on Chip Optical Detection in the Few Molecule Regime. Biosens. Bioelectron. 2020, 169, 112600. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Jones, H.B.L.; Frustaci, S.; Winter, S.; van der Kamp, M.W.; Arcus, V.L.; Pudney, C.R.; Vollmer, F. Sensing Enzyme Activation Heat Capacity at the Single-Molecule Level Using Gold-Nanorod-Based Optical Whispering Gallery Modes. ACS Appl. Nano Mater. 2021, 4, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Jin, L. Boost the sensitivity of optical sensors with interface modes. Sci. Bull. 2022, 67, 777–778. [Google Scholar]
- Hunt, H.K.; Soteropulos, C.; Armani, A.M. Bioconjugation Strategies for Microtoroidal Optical Resonators. Sensors 2010, 10, 9317–9336. [Google Scholar] [CrossRef]
- Sanati, P.; Hashemi, S.S.; Bahadoran, M.; Babadi, A.A.; Akbari, E. Detection of Escherichia coli K12 in Water Using Slot Waveguide in Cascaded Ring Resonator. Silicon 2022, 14, 851–857. [Google Scholar] [CrossRef]
- Kasper, L.; Zein Al-Din, A.; Bruns, J.; Volkmer, R.; Petermann, K. Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators. Eng. Proc. 2021, 6, 22. [Google Scholar]
- Soni, V.; Chang, C.W.; Xu, X.; Wang, C.; Yan, H.; D Agati, M.; Tu, L.W.; Chen, Q.Y.; Tian, H.; Chen, R.T. Portable Automatic Microring Resonator System Using a Subwavelength Grating Metamaterial Waveguide for High-Sensitivity Real-Time Optical-Biosensing Applications. IEEE Trans. Biomed. Eng. 2021, 68, 1894–1902. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Steiner, D.J.; Abedin, M.; Bryan, M.R.; Shanahan, C.; Tokranova, N.; Young, E.; Klose, A.M.; Zavriyev, A.; Judy, N.; et al. Disposable Photonics for Cost-Effective Clinical Bioassays: Application to COVID-19 Antibody Testing. Lab Chip 2021, 21, 2913–2921. [Google Scholar] [CrossRef]
- Iqbal, M.; Burlingame, R.W.; Romero, R.; Wang, A.; Grove, T.; Gleeson, M.A. Silicon Photonic Micro-Ring Resonators for Drug Screening and Kinetic Analysis. In Label-Free Biosensor Methods in Drug Discovery; Springer: New York, NY, USA, 2015; pp. 133–153. [Google Scholar]
- Estrada, I.A.; Burlingame, R.W.; Wang, A.P.; Chawla, K.; Grove, T.; Wang, J.; Southern, S.O.; Iqbal, M.; Gunn, L.C.; Gleeson, M.A. Multiplex Detection of Pathogen Biomarkers in Human Blood, Serum, and Saliva Using Silicon Photonic Microring Resonators. In Proceedings of the Advances in Global Health through Sensing Technologies 2015, Baltimore, MD, USA, 13 May 2015; Volume 9490. [Google Scholar]
- Toropov, N.; Osborne, E.; Joshi, L.T.; Davidson, J.; Morgan, C.; Page, J.; Pepperell, J.; Vollmer, F. SARS-CoV-2 Tests: Bridging the Gap between Laboratory Sensors and Clinical Applications. ACS Sens. 2021, 6, 2815–2837. [Google Scholar] [CrossRef] [PubMed]
- Pongruengkiat, W.; Pechprasarn, S. Whispering-Gallery Mode Resonators for Detecting Cancer. Sensors 2017, 17, 2095. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Mohammed, M.U.; Khan, M.; Bin Yousuf, A.H.; Chowdhury, M.H. High-Quality Optical Ring Resonator-Based Biosensor for Cancer Detection. IEEE Sens. J. 2020, 20, 1867–1875. [Google Scholar] [CrossRef]
- Griol, A.; Peransi, S.; Rodrigo, M.; Hurtado, J.; Bellieres, L.; Ivanova, T.; Zurita, D.; Sánchez, C.; Recuero, S.; Hernández, A.; et al. Design and Development of Photonic Biosensors for Swine Viral Diseases Detection. Sensors 2019, 19, 3985. [Google Scholar] [CrossRef]
- Juan-Colás, J.; Krauss, T.F.; Johnson, S.D. Real-Time Analysis of Molecular Conformation Using Silicon Electrophotonic Biosensors. ACS Photonics 2017, 4, 2320–2326. [Google Scholar] [CrossRef]
- Toropov, N.; Cabello, G.; Serrano, M.P.; Gutha, R.R.; Rafti, M.; Vollmer, F. Review of Biosensing with Whispering-Gallery Mode Lasers. Light Sci. Appl. 2021, 10, 42. [Google Scholar] [CrossRef]
- Xavier, J.; Yu, D.; Vollmer, F.; Jones, C.; Zossimova, E. Quantum Nanophotonic and Nanoplasmonic Sensing: Towards Quantum Optical Bioscience Laboratories on Chip. Nanophotonics 2021, 10, 1387–1435. [Google Scholar] [CrossRef]
- Xavier, J.; Vincent, S.; Meder, F.; Vollmer, F. Advances in optoplasmonic sensors—Combining optical nano/microcavities and photonic crystals with plasmonic nanostructures and nanoparticles. Nanophotonics 2018, 7, 1–38. [Google Scholar] [CrossRef]
- Bonifacio, L.D.; Ozin, G.A.; Arsenault, A.C. Photonic Nose–Sensor Platform for Water and Food Quality Control. Small 2011, 7, 3153–3157. [Google Scholar] [CrossRef]
- Bonifacio, L.D.; Puzzo, D.P.; Breslav, S.; Willey, B.M.; McGeer, A.; Ozin, G.A. Towards the Photonic Nose: A Novel Platform for Molecule and Bacteria Identification. Adv. Mater. 2010, 22, 1351–1354. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Molding the Flow of Light. Princeton Univ. Press, Princeton, NJ [ua]. 2008. Available online: https://physics.mit.edu/wp-content/uploads/2021/01/physicsatmit_01_joannopolous.pdf (accessed on 23 September 2022).
- Di Falco, A.; O’faolain, L.; Krauss, T. Chemical sensing in slotted photonic crystal heterostructure cavities. Appl. Phys. Lett. 2009, 94, 063503. [Google Scholar] [CrossRef]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef]
- Pacholski, C. Photonic crystal sensors based on porous Silicon. Sensors 2013, 13, 4694–4713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Pedro, V.; Calvo, M.E.; Míguez, H.; Maquieira, Á. Nanoparticle Bragg reflectors: A smart analytical tool for biosensing. Biosens. Bioelectron. X 2019, 1, 100012. [Google Scholar] [CrossRef]
- Han, M.G.; Shin, C.G.; Jeon, S.J.; Shim, H.; Heo, C.J.; Jin, H.; Kim, J.W.; Lee, S. Full color tunable photonic crystal from crystalline colloidal arrays with an engineered photonic stop-band. Adv. Mater. 2012, 24, 6438–6444. [Google Scholar] [CrossRef]
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef]
- Li, M.; Liang, H.; Luo, R.; He, Y.; Lin, Q. High-Q 2D lithium niobate photonic crystal slab nanoresonators. Laser Photonics Rev. 2019, 13, 1800228. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, F.; Gao, R.; Jao, C.-Y.; Ma, C.; Li, J.; Li, X.; Guan, B.-O.; Cetin, A.E.; Chen, K. Rayleigh anomaly-enabled mode hybridization in gold nanohole arrays by scalable colloidal lithography for highly-sensitive biosensing. Nanophotonics 2022, 11, 507–517. [Google Scholar] [CrossRef]
- Bai, X.; Shuai, Y.; Lv, L.; Huang, S.; Xing, Y.; Zhao, J.; Luo, W.; Wu, C.; Yang, T.; Zhang, W. Mo/Ti multilayer Bragg reflector for LiNbO3 film bulk acoustic wave resonators. J. Appl. Phys. 2020, 128, 094503. [Google Scholar] [CrossRef]
- Grigoriev, S.; Napolskii, K.; Grigoryeva, N.; Vasilieva, A.; Mistonov, A.; Chernyshov, D.Y.; Petukhov, A.; Belov, D.; Eliseev, A.; Lukashin, A. Structural and magnetic properties of inverse opal photonic crystals studied by x-ray diffraction, scanning electron microscopy, and small-angle neutron scattering. Phys. Rev. B 2009, 79, 045123. [Google Scholar] [CrossRef]
- Sansone, L.; Campopiano, S.; Pannico, M.; Giordano, M.; Musto, P.; Iadicicco, A. Photonic bandgap influence on the SERS effect in metal-dielectric colloidal crystals optical fiber probe. Sens. Actuators B Chem. 2021, 345, 130149. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Shen, R.; Li, Z.; Yang, Y.; Yuan, Q. Point-of-care pathogen testing using photonic crystals and machine vision for diagnosis of urinary tract infections. Nano Lett. 2021, 21, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Benelarbi, D.; Bouchemat, T.; Bouchemat, M. Study of photonic crystal microcavities coupled with waveguide for biosensing applications. Opt. Quantum Electron. 2017, 49, 347. [Google Scholar] [CrossRef]
- Biswas, P.; Zhang, C.; Chen, Y.; Liu, Z.; Vaziri, S.; Zhou, W.; Sun, Y. A portable micro-gas chromatography with integrated photonic crystal slab sensors on chip. Biosensors 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Shinn, M.; Robertson, W. Surface plasmon-like sensor based on surface electromagnetic waves in a photonic band-gap material. Sens. Actuators B Chem. 2005, 105, 360–364. [Google Scholar] [CrossRef]
- Petrova, I.; Konopsky, V.; Nabiev, I.; Sukhanova, A. Label-Free Flow Multiplex Biosensing via Photonic Crystal Surface Mode Detection. Sci. Rep. 2019, 9, 8745. [Google Scholar] [CrossRef] [PubMed]
- Konopsky, V.; Mitko, T.; Aldarov, K.; Alieva, E.; Basmanov, D.; Moskalets, A.; Matveeva, A.; Morozova, O.; Klinov, D. Photonic crystal surface mode imaging for multiplexed and high-throughput label-free biosensing. Biosens. Bioelectron. 2020, 168, 112575. [Google Scholar] [CrossRef]
- Rostova, E.; Ben Adiba, C.; Dietler, G.; Sekatskii, S.K. Kinetics of antibody binding to membranes of living bacteria measured by a photonic crystal-based biosensor. Biosensors 2016, 6, 52. [Google Scholar] [CrossRef]
- Baker, J.E.; Sriram, R.; Miller, B.L. Two-dimensional photonic crystals for sensitive microscale chemical and biochemical sensing. Lab Chip 2015, 15, 971–990. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, L.; Fu, Z.; Sun, F.; Tian, H. Multiplexed simultaneous high sensitivity sensors with high-order mode based on the integration of photonic crystal 1 × 3 beam splitter and three different single-slot PCNCs. Sensors 2016, 16, 1050. [Google Scholar] [CrossRef]
- Yan, H.; Tang, N.; Jairo, G.A.; Chakravarty, S.; Blake, D.A.; Chen, R.T. High-sensitivity high-throughput chip based biosensor array for multiplexed detection of heavy metals. In Proceedings of the Frontiers in Biological Detection: From Nanosensors to Systems VIII, San Francisco, CA, USA, 13–18 February 2016; pp. 6–10. [Google Scholar]
- Wei, X.; Bian, F.; Cai, X.; Wang, Y.; Cai, L.; Yang, J.; Zhu, Y.; Zhao, Y. Multiplexed detection strategy for bladder cancer microRNAs based on photonic crystal barcodes. Anal. Chem. 2020, 92, 6121–6127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Bian, F.; Cai, L.; Zhao, Y. Encoded microneedle arrays for detection of skin interstitial fluid biomarkers. Adv. Mater. 2019, 31, 1902825. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, Y.; Liao, J.; He, B.; Liu, H. Bioinspired multistructured paper microfluidics for POCT. Lab Chip 2019, 19, 3602–3608. [Google Scholar] [CrossRef]
- Murray, W.A.; Barnes, W.L. Plasmonic materials. Adv. Mater. 2007, 19, 3771–3782. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lundström, I. Biosensing with surface plasmon resonance—How it all started. Biosens. Bioelectron. 1995, 10, i–ix. [Google Scholar] [CrossRef]
- Leung, P.T.; Pollard-Knight, D.; Malan, G.P.; Finlan, M.F. Modelling of particle-enhanced sensitivity of the surface-plasmon-resonance biosensor. Sens. Actuators B. Chem. 1994, 22, 175–180. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Schianti, J.N.; Souto, D.E.P.; Kubota, L.T.; Hernandez-Figueroa, H.E.; Gabrielli, L.H. Dielectric barrier discharge plasma treatment of modified SU-8 for biosensing applications. Biomed. Opt. Express 2018, 9, 2168. [Google Scholar] [CrossRef]
- Habib, A.; Zhu, X.; Can, U.I.; McLanahan, M.L.; Zorlutuna, P.; Yanik, A.A. Electro-plasmonic nanoantenna: A nonfluorescent optical probe for ultrasensitive label-free detection of electrophysiological signals. Sci. Adv. 2019, 5, eaav9786. [Google Scholar] [CrossRef]
- Neuzil, P.; Reboud, J. Palm-Sized Biodetection System Based on Localized Surface Plasmon Resonance. Anal. Chem. 2008, 80, 6100–6103. [Google Scholar] [CrossRef]
- Ruemmele, J.A.; Hall, W.P.; Ruvuna, L.K.; Van Duyne, R.P. A Localized Surface Plasmon Resonance Imaging Instrument for Multiplexed Biosensing. Anal. Chem. 2013, 85, 4560–4566. [Google Scholar] [CrossRef] [PubMed]
- Demirdjian, B.; Bedu, F.; Ranguis, A.; Ozerov, I.; Henry, C.R. Water Adsorption by a Sensitive Calibrated Gold Plasmonic Nanosensor. Langmuir 2018, 34, 5381–5385. [Google Scholar] [CrossRef]
- Klinghammer, S.; Uhlig, T.; Patrovsky, F.; Böhm, M.; Schütt, J.; Pütz, N.; Baraban, L.; Eng, L.M.; Cuniberti, G. Plasmonic Biosensor Based on Vertical Arrays of Gold Nanoantennas. ACS Sens. 2018, 3, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.L.; Teixeira, F.M.F.; Ferreira, G.S.C.; Versiani, A.F.; Andrade, L.M.; Ladeira, L.O.; Fonseca, F.G.; Ramirez, J.C. Computational Guided Method Applied to LSPR-Based Biosensor for Specific Detection of the Four-Serotypes of Dengue Virus in Seropositive Patients. Part. Part. Syst. Charact. 2022, 39, 2100157. [Google Scholar] [CrossRef]
- Behi, M.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanoassembled Peptide Biosensors for Rapid Detection of Matrilysin Cancer Biomarker. Small 2020, 16, 1905994. [Google Scholar] [CrossRef] [PubMed]
- Versiani, A.F.; Martins, E.M.N.; Andrade, L.M.; Cox, L.; Pereira, G.C.; Barbosa-Stancioli, E.F.; Nogueira, M.L.; Ladeira, L.O.; da Fonseca, F.G. Nanosensors based on LSPR are able to serologically differentiate dengue from Zika infections. Sci. Rep. 2020, 10, 11302. [Google Scholar] [CrossRef]
- Thilsted, A.H.; Pan, J.Y.; Wu, K.; Zór, K.; Rindzevicius, T.; Schmidt, M.S.; Boisen, A. Lithography-Free Fabrication of Silica Nanocylinders with Suspended Gold Nanorings for LSPR-Based Sensing. Small 2016, 12, 6745–6752. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, B.; Huang, Y.; Liao, Z.; Li, Z.; Li, S.; Wang, S.; Wen, W. Gold crescent nanodisk array for nanoantenna-enhanced sensing in subwavelength areas. Appl. Opt. 2014, 53, 7236. [Google Scholar] [CrossRef]
- Jahani, Y.; Arvelo, E.R.; Yesilkoy, F.; Koshelev, K.; Cianciaruso, C.; De Palma, M.; Kivshar, Y.; Altug, H. Imaging-based spectrometer-less optofluidic biosensors based on dielectric metasurfaces for detecting extracellular vesicles. Nat. Commun. 2021, 12, 4–13. [Google Scholar] [CrossRef]
- Yavas, O.; Svedendahl, M.; Dobosz, P.; Sanz, V.; Quidant, R. On-a-chip Biosensing Based on All-Dielectric Nanoresonators. Nano Lett. 2017, 17, 4421–4426. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chung, M.T.; McHugh, W.; Nidetz, R.; Li, Y.; Fu, J.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ryu, B.; Deng, Q.; Pan, B.; Song, Y.; Tian, Y.; Alam, H.B.; Li, Y.; Liang, X.; Kurabayashi, K. An Integrated Plasmo-Photoelectronic Nanostructure Biosensor Detects an Infection Biomarker Accompanying Cell Death in Neutrophils. Small 2020, 16, 1905611. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, X.; Zhang, C.; Cerjan, B.; Zhou, L.; Tseng, M.L.; Zhang, Y.; Alabastri, A.; Nordlander, P.; Halas, N.J. Nanogapped Au Antennas for Ultrasensitive Surface-Enhanced Infrared Absorption Spectroscopy. Nano Lett. 2017, 17, 5768–5774. [Google Scholar] [CrossRef]
- Yesilkoy, F.; Arvelo, E.R.; Jahani, Y.; Liu, M.; Tittl, A.; Cevher, V.; Kivshar, Y.; Altug, H. Ultrasensitive hyperspectral imaging and biodetection enabled by dielectric metasurfaces. Nat. Photonics 2019, 13, 390–396. [Google Scholar] [CrossRef]
- Calvo-Lozano, O.; Aviñó, A.; Friaza, V.; Medina-Escuela, A.; SHuertas, C.; Calderón, E.J.; Eritja, R.; Lechuga, L.M. Fast and Accurate Pneumocystis Pneumonia Diagnosis in Human Samples Using a Label-Free Plasmonic Biosensor. Nanomaterials 2020, 10, 1246. [Google Scholar] [CrossRef]
- Ortega, M.A.; Rodríguez-Comas, J.; Yavas, O.; Velasco-Mallorquí, F.; Balaguer-Trias, J.; Parra, V.; Novials, A.; Servitja, J.M.; Quidant, R.; Ramón-Azcón, J. In Situ LSPR Sensing of Secreted Insulin in Organ-on-Chip. Biosensors 2021, 11, 138. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, J.C.; Grajales García, D.; Maldonado, J.; Fernández-Gavela, A. Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration. Chemosensors 2022, 10, 398. https://doi.org/10.3390/chemosensors10100398
Ramirez JC, Grajales García D, Maldonado J, Fernández-Gavela A. Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration. Chemosensors. 2022; 10(10):398. https://doi.org/10.3390/chemosensors10100398
Chicago/Turabian StyleRamirez, Jhonattan C., Daniel Grajales García, Jesús Maldonado, and Adrián Fernández-Gavela. 2022. "Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration" Chemosensors 10, no. 10: 398. https://doi.org/10.3390/chemosensors10100398
APA StyleRamirez, J. C., Grajales García, D., Maldonado, J., & Fernández-Gavela, A. (2022). Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration. Chemosensors, 10(10), 398. https://doi.org/10.3390/chemosensors10100398







