Dietary Diversity as a Risk Factor for Obesity in Algerian Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Dietary Assessment
2.3. Statistical Analyses
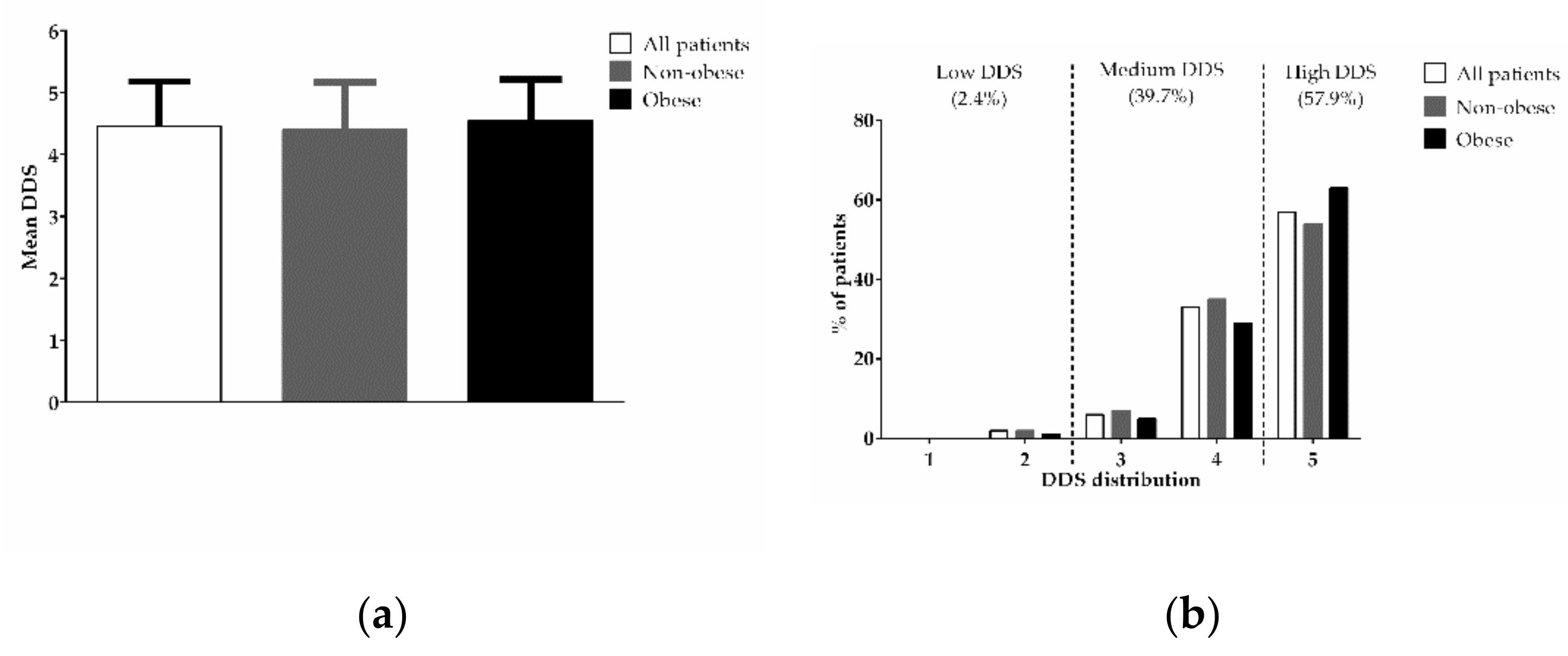
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarisco, G.; Leonetti, F. Covid-19 and diabesity: When a pandemia cross another pandemia. Eat. Weight Disord.—Stud. Anorex. Bulim. Obes. 2021, 26, 1283–1286. [Google Scholar] [CrossRef]
- Diaf, M.; Khaled, M.B.; Sellam, F. Impact of corpulence parameters and haemoglobin A1c on metabolic control in type 2 diabetic patients: Comparison of apolipoprotein B/A-I ratio with fasting and postprandial conventional lipid ratios. Libyan J. Med. 2015, 10, 27400. [Google Scholar] [CrossRef]
- Mansouri, E.H.; Reggabi, M. Association between type 2 diabetes and exposure to chlorinated persistent organic pollutants in Algeria: A case-control study. Chemosphere 2021, 264, 128596. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Boulaba, K.; Yousfi, A.; Taleb, N.; Difallah, B.; Negrichi, S. Associations between body mass index, waist circumference, waist circumference to-height ratio, and hypertension in an Algerian adult population. Environ. Sci. Pollut. Res. 2021, 28, 46514–46522. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D.; Dukessa, A.; Babusha, A. Prevalence and associated factors of overweight/obesity among type2 diabetic outpatients in Southwest Ethiopia. Heliyon 2021, 7, e06339. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Esmaillzadeh, A. Dietary diversity score is related to obesity and abdominal adiposity among Iranian female youth. Public Health Nutr. 2010, 14, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Oldewage-Theron, W.H.; Egal, A.A. A cross-sectional baseline survey investigating the relationship between dietary diversity and cardiovascular risk factors in women from the Vaal Region, South Africa. J. Nurs. Educ. Pract. 2013, 4, 50. [Google Scholar] [CrossRef]
- Jayawardena, R.; Byrne, N.M.; Soares, M.J.; Katulanda, P.; Yadav, B.; Hills, A.P. High dietary diversity is associated with obesity in Sri Lankan adults: An evaluation of three dietary scores. BMC Public Health 2013, 13, 314. [Google Scholar] [CrossRef] [Green Version]
- Azadbakht, L.; Mirmiran, P.; Esmaillzadeh, A.; Azizi, F. Dietary diversity score and cardiovascular risk factors in Tehranian adults. Public Health Nutr. 2006, 9, 728–736. [Google Scholar] [CrossRef]
- Ponce, X.; Ramirez, E.; Delisle, H. A More Diversified Diet among Mexican Men May Also Be More Atherogenic. J. Nutr. 2006, 136, 2921–2927. [Google Scholar] [CrossRef]
- Savy, M.; Martin-Prével, Y.; Danel, P.; Traissac, P.; Dabiré, H.; Delpeuch, F. Are dietary diversity scores related to the socio-economic and anthropometric status of women living in an urban area in Burkina Faso? Public Health Nutr. 2008, 11, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Ajani, S. An assessment of dietary diversity in six Nigerian states. Afr. J. Biomed. Res. 2010, 13, 161–167. [Google Scholar]
- Algerian Ministry of Health, Population, and Hospital Reform. Measurement of Risk Factors for Noncommunicable Diseases in Two Pilot Wilaya in Algeria; STEPS Survey Report; WHO: Geneva, Switzerland, 2005; Available online: https://www.who.int/ncds/surveillance/steps/STEPS_Report_Algeria.pdf?ua=1 (accessed on 21 August 2021).
- Willet, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation on Obesity; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [Green Version]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar] [CrossRef]
- Mann, J.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Kant, A.K.; Block, G.; Schatzkin, A.; Ziegler, R.G.; Nestle, M. Dietary diversity in the US population, NHANES II, 1976-1980. J. Am. Diet. Assoc. 1991, 91, 1526–1531. [Google Scholar] [CrossRef]
- Cheung, L.T.F.; Chan, R.S.M.; Ko, G.T.C.; Lau, E.S.H.; Chow, F.C.C.; Kong, A.P.S. Diet quality is inversely associated with obesity in Chinese adults with type 2 diabetes. Nutr. J. 2018, 17, 63. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, E.O.; Ter Goon, D.; Adeniyi, O.V. Central obesity and normal-weight central obesity among adults attending healthcare facilities in Buffalo City Metropolitan Municipality, South Africa: A cross-sectional study. J. Health Popul. Nutr. 2017, 36, 54. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.F.; Haregu, T.N.; Khayeka-Wandabwa, C.; Muthuri, S.K.; Kyobutungi, C. Magnitude and predictors of normal-weight central obesity– the AWI-Gen study findings. Glob. Health Action 2019, 12, 1685809. [Google Scholar] [CrossRef] [Green Version]
- Easton, J.F.; Sicilia, H.R.; Stephens, C.R. Classification of diagnostic subcategories for obesity and diabetes based on eating patterns. Nutr. Diet. 2019, 76, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.K.; Dos Santos, A.L.T.; Kirst, C.; Nunes, G.D.S.; De Franceschi, K.; De Azevedo, M.J.; Zelmanovitz, T. Dietary source of saturated fat and percentage body fat of patients with type 2 diabetes mellitus: A cross-sectional study. Food Sci. Nutr. 2018, 7, 195–204. [Google Scholar] [CrossRef]
- McCaffery, J.M.; Papandonatos, G.D.; Peter, I.; Huggins, G.S.; Raynor, H.; Delahanty, L.M.; Cheskin, L.J.; Balasubramanyam, A.; Wagenknecht, L.E.; Wing, R.R.; et al. Obesity susceptibility loci and dietary intake in the Look AHEAD Trial. Am. J. Clin. Nutr. 2012, 95, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Diaf, M.; Khaled, M.B.; Sellam, F. Correlation Between Dietary Fat Intake and Atherogenic Indices in Normal, Overweight and Obese Adults with or Without Type 2 Diabetes. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 347–360. [Google Scholar] [CrossRef] [Green Version]
| Variables | All Patients (n = 390) |
|---|---|
| Sex | |
| Male | 111 (28.5) |
| Female | 279 (71.5) |
| Age (years) | 57.1 ± 10.4 |
| <60 years | 231 (59.2) |
| ≥60 years | 159 (40.8) |
| Smoking (yes) | 11 (2.8) |
| Family history of diabetes (yes) | 280 (71.8) |
| Diabetes duration (years) | 8.8 ± 7.3 |
| <10 years | 238 (61.0) |
| ≥10 years | 152 (39.0) |
| Antidiabetic treatment strategies | |
| Dietary treatment alone | 11 (2.8) |
| Oral anti-diabetic drugs alone, any | 237 (60.8) |
| Insulin alone | 47 (12.1) |
| Oral anti-diabetic drugs plus insulin | 95 (24.4) |
| HbA1c (%) | 7.5 ± 1.7 |
| <7% | 168 (43.1) |
| ≥7% | 222 (59.9) |
| Total number of diabetes complications/comorbidities | |
| 0 | 152 (39.0) |
| 1–2 | 212 (54.4) |
| ≥3 | 26 (6.7) |
| Diabetes complications and comorbidities among participants | |
| Cardiovascular disease | 57 (14.6) |
| Retinopathy | 52 (13.3) |
| Neuropathy | 11 (2.8) |
| Nephropathy | 25 (6.4) |
| Hypertension | 184 (47.2) |
| BMI (kg/m2) | 29.4 ± 5.0 |
| Normal body weight | 79 (20.3) |
| Overweight | 150 (38.5) |
| Obese | 161 (41.3) |
| Waist circumference (cm) | 103.8 ± 12.5 |
| Normal | 25 (6.4) |
| At risk | 365 (93.5) |
| Hip circumference (cm) | 109.3 ± 12.7 |
| Waist-to-hip ratio | 0.9 ± 0.09 |
| Normal | 44 (11.3) |
| At risk | 346 (88.7) |
| Body fat (%) | 40.0 ± 8.8 |
| Fat mass index (kg/m2) | 12.1 ± 4.5 |
| Physical activity level | |
| Low | 238 (61.0) |
| Moderate | 136 (34.9) |
| High | 16 (4.1) |
| Independents Variables | Non-Obese (n = 229) | Obese (n = 161) | OR (95% CI) | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 87 (38.0) | 24 (14.9) | 1 | |
| Female | 142 (62.0) | 137 (85.1) | 3.497 (2.102–5.820) | <0.0001 |
| Age (years) | 57.9 ± 9.8 | 56.0 ± 11.0 | 0.982 (0.963–1.001) | 0.070 |
| <60 years | 128 (55.9) | 103 (64.0) | 1 | |
| ≥60 years | 101 (44.1) | 58 (36.0) | 0.714 (0.472–1.080) | 0.110 |
| Smoking | ||||
| No | - | - | 1 | |
| Yes | 9 (3.9) | 2 (1.2) | 0.307 (0.066–1.442) | 0.135 |
| Family history of diabetes | ||||
| No | - | - | 1 | |
| Yes | 166 (72.5) | 114 (70.8) | 0.921 (0.589–1.439) | 0.716 |
| Diabetes duration (years) | 8.8 ± 6.6 | 8.9 ± 8.1 | 1.001 (0.974–1.029) | 0.949 |
| <10 years | 138 (60.3) | 100 (62.1) | 1 | |
| ≥10 years | 91 (39.7) | 61 (37.9) | 0.925 (0.611–1.400) | 0.712 |
| Antidiabetic treatment strategies | ||||
| Dietary treatment alone | 5 (2.2) | 6 (3.7) | 1 | |
| Oral anti-diabetic drugs alone, any | 144 (62.9) | 93 (57.8) | 0.538 (0.160–1.814) | 0.318 |
| Insulin alone | 30 (13.1) | 17 (10.6) | 0.472 (0.125–1.781) | 0.268 |
| Oral anti-diabetic drugs plus insulin | 50 (21.8) | 45 (28.0) | 0.750 (0.214–2.626) | 0.653 |
| HbA1c (%) | 7.5 ± 1.8 | 7.5 ± 1.6 | 0.991 (0.885–1.110) | 0.876 |
| <7% | 103 (45.0) | 65 (10.4) | 1 | |
| ≥7% | 126 (55.0) | 96 (59.6) | 1.207 (0.802–1.817) | 0.366 |
| Total number of diabetes complications/comorbidities | ||||
| 0 | 94 (41.0) | 58 (36.0) | 1 | |
| 1–2 | 121 (52.8) | 91 (56.5) | 1.219 (0.796–1.865) | 0.362 |
| ≥3 | 14 (6.1) | 12 (7.5) | 1.389 (0.601–3.210) | 0.442 |
| Diabetes complications and comorbidities among participants | ||||
| Cardiovascular disease | ||||
| No | - | - | 1 | |
| Yes | 33 (14.4) | 24 (14.9) | 1.040 (0.589–1.839) | 0.891 |
| Retinopathy | ||||
| No | - | - | 1 | |
| Yes | 34 (14.8) | 18 (11.2) | 0.722 (0.392–1.330) | 0.296 |
| Neuropathy | ||||
| No | - | - | 1 | |
| Yes | 5 (2.2) | 6 (3.7) | 1.734 (0.520–5.783) | 0.370 |
| Nephropathy | ||||
| No | - | - | 1 | |
| Yes | 15 (6.6) | 10 (6.2) | 0.945 (0.413–2.160) | 0.893 |
| Hypertension | ||||
| No | - | - | 1 | |
| Yes | 97 (42.4) | 87 (54.0) | 1.600 (1.066–2.401) | 0.023 |
| BMI (kg/m2) | 26.0 ± 2.5 | 34.3 ± 3.7 | - | |
| Normal body weight | 79 (34.5) | 0 (0.0) | - | |
| Overweight | 150 (65.5) | 0 (0.0) | - | |
| Obese | 0 (0.0) | 161 (100) | - | |
| Waist circumference (cm) | 97.8 ± 10.4 | 112.3 ± 10.1 | 1.155 (1.121–1.191) | <0.0001 |
| Normal | 82 (35.8) | 3 (1.9) | 1 | |
| At risk | 147 (64.2) | 158 (98.1) | 29.379 (9.084–95.017) | <0.0001 |
| Hip circumference (cm) | 103.6 ± 11.1 | 117.3 ± 10.2 | 1.160 (1.123–1.198) | <0.0001 |
| Waist-to-hip ratio | 0.9 ± 0.09 | 0.9 ± 0.08 | 3.732 (0.418–33.329) | 0.238 |
| Normal | 29 (12.7) | 15 (9.3) | 1 | |
| At risk | 200 (87.3) | 146 (90.7) | 1.411 (0.730–2.727) | 0.305 |
| Body fat (%) | 35.1 ± 6.7 | 47.0 ± 6.3 | 1.379 (1.289–1.475) | <0.0001 |
| Fat mass index (kg/m2) | 9.2 ± 2.3 | 16.2 ± 3.7 | 3.674 (2.694–5.010) | <0.0001 |
| Physical activity level | ||||
| Low | 115 (50.2) | 123 (76.4) | 1 | |
| Moderate | 102 (44.5) | 34 (21.1) | 0.312 (0.196–0.496) | <0.0001 |
| High | 12 (5.2) | 4 (2.5) | 0.312 (0.098–0.994) | 0.049 |
| All Patients (n = 390) | Non-Obese (n = 229) | Obese (n = 161) | p | |
|---|---|---|---|---|
| Proportion consuming | ||||
| Meat group | 272 (69.7) | 152 (66.4) | 120 (74.5) | 0.084 |
| Fruit group | 362 (92.8) | 212 (92.6) | 150 (93.2) | 0.824 |
| Vegetable group | 377 (96.7) | 219 (95.6) | 158 (98.1) | 0.175 |
| Dairy group | 350 (89.7) | 200 (87.3) | 150 (93.2) | 0.062 |
| Grain group | 381 (97.7) | 226 (98.7) | 155 (96.3) | 0.118 |
| Average grams consumed | ||||
| Meat group | 87.6 ± 82.7 | 80.2 ± 78.7 | 98.1 ± 87.1 | 0.049 |
| Fruit group | 140.1 ± 94.2 | 144.8 ± 100.2 | 133.3 ± 84.7 | 0.508 |
| Vegetable group | 253.4 ± 168.4 | 260.9 ± 180.3 | 242.7 ± 149.9 | 0.612 |
| Dairy group | 207.6 ± 141.3 | 197.6 ± 139.9 | 221.9 ± 142.6 | 0.154 |
| Grain group | 290.5 ± 140.4 | 287.7 ± 143.2 | 294.3 ± 136.6 | 0.588 |
| All Patients (n = 390) | Non-Obese (n = 229) | Obese (n = 161) | p | |
|---|---|---|---|---|
| Energy intake (EI) (Kcal/d) | 1411.4 ± 378.9 | 1401.3 ± 399.6 | 1425.8 ± 348.0 | 0.318 |
| Carbohydrate (g/d) | 209.6 ± 53.2 | 210.6 ± 57.9 | 208.1 ± 45.8 | 0.940 |
| Carbohydrate (%EI) | 60.6 ± 9.9 | 61.3 ± 10.2 | 59.5 ± 9.3 | 0.075 |
| %EI from carbohydrate (45–60) | 169 (43.3) | 89 (38.9) | 80 (49.7) | 0.034 |
| Protein (g/d) | 43.5 ± 22.6 | 41.2 ± 22.8 | 46.6 ± 22.2 | 0.023 |
| Protein (%EI) | 12.3 ± 5.6 | 11.5 ± 5.5 | 13.3 ± 5.6 | 0.006 |
| %EI from protein (10–20) | 212 (54.4) | 122 (53.3) | 90 (55.9) | 0.608 |
| Fat (g/d) | 44.3 ± 25.7 | 43.7 ± 26.3 | 45.1 ± 25.0 | 0.347 |
| Fat (%EI) | 27.1 ± 10.2 | 27.0 ± 10.4 | 27.2 ± 9.9 | 0.882 |
| %EI from fat (<35) | 301 (77.2) | 176 (76.9) | 125 (77.6) | 0.856 |
| Crude Model | p | Model 1 | p | Model 2 | p | Model 3 | p | |
|---|---|---|---|---|---|---|---|---|
| DDS | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 1.335 (0.997–1.786) | 0.052 | 1.376 (1.020–1.855) | 0.037 | 1.458 (1.073–1.981) | 0.016 | 1.426 (1.029–1.974) | 0.033 |
| Meat group | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 1.003 (1.000–1.005) | 0.036 | 1.003 (1.001–1.006) | 0.017 | 1.003 (1.001–1.006) | 0.017 | 1.003 (1.000–1.006) | 0.032 |
| Fruit group | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 0.999 (0.996–1.001) | 0.238 | 0.999 (0.997–1.001) | 0.439 | 0.999 (0.997–1.002) | 0.543 | 0.999 (0.996–1.001) | 0.382 |
| Vegetable group | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 0.999 (0.998–1.001) | 0.293 | 1.000 (0.998–1.001) | 0.536 | 1.000 (0.998–1.001) | 0.649 | 1.000 (0.998–1.001) | 0.473 |
| Dairy group | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 1.001 (1.000–1.003) | 0.097 | 1.001 (0.999–1.002) | 0.281 | 1.001 (0.999–1.002) | 0.379 | 1.000 (0.999–1.002) | 0.627 |
| Grain group | ||||||||
| Non-obese | 1 | 1 | 1 | 1 | ||||
| Obese | 1.000 (0.999–1.002) | 0.649 | 1.001 (0.999–1.003) | 0.213 | 1.001 (1.000–1.003) | 0.125 | 1.000 (0.999–1.003) | 0.289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounihi, A.; Saidi, H.; Bouazza, A.; Benbaibeche, H.; Azzouz, M.; Koceir, E.A. Dietary Diversity as a Risk Factor for Obesity in Algerian Patients with Type 2 Diabetes Mellitus. Healthcare 2021, 9, 1229. https://doi.org/10.3390/healthcare9091229
Bounihi A, Saidi H, Bouazza A, Benbaibeche H, Azzouz M, Koceir EA. Dietary Diversity as a Risk Factor for Obesity in Algerian Patients with Type 2 Diabetes Mellitus. Healthcare. 2021; 9(9):1229. https://doi.org/10.3390/healthcare9091229
Chicago/Turabian StyleBounihi, Abdenour, Hamza Saidi, Asma Bouazza, Hassiba Benbaibeche, Malha Azzouz, and Elhadj Ahmed Koceir. 2021. "Dietary Diversity as a Risk Factor for Obesity in Algerian Patients with Type 2 Diabetes Mellitus" Healthcare 9, no. 9: 1229. https://doi.org/10.3390/healthcare9091229






