An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Solutions and Reagents
2.3. Synthesis of Reduced Graphene Oxide with Copper Nanoparticles (rGO-CuNPs)
2.4. Electrode Preparation
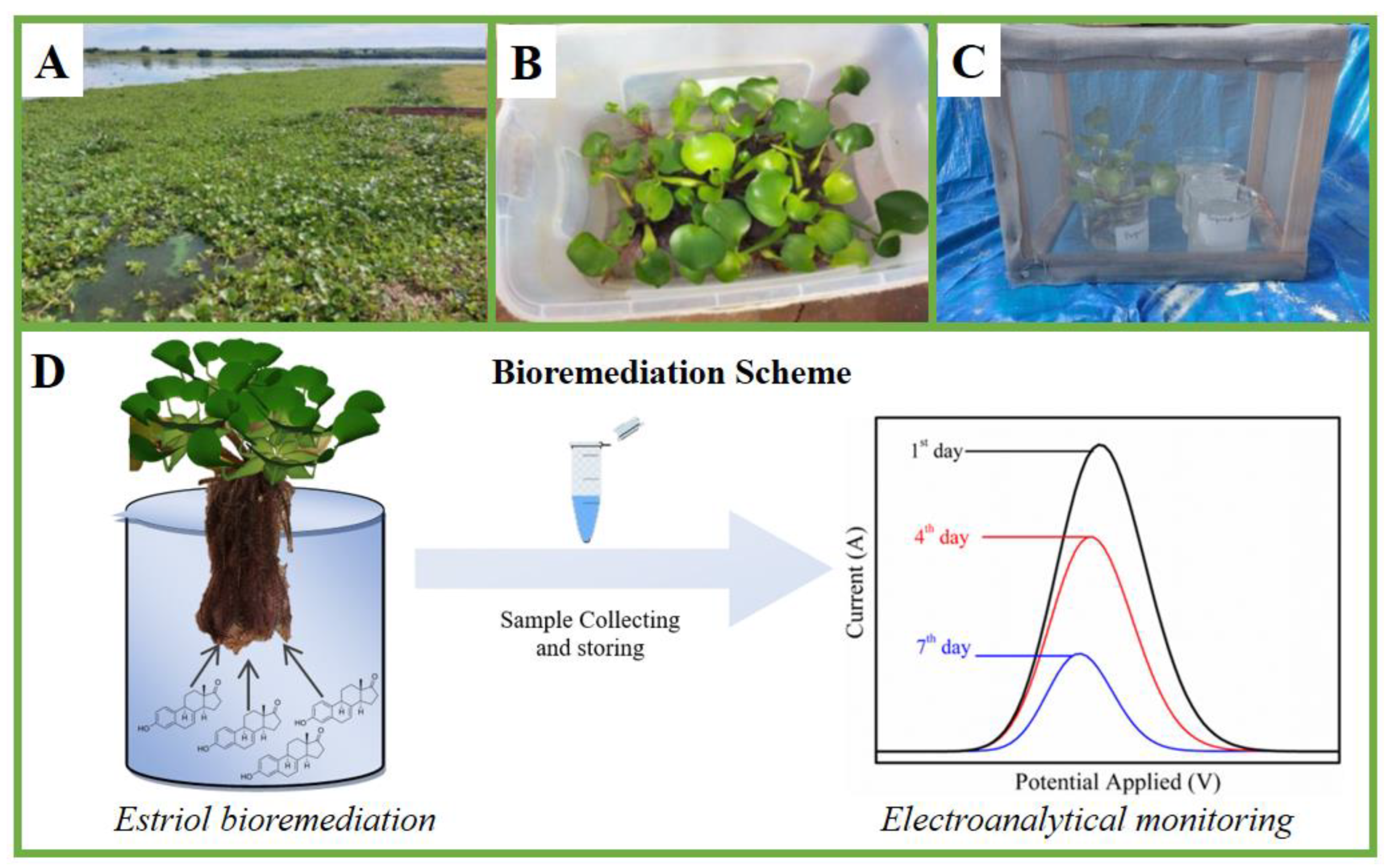
2.5. Obtaining the Plants and Setup of the Phytoremediation Experiment
2.6. Determination of Estriol in Real Samples
2.7. Statistical Analysis
3. Results
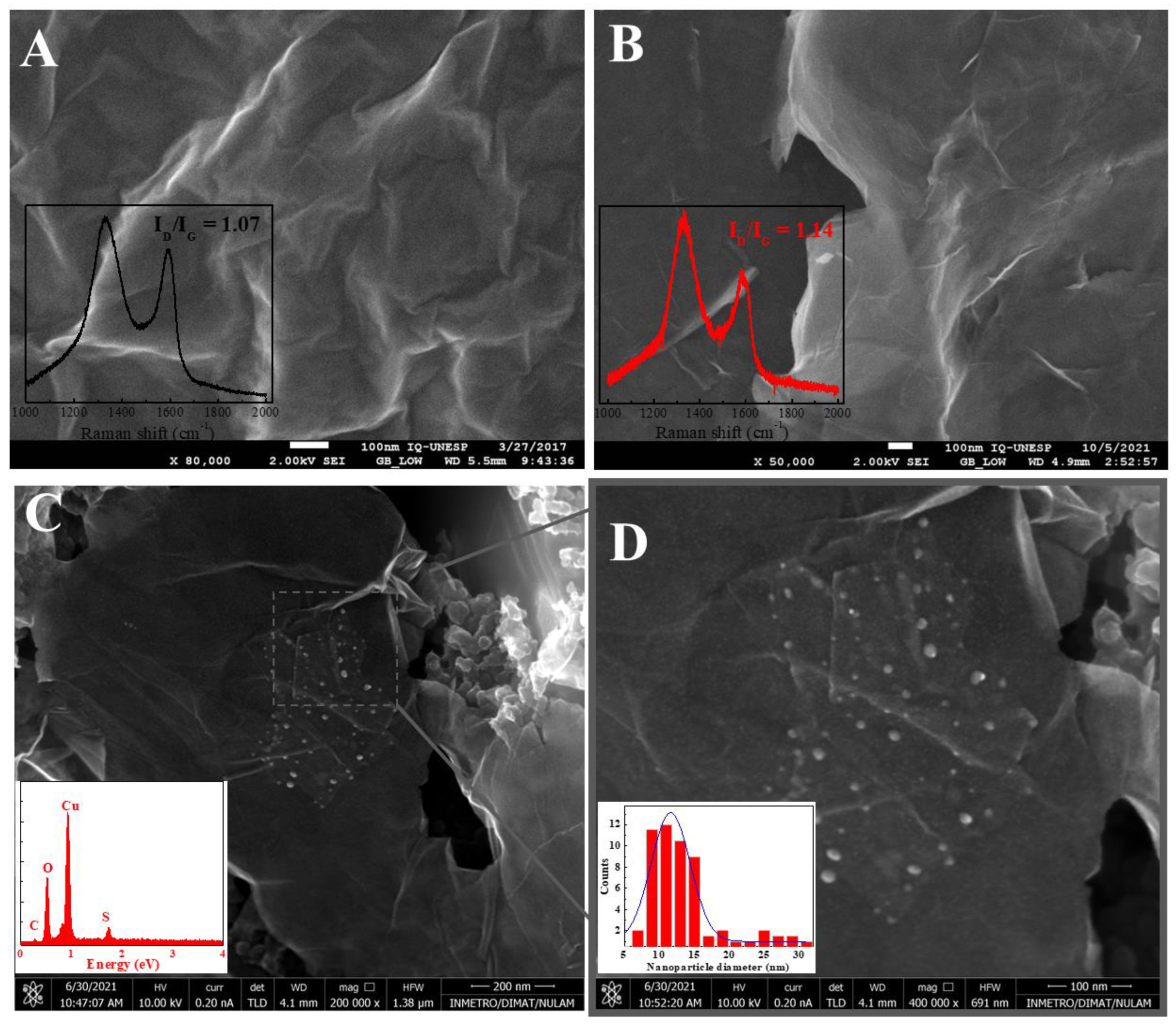
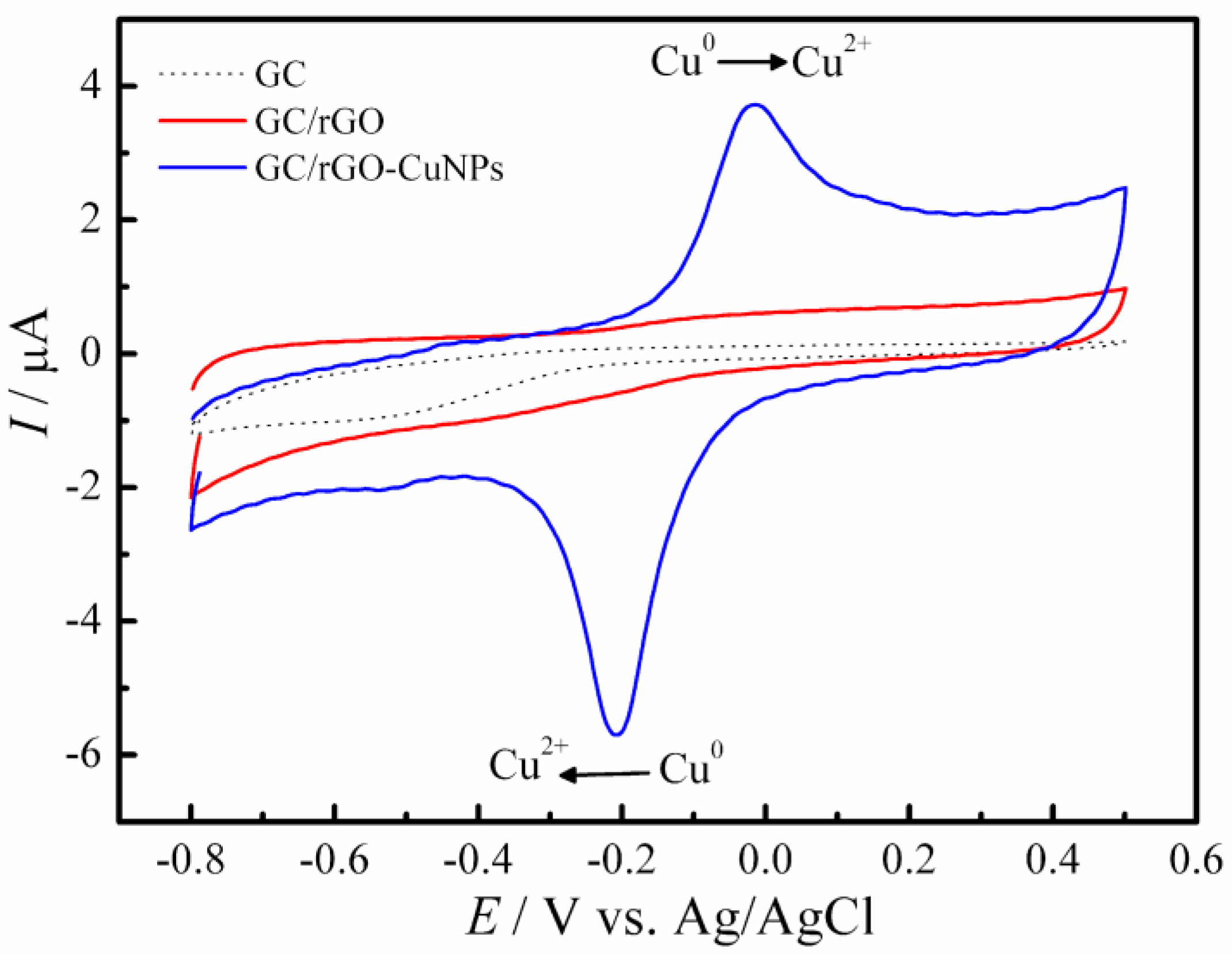
3.1. Morphological and Electrochemical Characterization of Materials
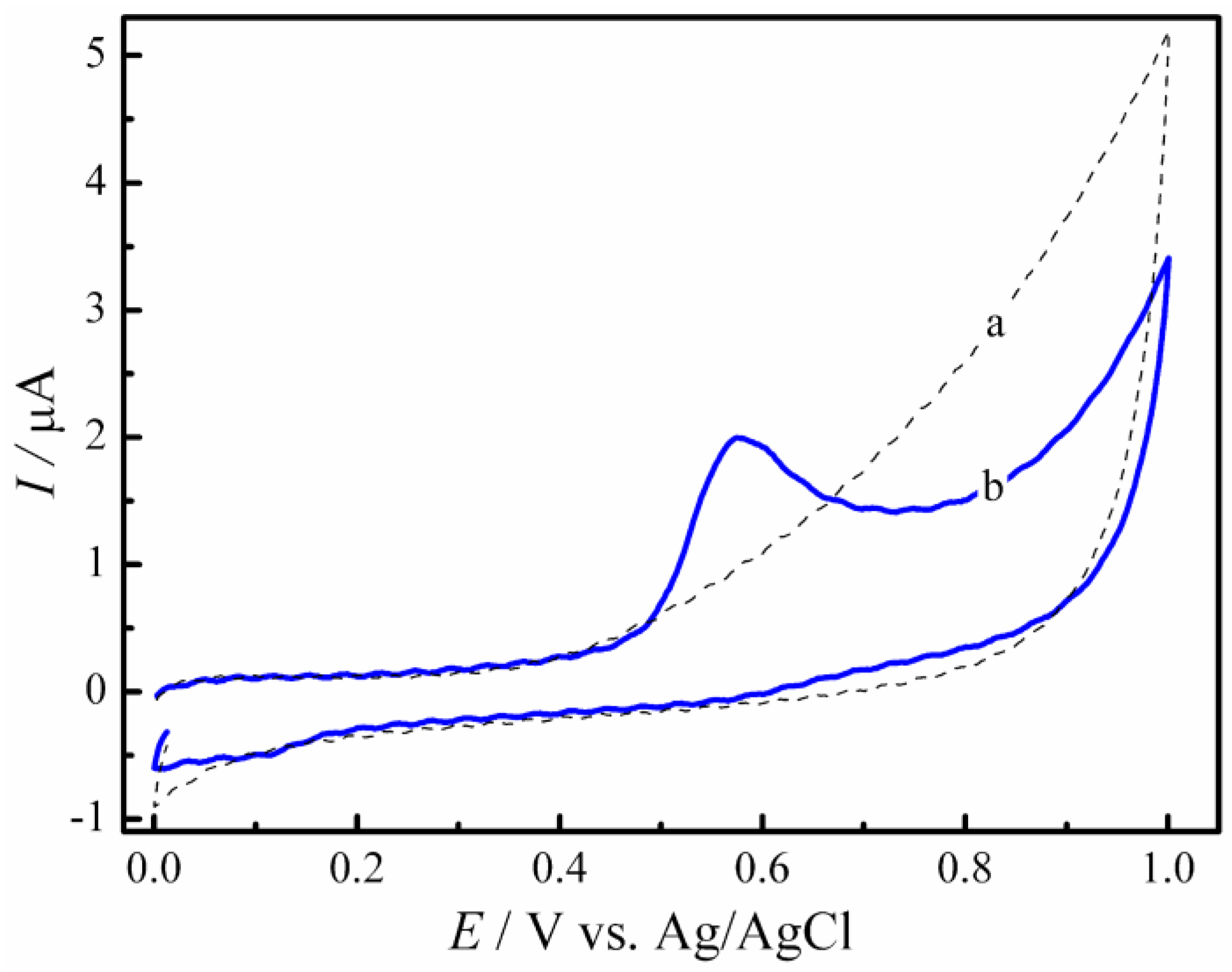
3.2. Electrochemical Behavior of GC/rGO-CuNPs Composite Electrode on the Estriol Oxidation Process
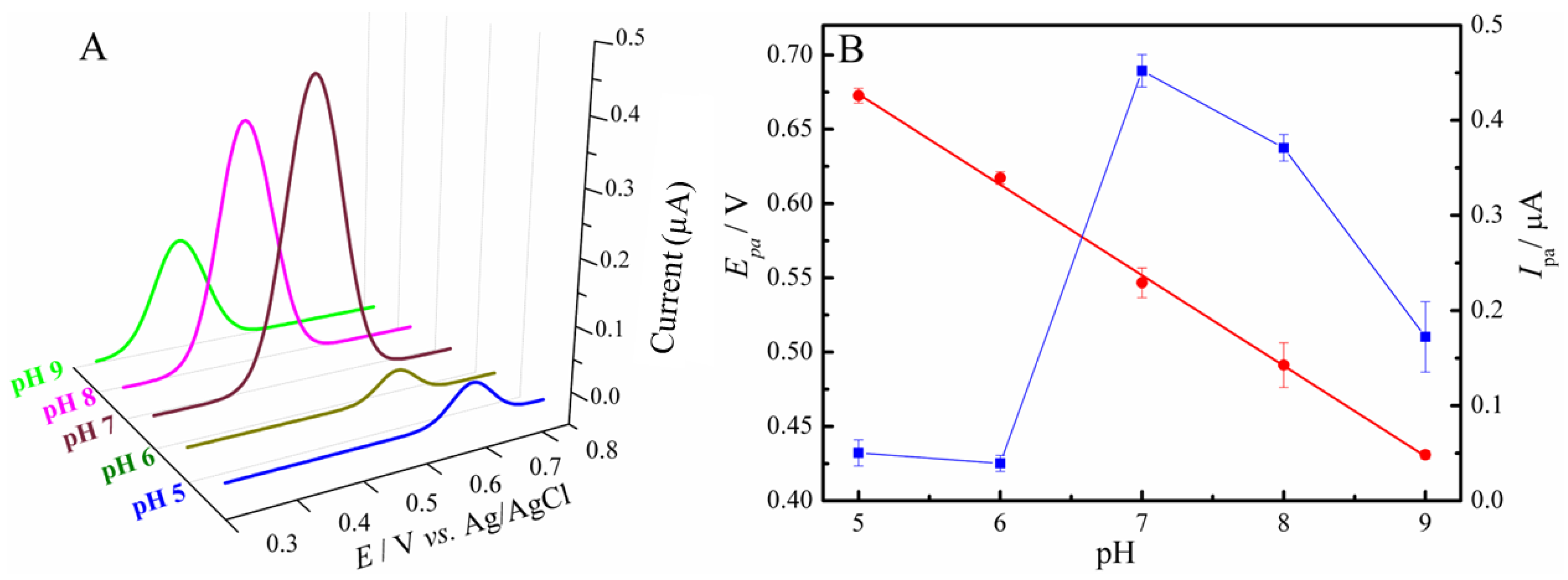
3.3. Optimization of Parameters
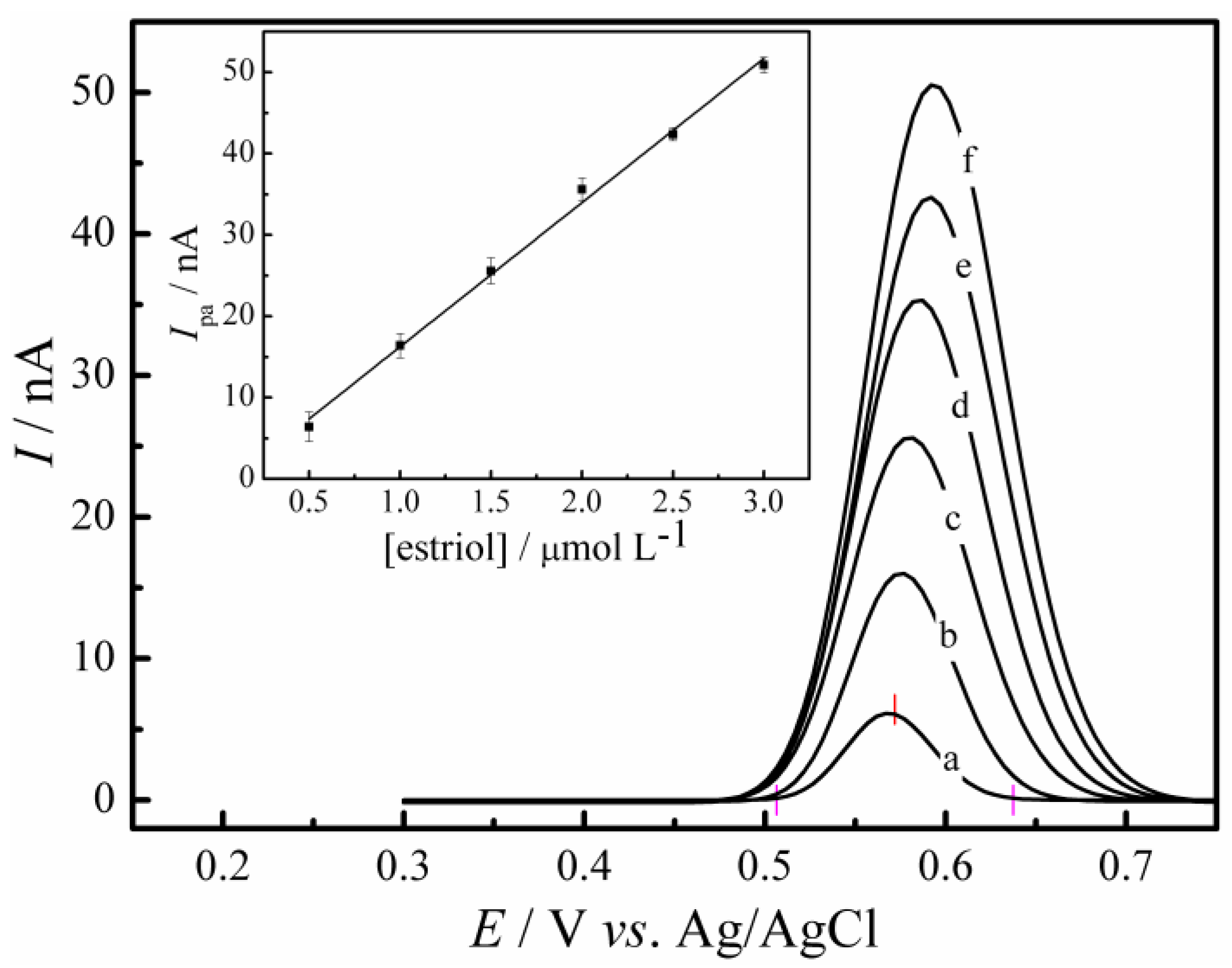
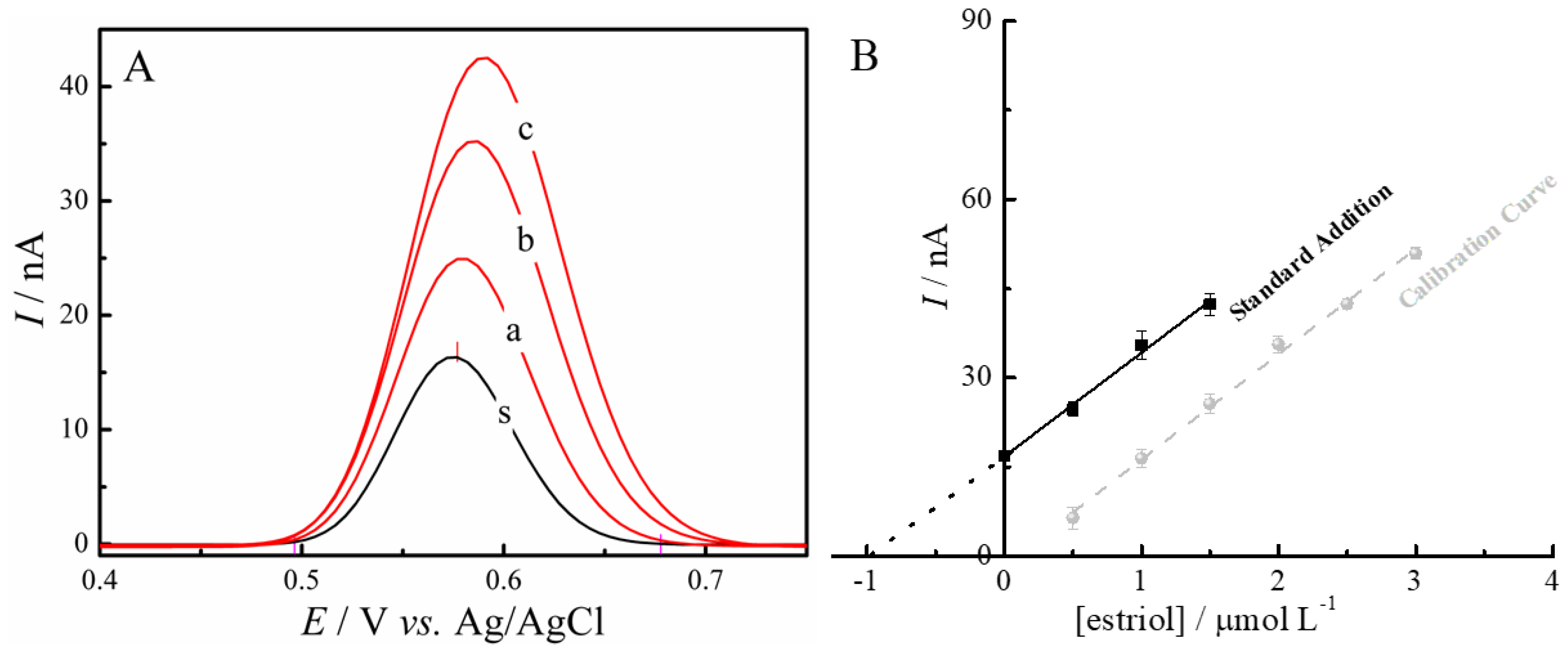
3.4. Analytical Characteristics
3.5. Determination of Estriol in Real Samples and Evaluation of the Efficiency of the Bioremediation Process
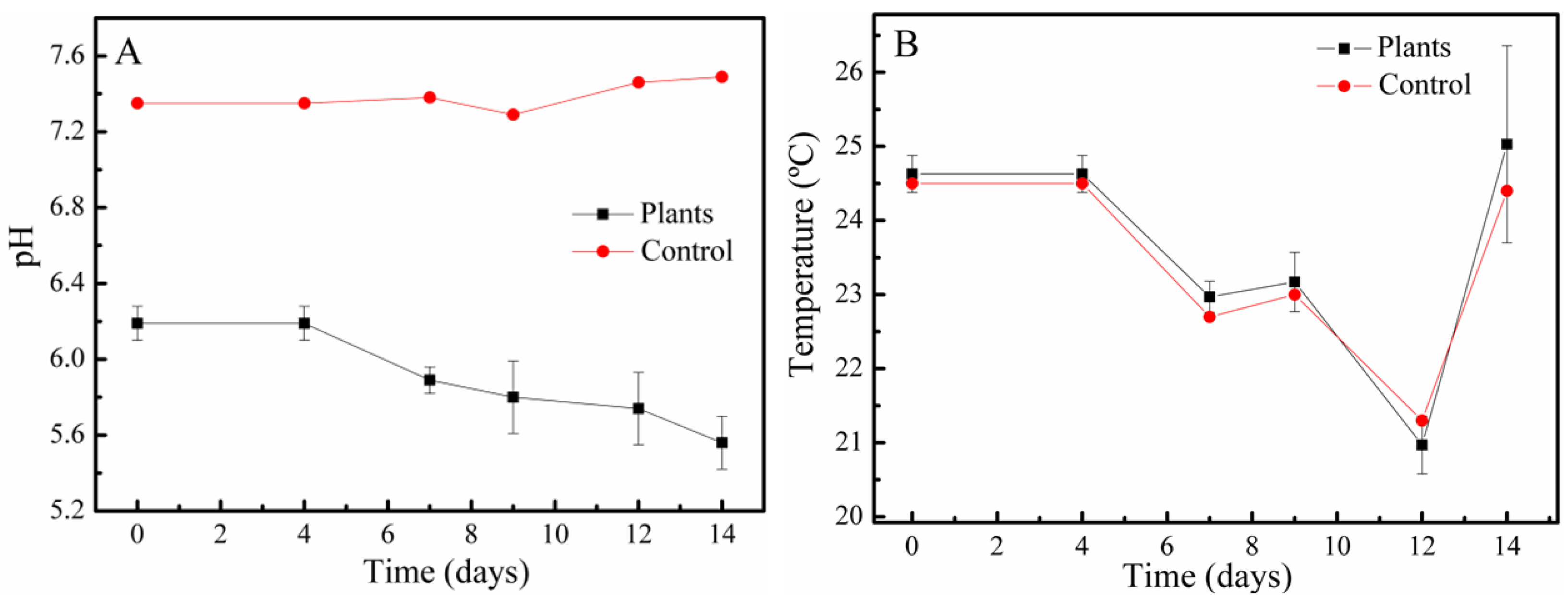
3.6. Physico-Chemical Parameters of the Bioremediation Process
3.7. Morphology Assessment of Water Hyacinth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, F.; Santana, E.R.; Spinelli, A. Ionic Liquid-Supported Magnetite Nanoparticles as Electrode Modifier Materials for Estrogens Sensing. Sci. Rep. 2020, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Cincotto, F.H.; Machado, S.A.S. A Synergistic Combination of Reduced Graphene Oxide and Antimony Nanoparticles for Estriol Hormone Detection. Sens. Actuators B Chem. 2015, 210, 453–459. [Google Scholar] [CrossRef]
- Manjunatha, J.G. Electroanalysis of Estriol Hormone Using Electrochemical Sensor. Sens. Bio-Sens. Res. 2017, 16, 79–84. [Google Scholar] [CrossRef]
- Donini, C.A.; da Silva, M.K.L.; Simões, R.P.; Cesarino, I. Reduced Graphene Oxide Modified with Silver Nanoparticles for the Electrochemical Detection of Estriol. J. Electroanal. Chem. 2018, 809, 67–73. [Google Scholar] [CrossRef]
- Gomes, E.S.; Leite, F.R.F.; Ferraz, B.R.L.; Mourão, H.A.J.L.; Malagutti, A.R. Voltammetric Sensor Based on Cobalt-Poly(Methionine)-Modified Glassy Carbon Electrode for Determination of Estriol Hormone in Pharmaceuticals and Urine. J. Pharm. Anal. 2019, 9, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Malaviya, P. Constructed Wetlands for Textile Wastewater Remediation: A Review on Concept, Pollutant Removal Mechanisms, and Integrated Technologies for Efficiency Enhancement. Chemosphere 2022, 290, 133358. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shah, M. Characterisation and Bioremediation of Wastewater: A Review Exploring Bioremediation as a Sustainable Technique for Pharmaceutical Wastewater. Groundw. Sustain. Dev. 2020, 11, 100383. [Google Scholar] [CrossRef]
- Pinto, É.S.M.; Dorn, M.; Feltes, B.C. The Tale of a Versatile Enzyme: Alpha-Amylase Evolution, Structure, and Potential Biotechnological Applications for the Bioremediation of n-Alkanes. Chemosphere 2020, 250, 126202. [Google Scholar] [CrossRef] [PubMed]
- Sayara, T.; Sánchez, A. Bioremediation of PAH-Contaminated Soils: Process Enhancement through Composting/Compost. Appl. Sci. 2020, 10, 3684. [Google Scholar] [CrossRef]
- Agarry, S.E.; Oghenejoboh, K.M.; Aworanti, O.A.; Arinkoola, A.O. Biocorrosion Inhibition of Mild Steel in Crude Oil-Water Environment Using Extracts of Musa Paradisiaca Peels, Moringa Oleifera Leaves, and Carica Papaya Peels as Biocidal-Green Inhibitors: Kinetics and Adsorption Studies. Chem. Eng. Commun. 2019, 206, 98–124. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.‘I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Madikizela, L.M. Removal of Organic Pollutants in Water Using Water Hyacinth (Eichhornia Crassipes). J. Environ. Manag. 2021, 295, 113153. [Google Scholar] [CrossRef] [PubMed]
- Correa dos Santos, N.M.; Monteiro, P.G.; Ferreira, E.A.; Alencar, B.T.B.; Cabral, C.M.; dos Santos, J.B. Use of Eichhornia Crassipes and Pistia Stratiotes for Environmental Services: Decontamination of Aquatic Environments with Atrazine Residues. Aquat. Bot. 2022, 176, 103470. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent Studies on Applications of Aquatic Weed Plants in Phytoremediation of Wastewater: A Review Article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Lin, Y.L.; Li, B.K. Removal of Pharmaceuticals and Personal Care Products by Eichhornia Crassipe and Pistia Stratiotes. J. Taiwan Inst. Chem. Eng. 2016, 58, 318–323. [Google Scholar] [CrossRef]
- Amos Sibeko, P.; Naicker, D.; Mdluli, P.S.; Madikizela, L.M. Naproxen, Ibuprofen, and Diclofenac Residues in River Water, Sediments and Eichhornia Crassipes of Mbokodweni River in South Africa: An Initial Screening. Environ. Forensics 2019, 20, 129–138. [Google Scholar] [CrossRef]
- Fernandes, V.Q.; Silva, M.K.L.; Cesarino, I. Determination of Isotretinoin (13-Cis-Retinoic Acid) Using a Sensor Based on Reduced Graphene Oxide Modified with Copper Nanoparticles. J. Electroanal. Chem. 2020, 856, 113692. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.gbif.org/pt/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 2 September 2022).
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical Deposition of Nickel Graphene Composite Coatings Effect of Deposition Temperature on Its Surface Morphology and Corrosion Resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Multiscale Patterning of Graphene Oxide and Reduced Graphene Oxide for Flexible Supercapacitors. Carbon N. Y. 2015, 92, 305–310. [Google Scholar] [CrossRef]
- Setznagl, S.; Cesarino, I. Copper Nanoparticles and Reduced Graphene Oxide Modified a Glassy Carbon Electrode for the Determination of Glyphosate in Water Samples. Int. J. Environ. Anal. Chem. 2022, 102, 293–305. [Google Scholar] [CrossRef]
- da Silveira, J.P.; Piovesan, J.V.V.; Spinelli, A. Carbon Paste Electrode Modified with Ferrimagnetic Nanoparticles for Voltammetric Detection of the Hormone Estriol. Microchem. J. 2017, 133, 22–30. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Vicentini, F.C.; Janegitz, B.C.; Mendonça, C.D.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Constantino, C.J.L.; Machado, S.A.S.; et al. Sensitive Detection of Estriol Hormone in Creek Water Using a Sensor Platform Based on Carbon Black and Silver Nanoparticles. Talanta 2017, 174, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. A Systematic Approach to Standard Addition Methods in Instrumental Analysis. J. Chem. Educ. 1980, 57, 703–706. [Google Scholar] [CrossRef]
- Castillo Loría, K.; Emiliani, J.; Bergara, C.D.; Herrero, M.S.; Salvatierra, L.M.; Pérez, L.M. Effect of Daily Exposure to Pb-Contaminated Water on Salvinia Biloba Physiology and Phytoremediation Performance. Aquat. Toxicol. 2019, 210, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, W.; Qu, Y.; Li, C.; Tian, Y.; Feng, Y. Pilot-Scale Benthic Microbial Electrochemical System (BMES) for the Bioremediation of Polluted River Sediment. J. Power Sources 2017, 356, 430–437. [Google Scholar] [CrossRef]
- Dai, Y.N.; Dan, A.; Yang, Y.; Tam, N.F.Y.; Tai, Y.P.; Tang, X.Y. Factors Affecting Behavior of Phenolic Endocrine Disruptors, Estrone and Estradiol, in Constructed Wetlands for Domestic Sewage Treatment. Environ. Sci. Technol. 2016, 50, 11844–11852. [Google Scholar] [CrossRef]
- Franks, C.G.; Pearce, D.W.; Rood, S.B. A Prescription for Drug-Free Rivers: Uptake of Pharmaceuticals by a Widespread Streamside Willow. Environ. Manag. 2019, 63, 136–147. [Google Scholar] [CrossRef]
- Hakk, H.; Sikora, L.; Casey, F.X.M. Fate of Estrone in Laboratory-Scale Constructed Wetlands. Ecol. Eng. 2018, 111, 60–68. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Liaw, E.T.; Fan, K.M. Removal of Veterinary Antibiotics, Alkylphenolic Compounds, and Estrogens from the Wuluo Constructed Wetland in Southern Taiwan. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 151–160. [Google Scholar] [CrossRef]
- Herrera-Melián, J.A.; Guedes-Alonso, R.; Borreguero-Fabelo, A.; Santana-Rodríguez, J.J.; Sosa-Ferrera, Z. Study on the Removal of Hormones from Domestic Wastewaters with Lab-Scale Constructed Wetlands with Different Substrates and Flow Directions. Environ. Sci. Pollut. Res. 2018, 25, 20374–20384. [Google Scholar] [CrossRef]
- Phouthavong-Murphy, J.C.; Merrill, A.K.; Zamule, S.; Giacherio, D.; Brown, B.; Roote, C.; Das, P. Phytoremediation Potential of Switchgrass (Panicum Virgatum), Two United States Native Varieties, to Remove Bisphenol-A (BPA) from Aqueous Media. Sci. Rep. 2020, 10, 835. [Google Scholar] [CrossRef]
- Loffredo, E.; Picca, G.; Parlavecchia, M. Single and Combined Use of Cannabis Sativa L. and Carbon-Rich Materials for the Removal of Pesticides and Endocrine-Disrupting Chemicals from Water and Soil. Environ. Sci. Pollut. Res. 2021, 28, 3601–3616. [Google Scholar] [CrossRef] [PubMed]
- Bircher, S.; Card, M.L.; Zhai, G.; Chin, Y.P.; Schnoor, J.L. Sorption, Uptake, and Biotransformation of 17β-Estradiol, 17α-Ethinylestradiol, Zeranol, and Trenbolone Acetate by Hybrid Poplar. Environ. Toxicol. Chem. 2015, 34, 2906–2913. [Google Scholar] [CrossRef]
- Pi, N.; Ng, J.Z.; Kelly, B.C. Bioaccumulation of Pharmaceutically Active Compounds and Endocrine Disrupting Chemicals in Aquatic Macrophytes: Results of Hydroponic Experiments with Echinodorus Horemanii and Eichhornia Crassipes. Sci. Total Environ. 2017, 601–602, 812–820. [Google Scholar] [CrossRef]
- Santos, K.D.; Braga, O.C.; Vieira, I.C.; Spinelli, A. Electroanalytical Determination of Estriol Hormone Using a Boron-Doped Diamond Electrode. Talanta 2010, 80, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Optimization Range | Optimized Values |
|---|---|---|
| rGO-CuNPs concentration (mg/mL) | 0.01–0.04 | 0.02 mg/mL |
| pH | 5–9 | 7.0 |
| Pre-concentration potential (V) | −1.2–−0.5 | −1.1 V |
| Electrode | Linear Range | LOD (μmol L−1) | LOQ (μmol L−1) | Matrix Sample | Ref. |
|---|---|---|---|---|---|
| CPE/Fe3O4NPs 1 | 3.0–111.0 | 2.75 | 8.35 | Pharmaceutical samples | [24] |
| GC/CNB/AgNP 2 | 0.2–3.0 | 0.16 | 0.50 | Creek water | [25] |
| GC/rGO-SbNPs 3 | 0.2–1.4 | 0.005 | Not reported | Natural waters | [2] |
| GC/rGO-AgNPs 4 | 0.1–3.0 | 0.02 | Not reported | Synthetic Urine and Tap Water | [4] |
| GC/rGO-CuNPs | 0.5–3.0 | 0.17 | 0.56 | Bioremediation system water | This work |
| Days of Bioremediation | Exp. 1 (μmol L−1) | Exp. 2 (μmol L−1) | Exp. 3 (μmol L−1) | Control (μmol L−1) |
|---|---|---|---|---|
| 1st day | 1.00 | 1.00 | 1.00 | 1.00 |
| 4th day | 0.33 ± 0.06 | 0.62 ± 0.02 | 0.73 ± 0.02 | 1.10 ± 0.10 |
| 7th day | 0.14 ± 0.05 | 0.15 ± 0.03 | 0.23 ± 0.02 | 0.96 ± 0.04 |
| 9th day | No detection | No detection | No detection | 0.88 ± 0.03 |
| Plants | Fresh Weight (g) | Root Length (cm) 1 | Petiole Length (cm) | Leaf Size (cm) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Exp. 1 | 31.35 | 38.7 | 15.0 | 19.0 | 8.38 ± 1.70 | 9.50 ± 3.42 | 5.40 ± 1.52 | 5.90 ± 1.60 |
| Exp. 2 | 36.88 | 52.1 | 16.0 | 19.5 | 9.40 ± 3.21 | 10.70 ± 2.73 | 6.42 ± 1.46 | 6.58 ± 1.11 |
| Exp. 3 | 37.90 | 44.7 | 15.5 | 21.0 | 7.75 ± 2.06 | 8.25 ± 2.50 | 5.88 ± 0.63 | 6.5 ± 1.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, F.C.; Silva, M.K.L.; Cesarino, I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors 2022, 10, 395. https://doi.org/10.3390/chemosensors10100395
Barreto FC, Silva MKL, Cesarino I. An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors. 2022; 10(10):395. https://doi.org/10.3390/chemosensors10100395
Chicago/Turabian StyleBarreto, Francisco Contini, Martin Kassio Leme Silva, and Ivana Cesarino. 2022. "An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation" Chemosensors 10, no. 10: 395. https://doi.org/10.3390/chemosensors10100395
APA StyleBarreto, F. C., Silva, M. K. L., & Cesarino, I. (2022). An Electrochemical Sensor Based on Reduced Graphene Oxide and Copper Nanoparticles for Monitoring Estriol Levels in Water Samples after Bioremediation. Chemosensors, 10(10), 395. https://doi.org/10.3390/chemosensors10100395








