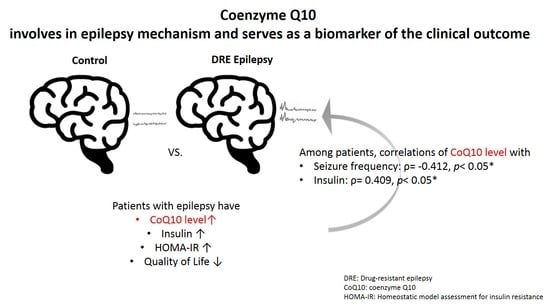
Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Sample Collection
2.3. Creatine Phosphokinase (CPK) Level
2.4. Fasting Sugar Profiles
2.5. Serum Vitamin CoQ10 Level
2.6. World Health Organization Quality of Life (WHOQoL)
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Kanner, A.M. Management of psychiatric and neurological comorbidities in epilepsy. Nat. Rev. Neurol. 2016, 12, 106. [Google Scholar] [CrossRef]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L.; Buckley, S.; Zahagun, R.; Rizvi, S. A validation of the new definition of drug-resistant epilepsy by the International League against Epilepsy. Epilepsia 2014, 55, 829–834. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization_Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 19 August 2020).
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-Resistant Epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekh-Ahmad, T.; Lieb, A.; Kovac, S.; Gola, L.; Christian Wigley, W.; Abramov, A.Y.; Walker, M.C. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019, 26, 101278. [Google Scholar] [CrossRef] [PubMed]
- Sattarinezhad, E.; Shafaroodi, H.; Sheikhnouri, K.; Mousavi, Z.; Moezi, L. The effects of coenzyme Q10 on seizures in mice: The involvement of nitric oxide. Epilepsy Behav. 2014, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.K. Coenzyme Q10 enhances the anticonvulsant effect of phenytoin in pilocarpine-induced seizures in rats and ameliorates phenytoin-induced cognitive impairment and oxidative stress. Epilepsy Behav. 2011, 22, 671–677. [Google Scholar] [CrossRef]
- Mancuso, M.; Orsucci, D.; Volpi, L.; Calsolaro, V.; Siciliano, G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr. Drug Targets 2010, 11, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Alcázar-Fabra, M.; Trevisson, E.; Brea-Calvo, G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018, 62, 377–398. [Google Scholar]
- Beal, M.F. Therapeutic effects of coenzyme Q10 in neurodegenerative diseases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 382, pp. 473–487. [Google Scholar]
- Dumont, M.; Kipiani, K.; Yu, F.; Wille, E.; Katz, M.; Calingasan, N.Y.; Gouras, G.K.; Lin, M.T.; Beal, M.F. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Mezawa, M.; Takemoto, M.; Onishi, S.; Ishibashi, R.; Ishikawa, T.; Yamaga, M.; Fujimoto, M.; Okabe, E.; He, P.; Kobayashi, K. The reduced form of coenzyme Q10 improves glycemic control in patients with type 2 diabetes: An open label pilot study. Biofactors 2012, 38, 416–421. [Google Scholar] [CrossRef]
- Bloch-Damti, A.; Bashan, N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef]
- McDonald, T.; Puchowicz, M.; Borges, K. Impairments in Oxidative Glucose Metabolism in Epilepsy and Metabolic Treatments Thereof. Front. Cell. Neurosci. 2018, 12, 274. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wu, J.; Guo, R.; Peng, Y.; Zheng, W.; Liu, D.; Song, Z. Glycolysis in energy metabolism during seizures. Neural Regen. Res. 2013, 8, 1316–1326. [Google Scholar] [PubMed]
- Chou, I.C.; Wang, C.-H.; Lin, W.-D.; Tsai, F.-J.; Lin, C.-C.; Kao, C.-H. Risk of epilepsy in type 1 diabetes mellitus: A population-based cohort study. Diabetologia 2016, 59, 1196–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauwecker, P.E. The effects of glycemic control on seizures and seizure-induced excitotoxic cell death. BMC Neurosci. 2012, 13, 94. [Google Scholar] [CrossRef] [Green Version]
- Leonardo, L.; Carlo, D.B.; Jinane, F.; Francesca, B.; Simona, P.; Silvia, G.; Sara, C.; Mario, M.; Massimiliano, P.; Anna Teresa, G. Focal epileptic seizure induced by transient hypoglycaemia in insulin-treated diabetes. Epileptic Disord. 2010, 12, 84–87. [Google Scholar]
- Casagrande, D.; Waib, P.H.; Jordão Júnior, A.A. Mechanisms of action and effects of the administration of Coenzyme Q10 on metabolic syndrome. J. Nutr. Intermed. Metab. 2018, 13, 26–32. [Google Scholar] [CrossRef]
- Ganesan, S.; Ito, M.K. Coenzyme Q10 Ameliorates the Reduction in GLUT4 Transporter Expression Induced by Simvastatin in 3T3-L1 Adipocytes. Metab. Syndr. Relat. Disord. 2013, 11, 251–255. [Google Scholar] [CrossRef]
- Gholnari, T.; Aghadavod, E.; Soleimani, A.; Hamidi, G.A.; Sharifi, N.; Asemi, Z. The Effects of Coenzyme Q10 Supplementation on Glucose Metabolism, Lipid Profiles, Inflammation, and Oxidative Stress in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2018, 37, 188–193. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Fisher, R.S.; Boas, W.V.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Matthews, D.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Chung, C.W.; Yu, C.F.; Wang, J.D. Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J. Med. Assoc. 2002, 101, 342–351. [Google Scholar]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Momin, A.; Bankar, M.; Bhoite, G. Determination of HOMA IR cut off value, and efficiency of lipids and lipoprotein ratios as discriminator of insulin resistance in type 2 Diabetes Mellitus patients. IOSR J. Pharm. Biol. Sci. 2014, 4, 9–14. [Google Scholar]
- Lee, C.; Shih, A.; Woo, Y.; Fong, C.; Leung, O.; Janus, E.; Cheung, B.; Lam, K. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: A 15-year prospective study in Chinese. PLoS ONE 2016, 11, e0163424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, S.; Yoshimitsu, S.; Hamachi, T.; Abe, H.; Ohnaka, K.; Kono, S. Waist circumference and insulin resistance: A cross-sectional study of Japanese men. BMC Endocr. Disord. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, M.; Kumar, A. Neuroprotective mechanism of Coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: Possible role of microglia inhibition. Pharmacol. Rep. 2016, 68, 1301–1311. [Google Scholar] [CrossRef]
- Nagib, M.M.; Tadros, M.G.; Rahmo, R.M.; Sabri, N.A.; Khalifa, A.E.; Masoud, S.I. Ameliorative Effects of α-Tocopherol and/or Coenzyme Q10 on Phenytoin-Induced Cognitive Impairment in Rats: Role of VEGF and BDNF-TrkB-CREB Pathway. Neurotox. Res. 2019, 35, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Keskin Güler, S.; Güneş, N.; ÇOKAL, B.G.; Yoldaş, T.; Söker, E.B. Development of Insulin Resistance in Patients with Epilepsy During Valproate and Carbamazepine Monotherapy. Epilepsi J. Turk. Epilepsi Soc. 2016, 22, 102–107. [Google Scholar]
- Çiçek, N.P.; Kamaşak, T.; Serin, M.; Okten, A.; Alver, A.; Cansu, A. The effects of valproate and topiramate use on serum insulin, leptin, neuropeptide Y and ghrelin levels in epileptic children. Seizure 2018, 58, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrotti, A.; D’Egidio, C.; Mohn, A.; Coppola, G.; Chiarelli, F. Weight gain following treatment with valproic acid: Pathogenetic mechanisms and clinical implications. Obes. Rev. 2011, 12, e32–e43. [Google Scholar] [CrossRef]
- Najafi, M.R.; Bazooyar, B.; Zare, M.; Aghaghazvini, M.R.; Ansari, B.; Rajaei, A.; Dashti, M. The Investigation of Insulin Resistance in Two Groups of Epileptic Patients Treated with Sodium Valproate and Carbamazepine. Adv. Biomed. Res. 2017, 6, 25. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Nair, D.R. The effects of antiepileptic drugs on vascular risk factors: A narrative review. Seizure 2014, 23, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.H.; Sung, P.-S.; Liao, W.C.; Chang, A.Y.W.; Hsiao, Y.-H.; Fu, T.-F.; Huang, C.-Y.; Huang, C.-W. An Open Pilot Study of the Effect and Tolerability of Add-On Multivitamin Therapy in Patients with Intractable Focal Epilepsy. Nutrients 2020, 12, 2359. [Google Scholar] [CrossRef]
- Żarnowska, I.; Wróbel-Dudzińska, D.; Tulidowicz-Bielak, M.; Kocki, T.; Mitosek-Szewczyk, K.; Gasior, M.; Turski, W.A. Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy. Seizure Eur. J. Epilepsy 2019, 69, 265–272. [Google Scholar] [CrossRef]
| AED-Resistant EP a (n = 33) | Control (n = 33) | Comparison | ||||
|---|---|---|---|---|---|---|
| Characteristics | Mean ± SD | Mean ± SD | t/χ2 | 95% CI | p-Value | |
| Lower | Upper | |||||
| Gender (male, %) | 11 (33.3) | 11 (33.3) | 0.00 | - | - | 1.000 |
| Age, years | 41.42 ± 10.22 | 40.10 ± 10.32 | 0.52 | −3.73 | 6.37 | 0.603 |
| BMI, kg/m2 | 23.14 ± 4.30 | 23.90 ± 3.66 | −0.77 | −2.73 | 1.22 | 0.445 |
| CoQ10, ng/mL | 2910.4 ± 1163.7 | (normal range: 360~1590) | ||||
| CPK, IU/L | 125.53 ± 60.39 | (normal range: 30~223) | ||||
| Blood sugar profile | ||||||
| AC glucose, mg/dL | 89.81 ± 16.08 | 88.88 ± 6.73 | 0.31 | −5.14 | 7.01 | 0.760 |
| Insulin, uIU/mL | 9.46 ± 5.50 | 6.60 ± 3.39 | 2.53 | 0.60 | 5.12 | 0.014 * |
| HOMA-IR | 2.12 ± 1.28 | 1.47 ± 0.80 | 2.47 | 0.13 | 1.18 | 0.016 * |
| HOMA-Beta, % | 143.24 ± 84.14 | 92.54 ± 46.07 | 3.03 | 17.22 | 84.19 | 0.004 * |
| HbA1c, % | 5.25 ± 0.60 | 5.59 ± 0.31 | −2.82 | −0.58 | −0.10 | 0.007 * |
| McAuley Index | 8.28 ± 2.23 | 8.92 ± 1.87 | −1.21 | −1.71 | 0.42 | 0.233 |
| WHOQoL score | ||||||
| Physical domain | 12.94 ± 2.42 | 14.56 ± 1.62 | −3.21 | −2.64 | −0.61 | 0.002 * |
| Psychological domain | 13.52 ± 2.20 | 13.56 ± 1.49 | −0.09 | −0.97 | 0.89 | 0.931 |
| Social domain | 13.91 ± 2.48 | 13.88 ± 1.76 | 0.06 | −1.03 | 1.09 | 0.955 |
| Environment domain | 14.11 ± 2.14 | 14.09 ± 1.69 | 0.06 | −0.92 | 0.98 | 0.955 |
| Integrated QOL | 3.27 ± 0.94 | 3.27 ± 0.57 | 0.00 | −0.39 | 0.39 | 1.000 |
| Integrated Health | 2.94 ± 0.79 | 3.03 ± 0.30 | −0.62 | −0.39 | 0.21 | 0.540 |
| Total scores | 93.73 ± 12.47 | 97.70 ± 8.94 | −1.48 | −9.33 | 1.40 | 0.145 |
| Parameters | CoQ10 | CPK | ||
|---|---|---|---|---|
| σ | Spearman’s p | σ | Spearman’s p | |
| Seizure frequency, episode/28 days | −0.412 | 0.037 * | −0.385 | 0.039 * |
| BMI, kg/m2 | 0.193 | 0.344 | 0.207 | 0.273 |
| CPK, IU/L | 0.231 | 0.257 | - | - |
| Blood sugar profile | ||||
| AC glucose, mg/dL | 0.013 | 0.949 | 0.117 | 0.538 |
| Insulin, µIU/mL | 0.409 | 0.038 * | −0.322 | 0.082 |
| HOMA-IR | 0.378 | 0.057 | −0.242 | 0.197 |
| HOMA-Beta, % | 0.299 | 0.138 | −0.391 | 0.033 * |
| HbA1c, % | −0.168 | 0.412 | 0.014 | 0.940 |
| McAuley Index | −0.368 | 0.065 | 0.382 | 0.041 * |
| HOMA-IR > 2.4 (n = 9) | HOMA-IR ≤ 2.4 (n = 24) | Comparison | ||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Mean ± SD | Mean ± SD | t/χ2 | 95% CI | p-Value | |
| Lower | Upper | |||||
| Gender (male, %) | 2 (25.0) | 9 (37.5) | 0.42 | 0.519 | ||
| Age, years | 41.56 ± 11.24 | 41.88 ± 9.50 | −0.08 | −7.83 | 7.20 | 0.933 |
| Seizure frequency, episode/28 days | 1.71 ± 1.31 | 8.58 ± 7.88 | −1.87 | −14.47 | 0.72 | 0.074 |
| BMI, kg/m2 | 27.04 ± 5.73 | 21.84 ± 2.79 | 2.47 | 0.36 | 10.04 | 0.038 * |
| CoQ10, ng/mL | 4387.00 ± 1351.67 | 2612.12 ± 826.84 | 3.82 | 814.62 | 2735.15 | 0.001 * |
| CPK, IU/L | 108.33 ± 41.18 | 129.83 ± 64.30 | −0.78 | −78.36 | 35.36 | 0.445 |
| Blood sugar profile | ||||||
| AC glucose, mg/dL | 98.38 ± 27.39 | 86.96 ± 9.29 | 1.16 | −11.58 | 34.41 | 0.283 |
| Insulin, µIU/mL | 16.23 ± 6.97 | 7.20 ± 2.18 | 3.60 | 3.18 | 14.88 | 0.008 * |
| HOMA-IR | 3.80 ± 1.43 | 1.56 ± 0.53 | 4.31 | 1.03 | 3.44 | 0.003 * |
| HOMA-Beta, % | 222.22 ± 130.59 | 116.91 ± 38.21 | 2.25 | −4.20 | 214.82 | 0.057 |
| HbA1c, % | 5.57 ± 0.79 | 5.15 ± 0.50 | 1.81 | −0.06 | 0.91 | 0.081 |
| McAuley Index | 5.57 ± 0.68 | 9.07 ± 1.87 | −7.62 | −4.45 | −2.56 | <0.001 * |
| WHOQoL score | ||||||
| Physical domain | 11.21 ± 2.26 | 13.29 ± 2.05 | −2.42 | −3.83 | −0.32 | 0.022 * |
| Psychological domain | 12.33 ± 1.82 | 13.67 ± 1.94 | −1.71 | −2.93 | 0.26 | 0.097 |
| Social domain | 12.67 ± 2.21 | 14.07 ± 2.21 | −1.55 | −3.25 | 0.44 | 0.131 |
| Environment domain | 13.11 ± 1.94 | 14.22 ± 1.89 | −1.43 | −2.70 | 0.48 | 0.163 |
| Integrated QOL | 3.00 ± 1.20 | 3.29 ± 0.81 | −0.78 | −1.05 | 0.47 | 0.440 |
| Integrated Health | 2.88 ± 1.13 | 2.96 ± 0.69 | −0.25 | −0.76 | 0.60 | 0.803 |
| Total scores | 86.18 ± 10.69 | 96.25 ± 12.18 | −2.08 | −19.95 | −0.19 | 0.046 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.-C.; Huang, C.-W.; Hsiao, Y.-H.; Sung, P.-S.; Fu, T.-F.; Chang, A.Y.W.; Chang, H.H. Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy. Healthcare 2021, 9, 1118. https://doi.org/10.3390/healthcare9091118
Liao W-C, Huang C-W, Hsiao Y-H, Sung P-S, Fu T-F, Chang AYW, Chang HH. Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy. Healthcare. 2021; 9(9):1118. https://doi.org/10.3390/healthcare9091118
Chicago/Turabian StyleLiao, Wei-Chen, Chin-Wei Huang, Ya-Hsin Hsiao, Pi-Shan Sung, Tzu-Fun Fu, Alice Y. W. Chang, and Hui Hua Chang. 2021. "Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy" Healthcare 9, no. 9: 1118. https://doi.org/10.3390/healthcare9091118
APA StyleLiao, W.-C., Huang, C.-W., Hsiao, Y.-H., Sung, P.-S., Fu, T.-F., Chang, A. Y. W., & Chang, H. H. (2021). Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy. Healthcare, 9(9), 1118. https://doi.org/10.3390/healthcare9091118