Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors
Abstract
:1. Introduction
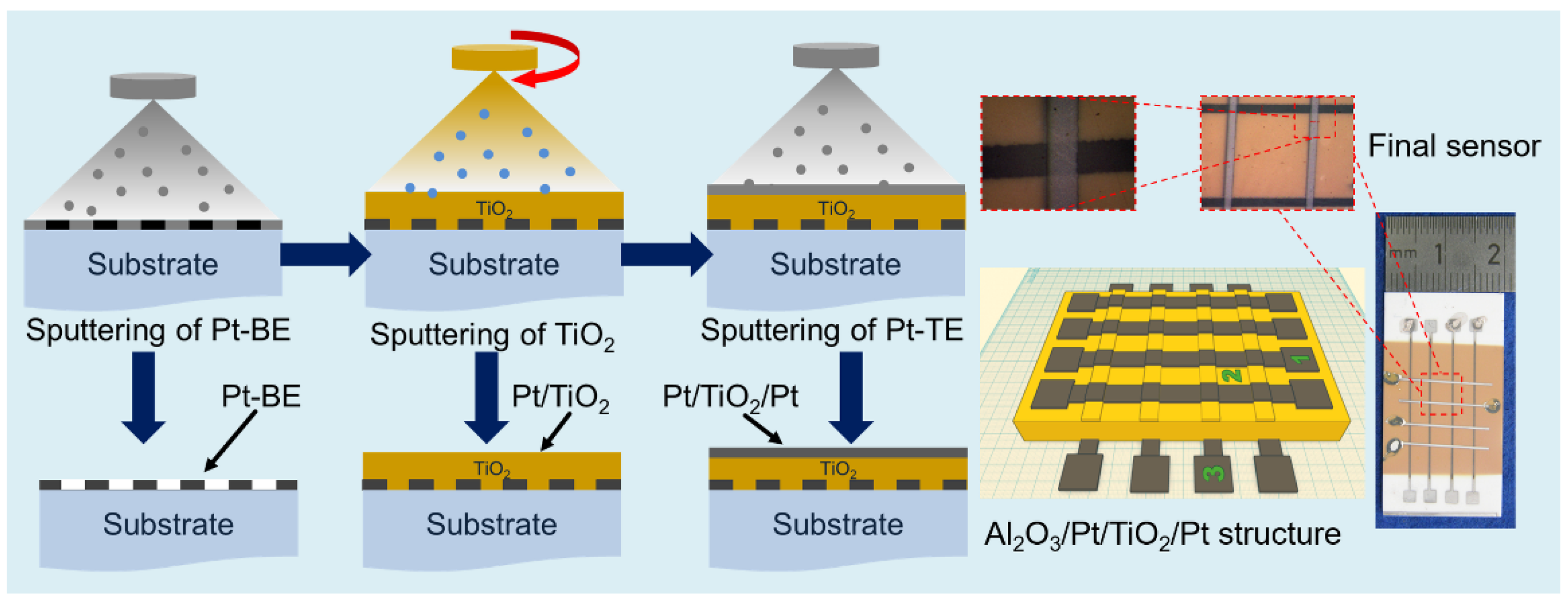
2. Materials and Methods
3. Results and Discussion
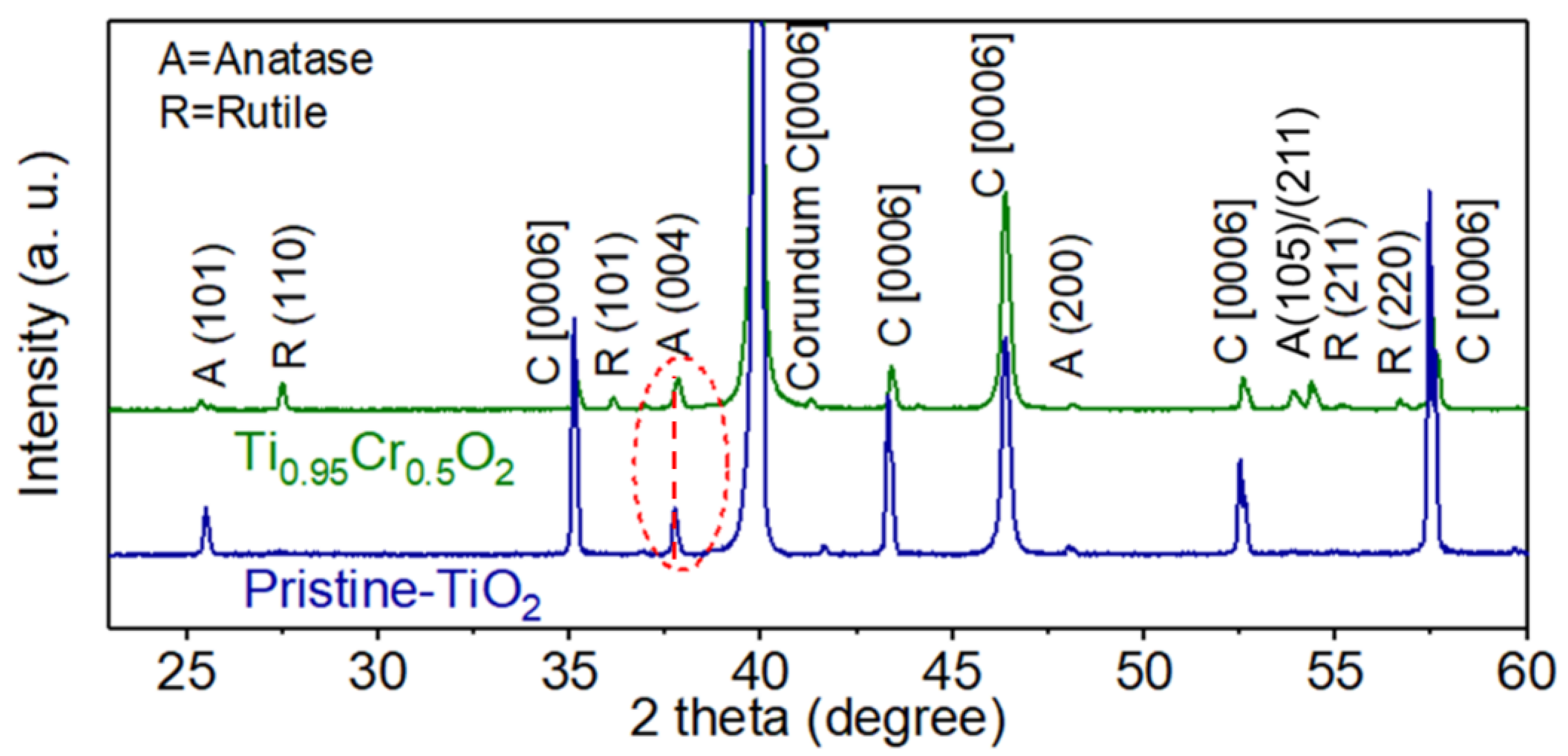
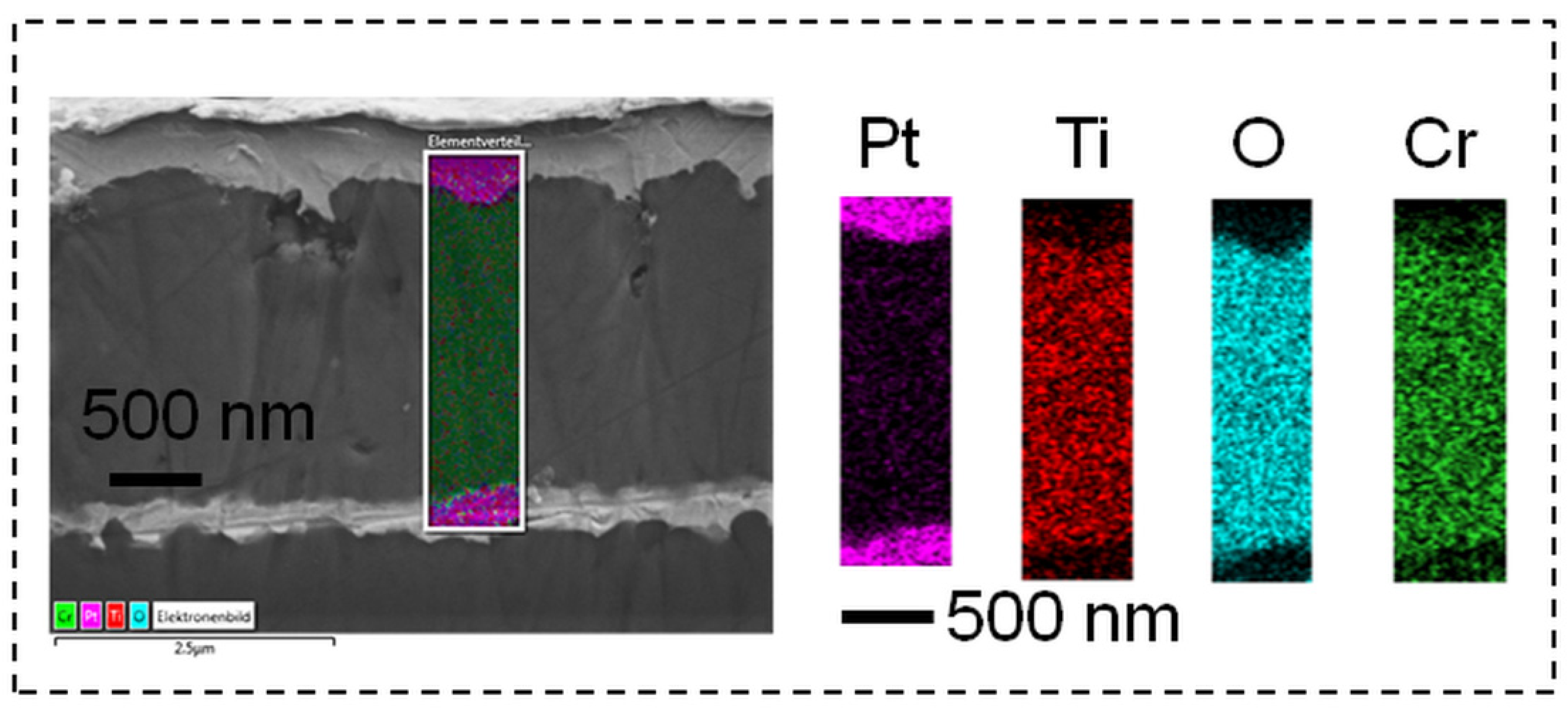
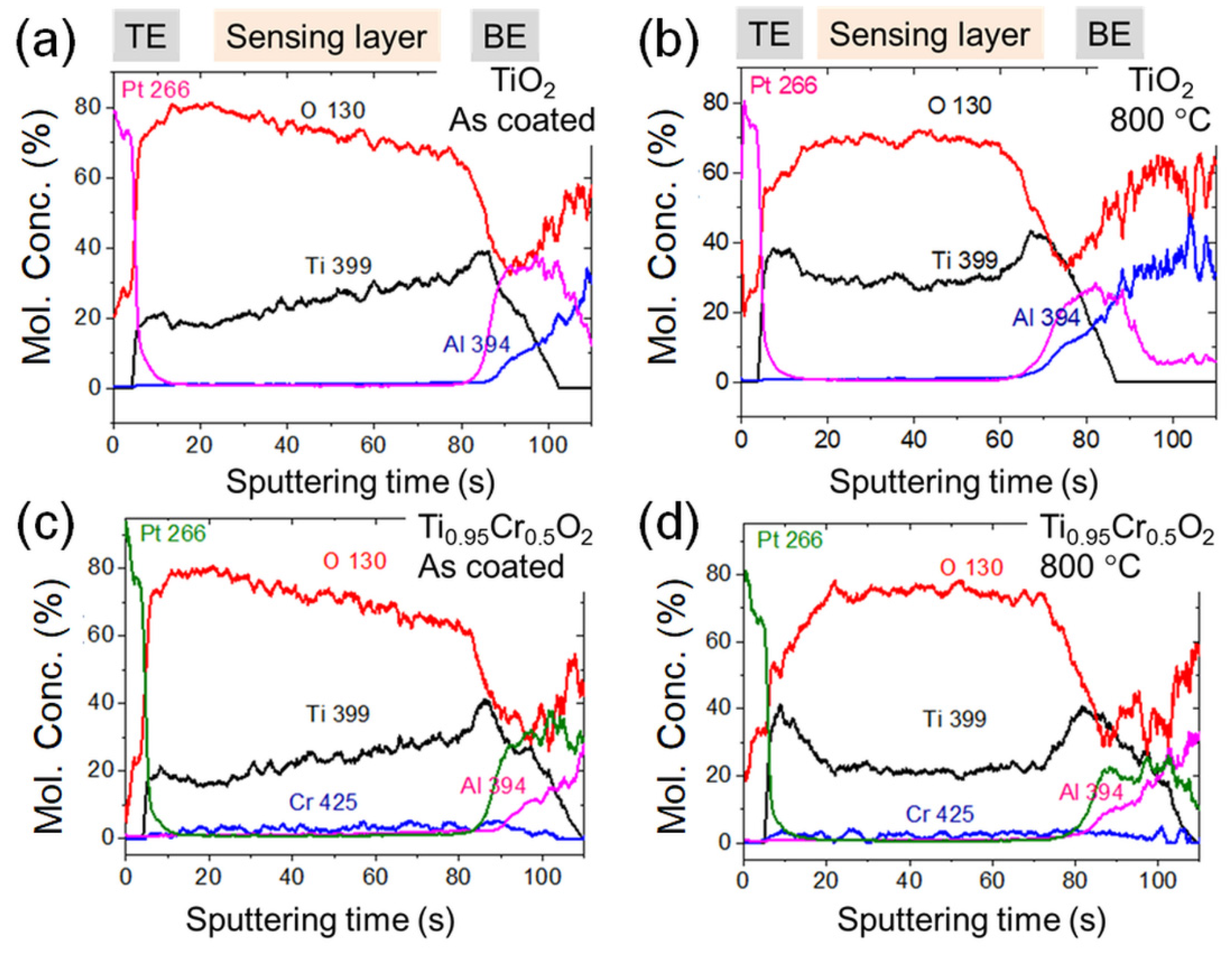
3.1. Characterization
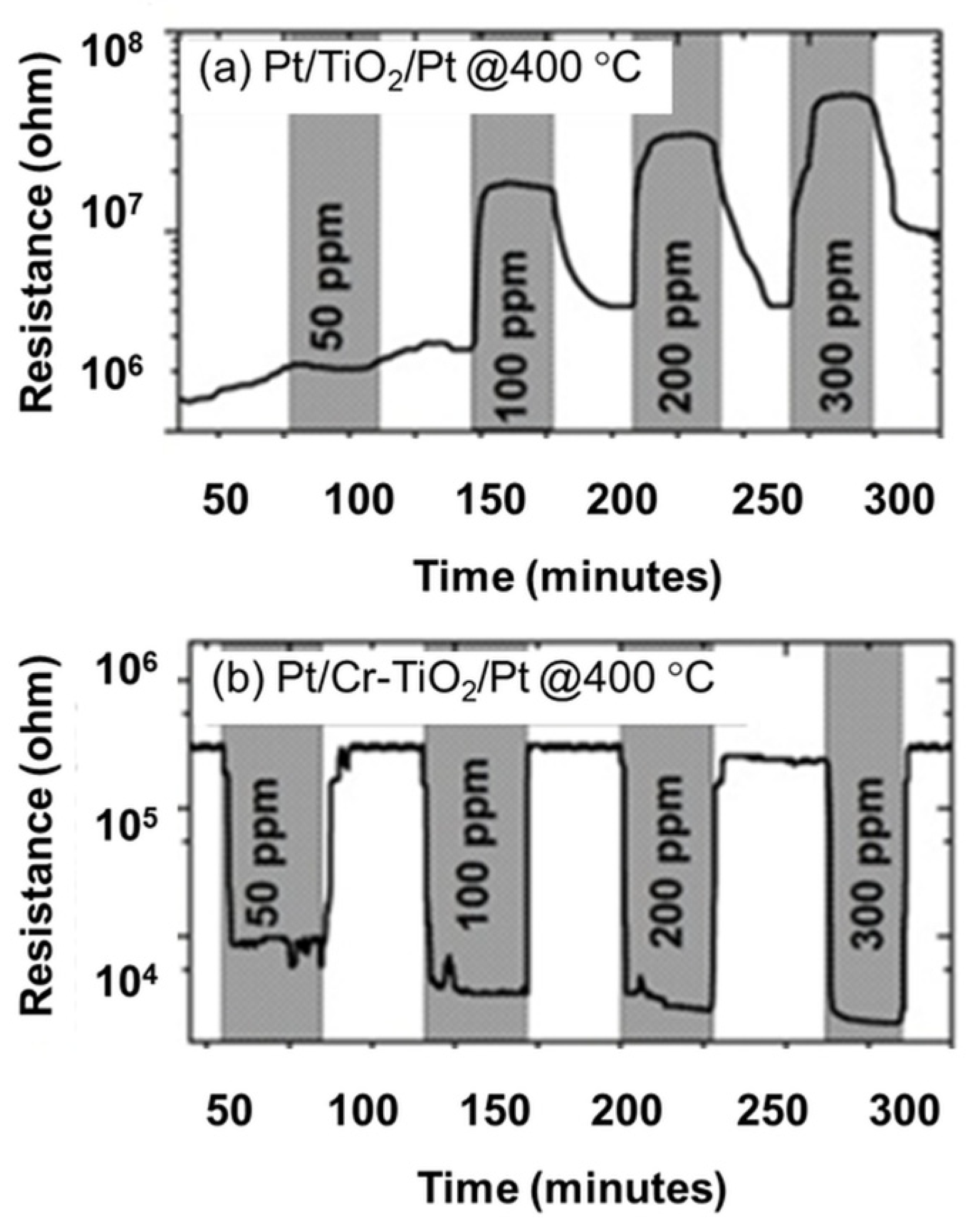
3.2. Sensor Performance
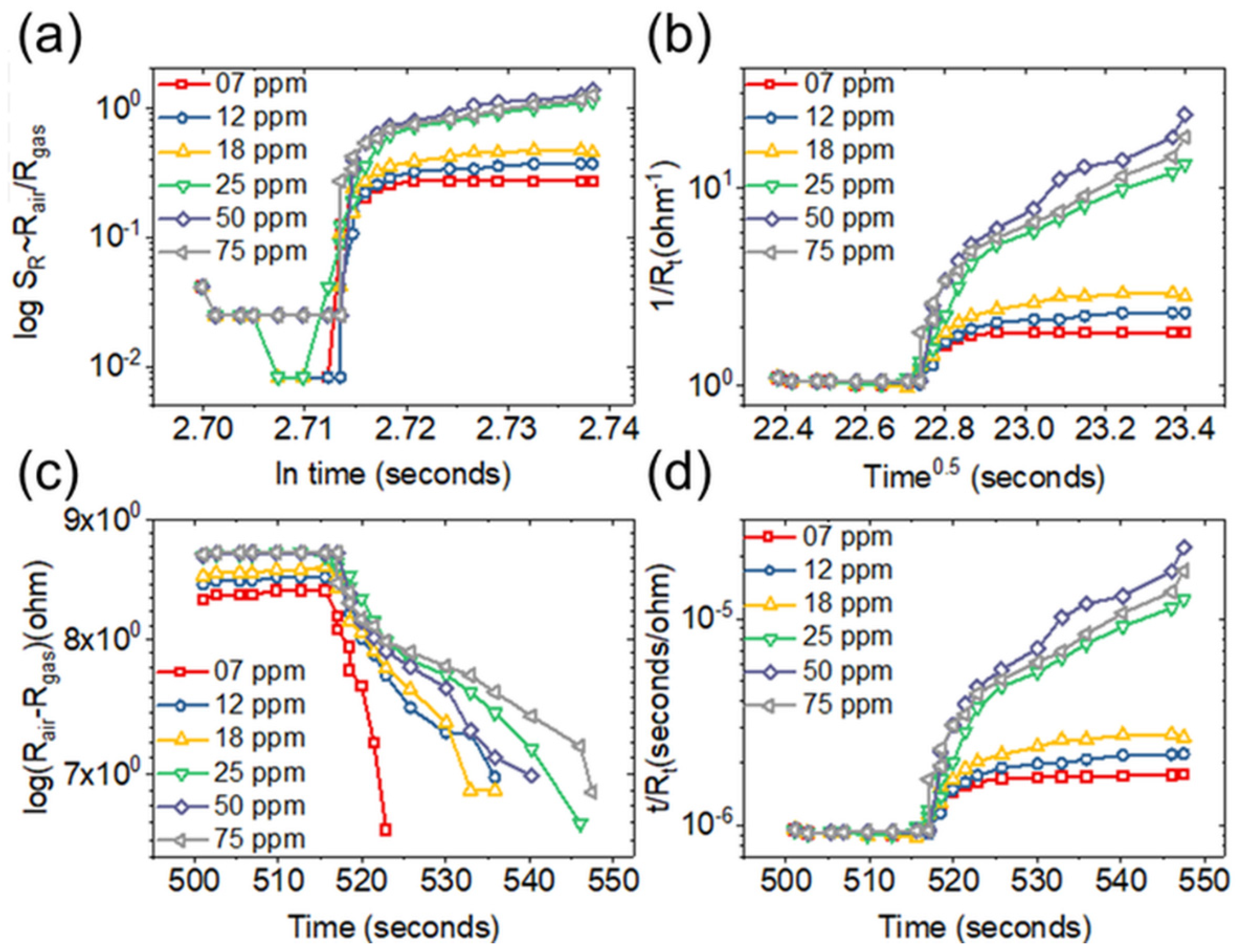
3.3. Adsorption Kinetics
3.4. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myasoedova, T.N.; Plugotarenko, N.K.; Moiseeva, T.A. Copper-Containing Films Obtained by the Simple Citrate Sol–Gel Route for NO2 Detection: Adsorption and Kinetic Study. Chemosensors 2020, 8, 79. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2-Sensing Properties of Au-Loaded Porous In2O3 Gas Sensors at Low Operating Temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Levy, J.I. Impact of residential nitrogen dioxide exposure on personal exposure: An international study. J. Air Waste Manag. Assoc. 1998, 48, 553–560. [Google Scholar] [CrossRef]
- Tomeček, D.; Piliai, L.; Hruška, M.; Fitl, P.; Gadenne, V.; Vorokhta, M.; Matolínová, I.; Vrňata, M. Study of Photoregeneration of Zinc Phthalocyanine Chemiresistor after Exposure to Nitrogen Dioxide. Chemosensors 2021, 9, 237. [Google Scholar] [CrossRef]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique. J. Mater. Sci. Mater. Electron. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Comini, E. Shelf Life Study of NiO Nanowire Sensors for NO2 Detection. Electron. Mater. Lett. 2019, 15, 743–749. [Google Scholar] [CrossRef]
- Teoh, L.G.; Hung, I.M.; Shieh, J.; Lai, W.H.; Hon, M.H. High sensitivity semiconductor NO2 gas sensor based on mesoporous WO3 thin film. Electrochem. Solid State Lett. 2003, 6, G108. [Google Scholar] [CrossRef] [Green Version]
- Barsan, N.; Koziej, D.; Weimar, U.; Chemical, A.B. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Sholehah, A.; Faroz, D.F.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Synthesis of ZnO flakes on flexible substrate and its application on ethylene sensing at room temperature. Chemosensors 2020, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Galstyan, V.; Comini, E.; Ponzoni, A.; Sberveglieri, V.; Sberveglieri, G. ZnO quasi-1D nanostructures: Synthesis, modeling, and properties for applications in conductometric chemical sensors. Chemosensors 2016, 4, 6. [Google Scholar] [CrossRef]
- Saruhan, B.; Yüce, A.; Gönüllü, Y.; Kelm, K. Effect of Al doping on NO2 gas sensing of TiO2 at elevated temperatures. Sens. Actuators B Chem. 2013, 187, 586–597. [Google Scholar] [CrossRef]
- Haidry, A.A.; Durina, P.; Tomasek, M.; Gregus, J.; Schlosser, P.; Mikula, M.; Truhly, M.; Roch, T.; Plecenik, T.; Pidik, A. Effect of post-deposition annealing treatment on the structural, optical and gas sensing properties of TiO2 thin films. Key Eng. Mater. 2012, 510–511, 467–474. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sens. Actuators B Chem. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Li, G.; Du, W.; Li, R.; Liu, Y.; Cheng, Z.; Xu, J. Biomimetic synthesis of zeolitic imidazolate frameworks and their application in high performance acetone gas sensors. Sens. Actuators B Chem. 2020, 302, 127187. [Google Scholar] [CrossRef]
- Yang, X.; Hao, X.; Liu, T.; Liu, F.; Wang, B.; Ma, C.; Liang, X.; Yang, C.; Zhu, H.; Zheng, J.; et al. CeO2-based mixed potential type acetone sensor using La1−xSrxCoO3 sensing electrode. Sens. Actuators B Chem. 2018, 269, 118–126. [Google Scholar] [CrossRef]
- Abdelghani, R.; Hassan, H.S.; Morsi, I.; Kashyout, A. Nano-architecture of highly sensitive SnO2–based gas sensors for acetone and ammonia using molecular imprinting technique. Sens. Actuators B Chem. 2019, 297, 126668. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.; Yamazaki, T.; Kikuta, T. Effect of micro-electrode geometry on NO2 gas-sensing characteristics of one-dimensional tin dioxide nanostructure microsensors. Sens. Actuators B Chem. 2011, 156, 784–790. [Google Scholar] [CrossRef]
- Tamaki, J.; Miyaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuators B Chem. 2005, 108, 202–206. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Grancic, B.; Roch, T.; Gregor, M.; Plecenik, A. The effect of Nb doping on hydrogen gas sensing properties of capacitor-like Pt/Nb-TiO2/Pt hydrogen gas sensors. J. Alloys Compd. 2019, 806, 1052–1059. [Google Scholar] [CrossRef]
- Capone, S.; Siciliano, P.; Quaranta, F.; Rella, R.; Epifani, M.; Vasanelli, L. Moisture influence and geometry effect of Au and Pt electrodes on CO sensing response of SnO2 microsensors based on sol–gel thin film. Sens. Actuators B Chem. 2001, 77, 503–511. [Google Scholar] [CrossRef]
- Haidry, A.A.; Xie, L.; Wang, Z.; Zavabeti, A.; Li, Z.; Plecenik, T.; Gregor, M.; Roch, T.; Plecenik, A. Remarkable improvement in hydrogen sensing characteristics with Pt/TiO2 interface control. ACS Sens. 2019, 4, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Haidry, A.A.; Cetin, C.; Kelm, K.; Saruhan, B. Sensing mechanism of low temperature NO2 sensing with top–bottom electrode (TBE) geometry. Sens. Actuators B Chem. 2016, 236, 874–884. [Google Scholar] [CrossRef]
- Bakri, A.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.; Said, N.; Abdullah, S.; Rahim, M. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. AIP Conf. Proc. 2017, 1788, 030030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, C. High Temperature Stable Anatase Phase Titanium Dioxide Films Synthesized by Mist Chemical Vapor Deposition. Nanomaterials 2020, 10, 911. [Google Scholar] [CrossRef]
- Sertel, B.C.; Efkere, H.I.; Ozcelik, S. Gas Sensing Properties of Cr Doped TiO2 Films Against Propane. IEEE Sens. 2020, 20, 13436–13443. [Google Scholar] [CrossRef]
- Su, T.-Y.; Chen, Y.-Z.; Wang, Y.-C.; Tang, S.-Y.; Shih, Y.-C.; Cheng, F.; Wang, Z.M.; Lin, H.-N.; Chueh, Y.-L. Highly sensitive, selective and stable NO2 gas sensors with a ppb-level detection limit on 2D-platinum diselenide films. J. Mater. Chem. C 2020, 8, 4851–4858. [Google Scholar] [CrossRef]
- Lee, S.P. Electrodes for semiconductor gas sensors. Sensors 2017, 17, 683. [Google Scholar] [CrossRef]
- Li, Z.; Xing, L.; Zhang, Z. Photocatalytic Properties of Columnar Nanostructured Films Fabricated by Sputtering Ti and Subsequent Annealing. Adv. Mater. Sci. Eng. 2012, 2012, 413638. [Google Scholar] [CrossRef] [Green Version]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Czapla, A.; Lubecka, M.; Gwizdz, P.; Schneider, K.; Zakrzewska, K.; Michalow, K.; Graule, T.; Reszka, A.; Rekas, M.; et al. Nanopowders of chromium doped TiO2 for gas sensors. Sens. Actuators B Chem. 2012, 175, 163–172. [Google Scholar] [CrossRef]
- Suhak, Y.; Roshchupkin, D.; Redkin, B.; Kabir, A.; Jerliu, B.; Ganschow, S.; Fritze, H. Correlation of Electrical Properties and Acoustic Loss in Single Crystalline Lithium Niobate-Tantalate Solid Solutions at Elevated Temperatures. Crystals 2021, 11, 398. [Google Scholar] [CrossRef]
- Perini, N.; Prado, A.; Sad, C.; Castro, E.; Freitas, M. Electrochemical impedance spectroscopy for in situ petroleum analysis and water-in-oil emulsion characterization. Fuel 2012, 91, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Dobromir, M.; Konrad-Soare, C.T.; Stoian, G.; Semchenko, A.; Kovalenko, D.; Luca, D. Surface Wettability of ZnO-Loaded TiO2 Nanotube Array Layers. Nanomaterials 2020, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Smecca, E.; Sanzaro, S.; Galati, C.; Renna, L.; Gervasi, L.; Santangelo, A.; Condorelli, G.G.; Grosso, D.; Bottein, T.; Mannino, G. Porous Gig-Lox TiO2 Doped with N2 at Room Temperature for P-Type Response to Ethanol. Chemosensors 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Saruhan, B.; Haidry, A.A.; Yüce, A.; Ciftyürek, E.; Mondragón Rodríguez, G.C. A Double Layer Sensing Electrode “BaTi(1−X)RhxO3/Al-Doped TiO2” for NO2 Detection above 600 °C. Chemosensors 2016, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.M.; Sakai, G.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Cr-doped TiO2 gas sensor for exhaust NO2 monitoring. Sens. Actuators B Chem. 2003, 93, 509–518. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Thévenet, F.; Gravejat, P.; Rousseau, A. Investigation of NO and NO2 adsorption mechanisms on TiO2 at room temperature. Appl. Catal. B Environ. 2013, 142, 196–204. [Google Scholar] [CrossRef]
- Hope, G.A.; Bard, A.J. Platinum/titanium dioxide (rutile) interface. Formation of ohmic and rectifying junctions. J. Phys. Chem. 1983, 87, 1979–1984. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, S.-J.; Wu, C.-H.; Wu, R.-J.J.S.; Physical, A.A. Synthesis of TiO2 nanowires for rapid NO2 detection. Sens. Actuators A Phys. 2018, 272, 288–294. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Gorobtsov, P.Y.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensing properties of nanostructured TiO2–xZrO2 thin films obtained by the sol–gel method. J. Sol-Gel Sci. Technol. 2019, 92, 415–426. [Google Scholar] [CrossRef]


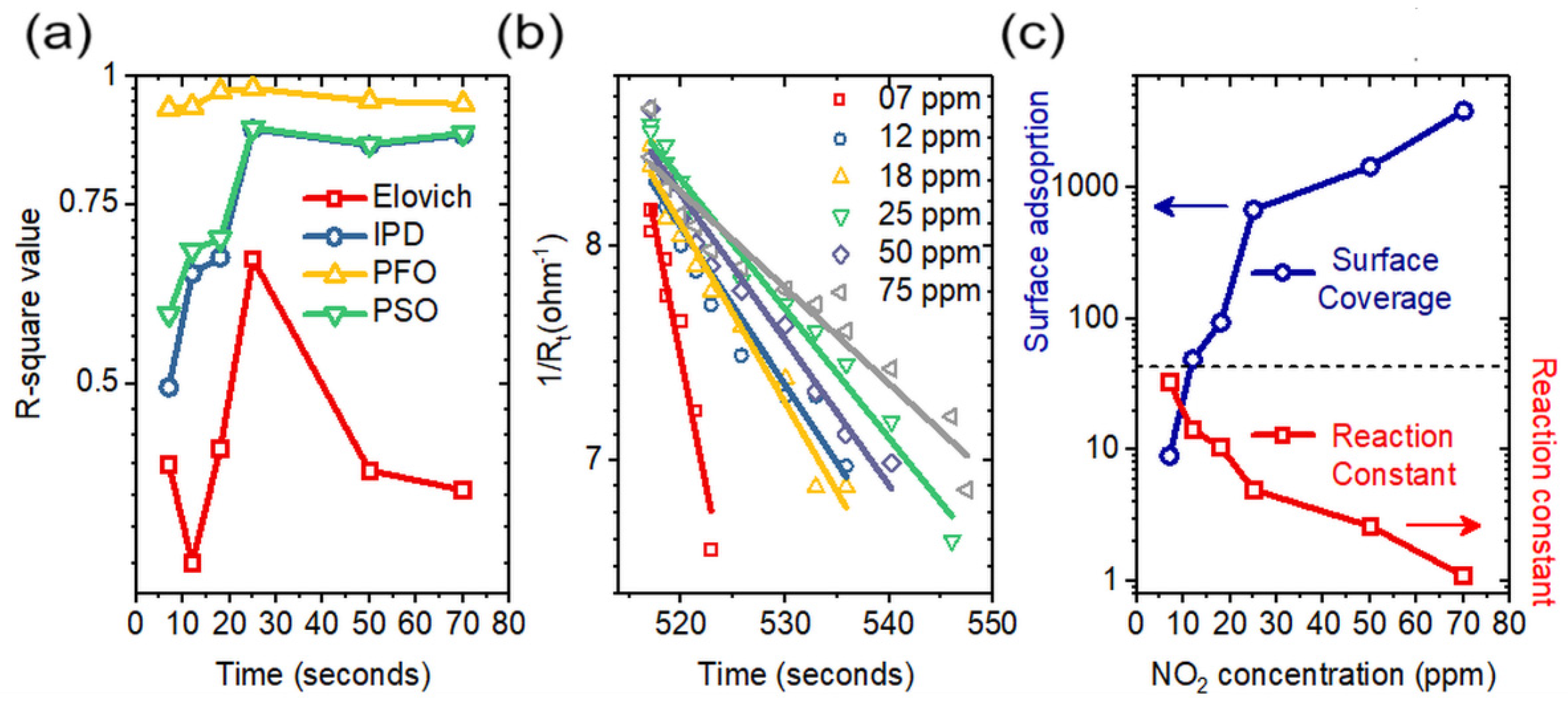

| NO2 Concentration (ppm) | R2 Value | PFO | ||||
|---|---|---|---|---|---|---|
| Elovich Model | IPD Model | PFO Model | PSO Model | Reaction Constant | Surface Coverage | |
| 7 | 0.41688 | 0.49611 | 0.93143 | 0.58517 | 3.26 × 108 | 9.03404 |
| 12 | 0.33381 | 0.64163 | 0.9351 | 0.67651 | 1.41 × 108 | 48.91089 |
| 18 | 0.43138 | 0.66521 | 0.96841 | 0.69388 | 1.05 × 108 | 93.6908 |
| 25 | 0.66231 | 0.88895 | 0.97402 | 0.8918 | 4.93 × 107 | 678.57839 |
| 50 | 0.41089 | 0.85677 | 0.9472 | 0.85997 | 2.6 × 107 | 1450.98803 |
| 70 | 0.39329 | 0.87706 | 0.94101 | 0.88094 | 1.09 × 107 | 3866.0941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidry, A.A.; Fatima, Q.; Mehmood, A.; Shahzad, A.; Ji, Y.; Saruhan, B. Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors. Chemosensors 2022, 10, 11. https://doi.org/10.3390/chemosensors10010011
Haidry AA, Fatima Q, Mehmood A, Shahzad A, Ji Y, Saruhan B. Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors. Chemosensors. 2022; 10(1):11. https://doi.org/10.3390/chemosensors10010011
Chicago/Turabian StyleHaidry, Azhar Ali, Qawareer Fatima, Ahmar Mehmood, Asim Shahzad, Yinwen Ji, and Bilge Saruhan. 2022. "Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors" Chemosensors 10, no. 1: 11. https://doi.org/10.3390/chemosensors10010011
APA StyleHaidry, A. A., Fatima, Q., Mehmood, A., Shahzad, A., Ji, Y., & Saruhan, B. (2022). Adsorption Kinetics of NO2 Gas on Pt/Cr-TiO2/Pt-Based Sensors. Chemosensors, 10(1), 11. https://doi.org/10.3390/chemosensors10010011








