Gender-Related Differences in Chronic Kidney Disease-Associated Vascular Calcification Risk and Potential Risk Mediators: A Scoping Review
Abstract
1. Vascular Calcification (VC): An Introduction
2. Risk Factors of CKD-Associated VC: Gender Matters?
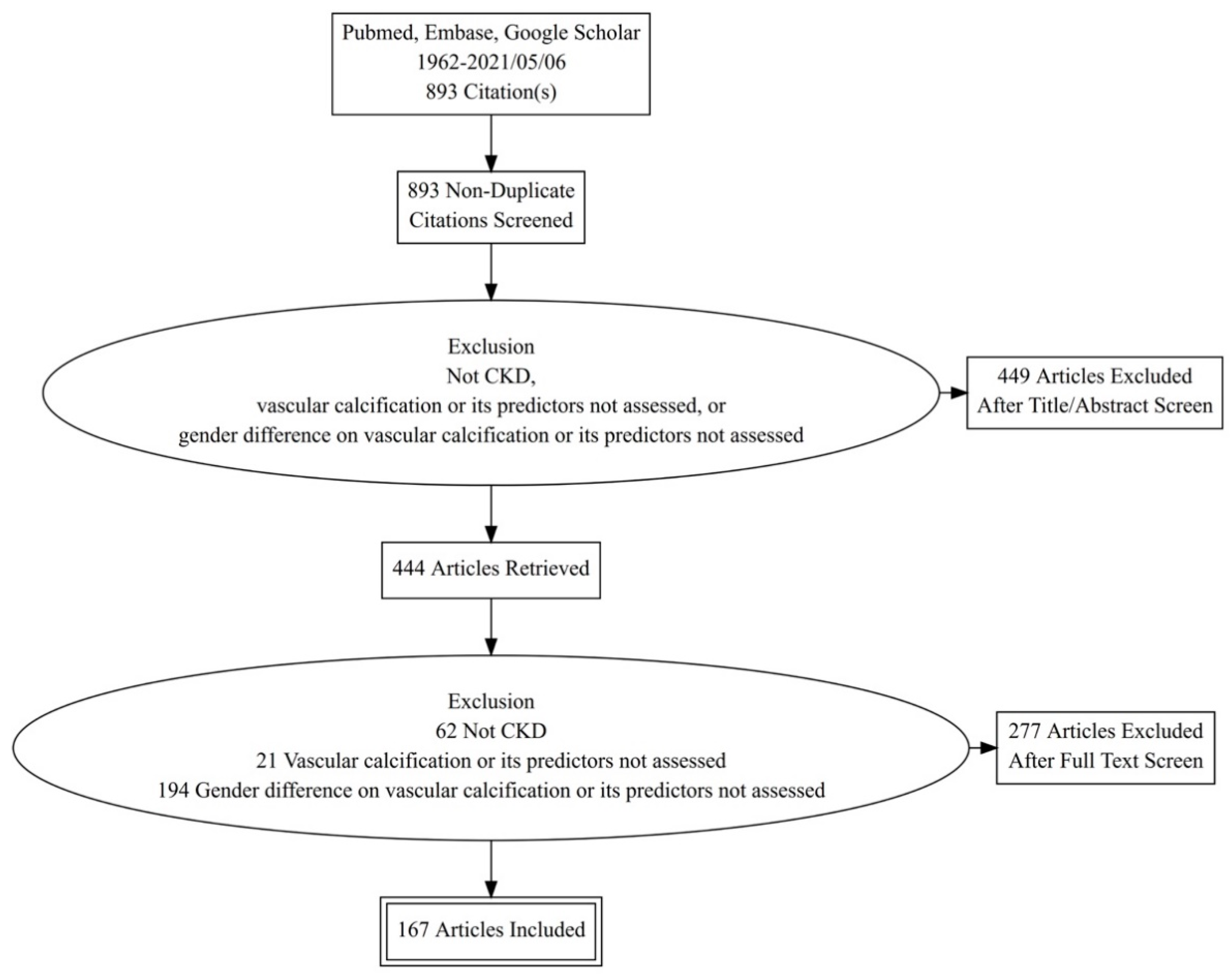
3. Literature Search Strategy
4. Summary of Findings
4.1. Gender-Related Differences in the Prevalence and/or Severity of CKD-Associated VC
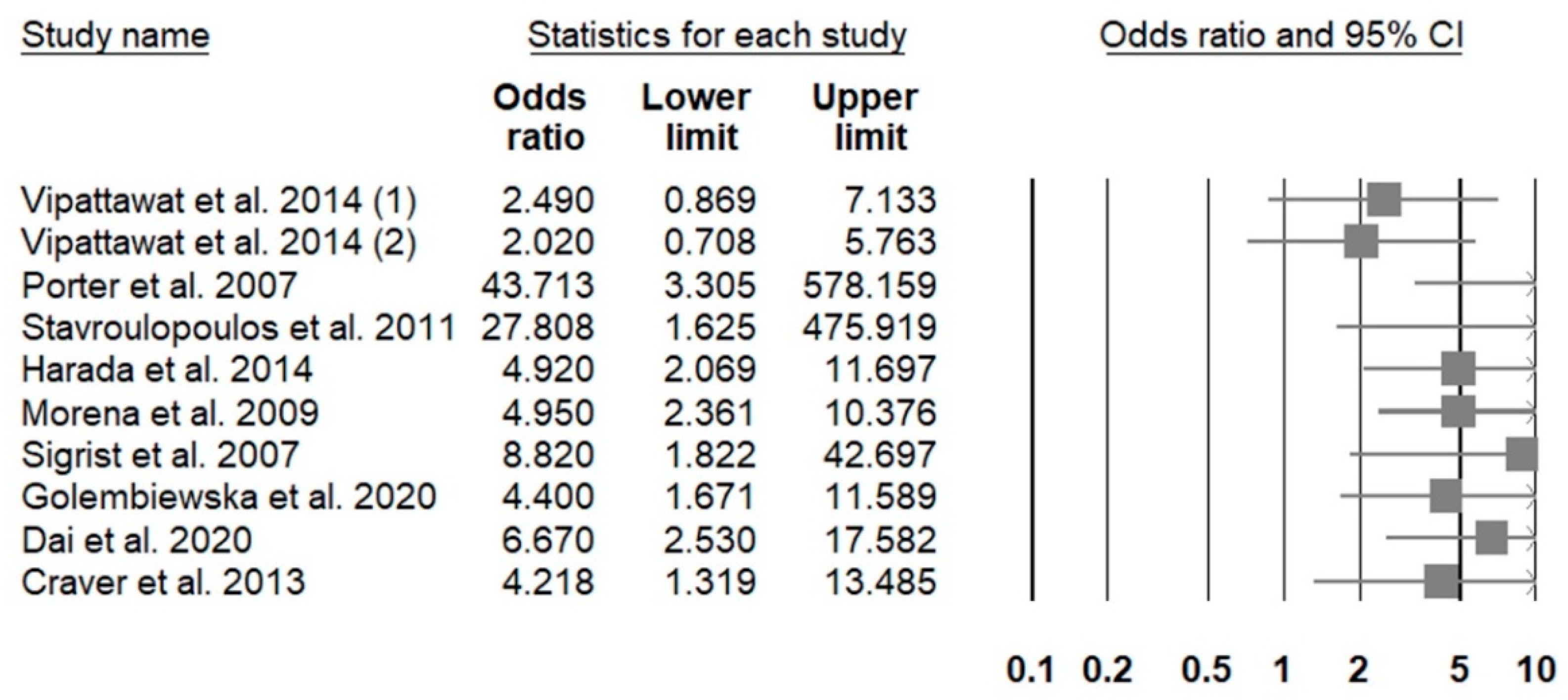
4.2. Gender-Related Differences in the Adjusted Risk of CKD-Associated VC Presence or Severity
4.3. Potential Mediators of Gender-Related Differences in CKD-Associated VC Risk
5. Overall Interpretations
5.1. Intimal or Non-Intimal Calcification: A Potential Consideration for Gender-Related Differences in CKD-Associated VC Risk
5.2. Reports Favoring a Higher Risk of CKD-Associated VC in Males: Plausible Mediators
5.3. Reports Favoring Higher Risk of CKD-Associated VC in Females: Clinical Implications and Plausible Mediators
5.4. Gender-Related Differences in Osteoprotegerin Levels and CKD-Associated VC
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yano, Y.; O’Donnell, C.J.; Kuller, L.; Kavousi, M.; Erbel, R.; Ning, H.; D’Agostino, R.; Newman, A.B.; Nasir, K.; Hofman, A.; et al. Association of Coronary Artery Calcium Score vs. Age with Cardiovascular Risk in Older Adults: An Analysis of Pooled Population-Based Studies. JAMA Cardiol. 2017, 2, 986–994. [Google Scholar] [CrossRef]
- Chao, C.-T.; Han, D.-S.; Huang, J.-W. Circulating microRNA-125b Levels Are Associated with the Risk of Vascular Calcification in Healthy Community-Dwelling Older Adults. Front. Cardiovasc. Med. 2021, 8, 624313. [Google Scholar] [CrossRef] [PubMed]
- Miedema, M.D.; Dardari, Z.A.; Nasir, K.; Blankstein, R.; Knickelbine, T.; Oberembt, S.; Shaw, L.; Rumberger, J.; Michos, E.D.; Rozanski, A.; et al. Association of Coronary Artery Calcium with Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw. Open 2019, 2, e197440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-R.; Zhang, J.-J.; Xu, X.-X.; Wu, Y.-G. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2019, 41, 244–256. [Google Scholar] [CrossRef]
- Mencke, R.; van der Vaart, A.; Pasch, A.; Harms, G.; Waanders, F.; Bilo, H.J.G.; van Goor, H.; Hillebrands, J.-L.; van Dijk, P.R. Serum calcification propensity is associated with HbA1c in type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2021, 9, e002016. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, M.; Sidhu, M.S.; Bangalore, S.; Mathew, R.O. Accelerated and intensified calcific atherosclerosis and microvascular dysfunction in patients with chronic kidney disease. Rev. Cardiovasc. Med. 2020, 21, 157–162. [Google Scholar] [CrossRef]
- Lee, J.H.; Rizvi, A.; Hartaigh, B.Ó.; Han, D.; Park, M.W.; Roudsari, H.M.; Stuijfzand, W.J.; Gransar, H.; Lu, Y.; Callister, T.Q.; et al. The Predictive Value of Coronary Artery Calcium Scoring for Major Adverse Cardiac Events According to Renal Function (from the Coronary Computed Tomography Angiography Evaluation for Clinical Outcomes: An International Multicenter [CONFIRM] Registry). Am. J. Cardiol. 2019, 123, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Eelderink, C.; Te Velde-Keyzer, C.A.; Frenay, A.S.; Vermeulen, E.A.; Bachtler, M.; Aghagolzadeh, P.; van Dijk, P.R.; Gansevoort, R.T.; Vervloet, M.G.; Hillebrands, J.-A.; et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population: The PREVEND Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Cano-Megías, M.; Guisado-Vasco, P.; Bouarich, H.; de Arriba-de la Fuente, G.; De Sequera-Ortiz, P.; Álvarez-Sanz, C.; Rodríguez-Puyol, D. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: A prospective long-term follow-up study. BMC Nephrol. 2019, 20, 188. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, A.; Wei, F.; Chen, H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 12. [Google Scholar] [CrossRef]
- Chen, S.-C.; Teh, M.; Huang, J.-C.; Wu, P.-Y.; Chen, C.-Y.; Tsai, Y.-C.; Chiu, Y.-W.; Chang, J.-M.; Chen, H.-C. Increased Aortic Arch Calcification and Cardiomegaly is Associated with Rapid Renal Progression and Increased Cardiovascular Mortality in Chronic Kidney Disease. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Handy, C.E.; Desai, C.S.; Dardari, Z.A.; Al-Mallah, M.H.; Miedema, M.D.; Ouyang, P.; Budoff, M.J.; Blumenthal, R.S.; Nasir, K.; Blaha, M.J. The Association of Coronary Artery Calcium with Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging 2016, 9, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chao, C.; Huang, J.; Huang, K. Vascular Calcification as an Underrecognized Risk Factor for Frailty in 1783 Community-Dwelling Elderly Individuals. J. Am. Hear. Assoc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Yeh, H.-Y.; Tsai, Y.-T.; Chuang, P.-H.; Yuan, T.-H.; Huang, J.-W.; Chen, H.-W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019, 5, 145. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lu, C.-L.; Yuan, T.-H.; Liao, M.-T.; Chao, C.-T.; Lu, K.-C. The Epigenetic Landscape of Vascular Calcification: An Integrative Perspective. Int. J. Mol. Sci. 2020, 21, 980. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Yeh, H.-Y.; Chao, C.-T.; Chiang, C.-K. Superoxide Dismutase 2 (SOD2) in Vascular Calcification: A Focus on Vascular Smooth Muscle Cells, Calcification Pathogenesis, and Therapeutic Strategies. Oxidative Med. Cell. Longev. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S.; Srisuwarn, P. Mechanisms of Vascular Calcification in Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 417–426. [Google Scholar] [CrossRef]
- Chao, C.; Yeh, H.; Yuan, T.; Chiang, C.; Chen, H. MicroRNA-125b in vascular diseases: An updated systematic review of pathogenetic implications and clinical applications. J. Cell. Mol. Med. 2019, 23, 5884–5894. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.; Niccoli, G.; Gragnano, F.; Grove, E.L.; Vergallo, R.; Mikhailidis, D.P.; Patti, G.; Spaccarotella, C.; Katsiki, N.; Masiero, G.; et al. Are we ready for a gender-specific approach in interventional cardiology? Int. J. Cardiol. 2019, 286, 226–233. [Google Scholar] [CrossRef]
- Tani, S.; Matsuo, R.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Matsumoto, N.; Okumura, Y. Gender differences in the associations among fish intake, lifestyle, and non-HDL-C level in Japanese subjects over the age of 50 years: Anti-atherosclerotic effect of fish consumption. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1434–1444. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 3), S21–S29. [Google Scholar]
- Tao, X.-Y.; Zuo, A.-Z.; Wang, J.-Q.; Tao, F.-B. Effect of primary ovarian insufficiency and early natural menopause on mortality: A meta-analysis. Climacteric 2016, 19, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Chan, N.; Hsu, G.; Makaryus, A.N.; Chopra, M.; Cohen, S.L.; Makaryus, J.N. Sex Differences in Coronary Arterial Calcification in Symptomatic Patients. Am. J. Cardiol. 2021, 149, 16–20. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H.I. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Ahmed, S.; Neill, K.D.O.; Hood, A.F.; Evan, A.P.; Moe, S.M. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am. J. Kidney Dis. 2001, 37, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Alayoud, A.; El Amrani, M.; Belarbi, M.; El Kharras, A.; Chtioui, M.; Elfilali, K. Facteurs de risque de progression des calcifications des artères coronaires après 5 ans d’évolution en dialyse. Ann. Cardiol. d’Angéiol. 2020, 69, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, R.; Arabi, A.; Masrouji, R.; Daouk, M. Prevalence of peripheral vascular calcifications in patients on chronic hemodialysis at a tertiary care center in Beirut: A pilot study. Leban. Med. J. 2011, 59, 117–121. [Google Scholar]
- Asci, G.; Ok, E.; Savas, R.; Ozkahya, M.; Duman, S.; Töz, H.; Kayikcioglu, M.; Branscum, A.J.; Monier-Faugere, M.-C.; Herberth, J.; et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Avramovski, P.; Avramovska, M.; Sotiroski, K.; Sikole, A. Acute-phase proteins as promoters of abdominal aortic calcification in chronic dialysis patients. Saudi J. Kidney Dis. Transplant. 2019, 30, 376–386. [Google Scholar] [CrossRef]
- Bae, E.; Seong, E.Y.; Han, B.-G.; Kim, D.K.; Lim, C.S.; Kang, S.-W.; Park, C.W.; Kim, C.-D.; Shin, B.C.; Kim, S.G.; et al. Coronary artery calcification in Korean patients with incident dialysis. Hemodial. Int. 2017, 21, 367–374. [Google Scholar] [CrossRef]
- Ballotta, E.; Renon, L.; Toffano, M.; Piccoli, A.; Da Giau, G. Patency and limb salvage rates after distal revascularization to unclampable calcified outflow arteries. J. Vasc. Surg. 2004, 39, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Block, G.A.; Ferramosca, E.; Ratti, C.; Raggi, P. Integration of clinical and imaging data to predict death in hemodialysis patients. Hemodial. Int. 2013, 17, 12–18. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Mehta, R.C.; Scialla, J.J.; De Boer, I.H.; Hsu, C.-Y.; Go, A.S.; Dobre, M.A.; Chen, J.; Rao, P.S.; et al. Serum Calcification Propensity and Clinical Events in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 1562–1571. [Google Scholar] [CrossRef]
- Rao, N.; Chandra, A.; Raj, G.; Awasthi, N.P.; Srivastava, D. Evaluation of the relationship between blood cell parameters and vascular calcification in dialysis-dependent end-stage renal disease patients. Saudi J. Kidney Dis. Transplant. 2020, 31, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Ro, H.; Kim, S.; Lee, H.H.; Chung, W.; Jung, J.Y. Study on the relationship between serum 25-hydroxyvitamin D levels and vascular calcification in hemodialysis patients with consideration of seasonal variation in vitamin D levels. Atherosclerosis 2012, 220, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Yeh, H.-Y.; Tsai, Y.-T.; Chiang, C.-K.; Chen, H.-W. A combined microRNA and target protein-based panel for predicting the probability and severity of uraemic vascular calcification: A translational study. Cardiovasc. Res. 2021, 117, 1958–1973. [Google Scholar] [CrossRef]
- Chao, C.-T.; Liu, Y.-P.; Su, S.-F.; Yeh, H.-Y.; Chen, H.-Y.; Lee, P.-J.; Chen, W.-J.; Lee, Y.-M.; Huang, J.-W.; Chiang, C.-K.; et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arter. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef]
- Charitaki, E.; Davenport, A. Aortic pulse wave velocity in haemodialysis patients is associated with the prescription of active vitamin D analogues. J. Nephrol. 2014, 27, 431–437. [Google Scholar] [CrossRef]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.; Barany, P.; Jaminon, A.M.; Schurgers, L.; Heimbürger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Budoff, M.J.; Reilly, M.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients with Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-W.; Adler, S.G.; Budoff, M.J.; Takasu, J.; Ashai, J.; Mehrotra, R. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 2010, 77, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lee, Y.-K.; Cho, A.J.; Park, H.C.; Han, C.H.; Choi, M.-J.; Koo, J.-R.; Yoon, J.-W.; Noh, J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS ONE 2019, 14, e0216415. [Google Scholar] [CrossRef]
- Chue, C.D.; Wall, N.A.; Crabtree, N.J.; Zehnder, D.; Moody, W.E.; Edwards, N.C.; Steeds, R.; Townend, J.N.; Ferro, C.J. Aortic Calcification and Femoral Bone Density Are Independently Associated with Left Ventricular Mass in Patients with Chronic Kidney Disease. PLoS ONE 2012, 7, e39241. [Google Scholar] [CrossRef]
- Claes, K.J.; Heye, S.; Bammens, B.; Kuypers, D.R.; Meijers, B.; Naesens, M.; Vanrenterghem, Y.; Evenepoel, P. Aortic calcifications and arterial stiffness as predictors of cardiovascular events in incident renal transplant recipients. Transpl. Int. 2013, 26, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Manni, M.; Agnoli, A.; Balducci, A.; Dessi, M.; De Angelis, S.; Jankovic, L.; Mantella, D.; Morosetti, M.; Naticchia, A.; et al. Cardiac Calcifications: Fetuin???A and Other Risk Factors in Hemodialysis Patients. ASAIO J. 2006, 52, 150–156. [Google Scholar] [CrossRef]
- Coll, B.; Betriu, A.; Martinez-Alonso, M.; Amoedo, M.L.; Arcidiacono, M.V.; Borras, M.; Valdivielso, J.M.; Fernández, E. Large Artery Calcification on Dialysis Patients Is Located in the Intima and Related to Atherosclerosis. Clin. J. Am. Soc. Nephrol. 2011, 6, 303–310. [Google Scholar] [CrossRef]
- Craver, L.; Dusso, A.; Martinez-Alonso, M.; Sarro, F.; Valdivielso, J.M.; Fernández, E. A low fractional excretion of Phosphate/Fgf23 ratio is associated with severe abdominal Aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol. 2013, 14, 1–221. [Google Scholar] [CrossRef]
- Davis, B.; Marin, D.; Hurwitz, L.M.; Ronald, J.; Ellis, M.J.; Ravindra, K.V.; Collins, B.H.; Kim, C.Y. Application of a Novel CT-Based Iliac Artery Calcification Scoring System for Predicting Renal Transplant Outcomes. Am. J. Roentgenol. 2016, 206, 436–441. [Google Scholar] [CrossRef]
- Deloach, S.S.; Joffe, M.M.; Mai, X.; Goral, S.; Rosas, S.E. Aortic calcification predicts cardiovascular events and all-cause mortality in renal transplantation. Nephrol. Dial. Transplant. 2009, 24, 1314–1319. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Bortone, S.; Piscopo, C.; Grimaldi, P.; Cucciniello, E.; D’Avanzo, E.; Ernesto, A.; Fiorentino, M.; Nicola, C.; Vincenzoet, B. Cardiac Vascular Calcification and QT Interval in ESRD Patients: Is There a Link? Blood Purificat. 2006, 24, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S.; Vipattawat, K.; Phakdeekitcharoen, B.; Kitiyakara, C.; Sumethkul, V. Abdominal aorta and pelvic artery calcifications on plain radiographs may predict mortality in chronic kidney disease, hemodialysis and renal transplantation. Int. Urol. Nephrol. 2018, 50, 355–364. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Maoujoud, O.; Belarbi, M.; El Farouki, M.R.; Zajjari, Y.; Boukili, Y.; Bouzelmate, H.; Rbaibi, A.; El Kharras, A.; Salahedine, T.; et al. Dépistage et facteurs de risque des calcifications cardiaques chez l’hémodialysé: Apport du scanner multi-coupe ultra-rapide et de l’échocardiographie transthoracique. Ann. Cardiol. d’Angéiol. 2015, 64, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Etta, P.K.; Sharma, R.K.; Gupta, A. Study of chronic kidney disease-mineral bone disorders in newly detected advanced renal failure patients: A Hospital-based cross-sectional study. Saudi J. Kidney Dis. Transplant. 2017, 28, 874–885. [Google Scholar]
- Fabbian, F.; Catalano, C.; Orlandi, V.; Conte, M.M.; Lupo, A.; Catizone, L. Evaluation of aortic arch calcification in hemodialysis patients. J. Nephrol. 2005, 18, 289–293. [Google Scholar]
- Fayed, A.; Soliman, A.; El Mahdy, H.; Hamza, W.; Abdulazim, D.O.; Salem, M.M.; El Din, U.A.S.; on behalf of the Vascular Calcification Group (VCG). Intraoperative Arterial Biopsy in Incident Hemodialysis Patients: Differences Observed. Nephron 2019, 143, 54–61. [Google Scholar] [CrossRef]
- Gunen Yilmaz, S.; Yilmaz, F.; Bayrakdar, I.; Harorli, A. The Relationship between carotid artery calcification and pulp stone among hemodialysis patients: A retrospective study. Saudi J. Kidney Dis. Transplant. 2019, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Harada, P.H.N.; Canziani, M.E.F.; Lima, L.M.; Kamimura, M.A.; Rochitte, C.E.; Lemos, M.M.; Cuppari, L.; Filho, R.K.; Draibe, S.A.; Santos, R.D. Pericardial Fat Is Associated with Coronary Artery Calcification in Non-Dialysis Dependent Chronic Kidney Disease Patients. PLoS ONE 2014, 9, e114358. [Google Scholar] [CrossRef]
- He, L.; He, W.-Y.; La-Ta, A.; Yang, W.-L.; Zhang, A.-H. Lower Serum Irisin Levels Are Associated with Increased Vascular Calcification in Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 287–295. [Google Scholar] [CrossRef]
- He, J.; Reilly, M.; Yang, W.; Chen, J.; Go, A.S.; Lash, J.P.; Rahman, M.; DeFilippi, C.; Gadegbeku, C.; Kanthety, R.; et al. Risk Factors for Coronary Artery Calcium Among Patients with Chronic Kidney Disease (from the Chronic Renal Insufficiency Cohort Study). Am. J. Cardiol. 2012, 110, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-S.; Lin, Y.-L.; Wang, C.-H.; Lai, Y.-H.; Kuo, C.-H.; Subeq, Y.-M.; Hsu, B.-G. Serum osteoprotegerin is an independent marker of central arterial stiffness as assessed using carotid–femoral pulse wave velocity in hemodialysis patients: A cross sectional study. BMC Nephrol. 2019, 20, 184. [Google Scholar] [CrossRef]
- Al Humoud, H.; Al-Hilali, N.; Ahmad, A.A.M.H.; Ninan, V.T.; Nampoory, M.R.N.; Rizk, A.M.; Ali, J.H.; Johny, K.V. Vascular Calcification in Dialysis Patients. Transplant. Proc. 2005, 37, 4183–4186. [Google Scholar] [CrossRef]
- Jankovic, A.; Damjanovic, T.; Djuric, Z.; Marinkovic, J.; Schlieper, G.; Djuric, P.; Dragovic, J.T.; Bulatovic, A.; Mitrovic, M.; Popovic, J.; et al. Calcification in arteriovenous fistula blood vessels may predict arteriovenous fistula failure: A 5-year follow-up study. Int. Urol. Nephrol. 2017, 49, 881–887. [Google Scholar] [CrossRef]
- Janković, A.; Damjanovic, T.; Djuric, Z.; Marinkovic, J.; Schlieper, G.; Tosic-Dragovic, J.; Djuric, P.; Popovic, J.; Floege, J.; Dimkovic, N. Impact of Vascular Calcifications on Arteriovenous Fistula Survival in Hemodialysis Patients: A Five-Year Follow-Up. Nephron 2015, 129, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jansson, H.; Saeed, A.; Svensson, M.K.; Finnved, K.; Hellström, M.; Guron, G. Impact of Abdominal Aortic Calcification on Central Haemodynamics and Decline of Glomerular Filtration Rate in Patients with Chronic Kidney Disease Stages 3 and 4. Kidney Blood Press. Res. 2019, 44, 950–960. [Google Scholar] [CrossRef]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef]
- Jean, G.; Bresson, E.; Terrat, J.-C.; Vanel, T.; Hurot, J.-M.; Lorriaux, C.; Mayor, B.; Chazot, C. Peripheral vascular calcification in long-haemodialysis patients: Associated factors and survival consequences. Nephrol. Dial. Transplant. 2009, 24, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Bresson, É.; Lorriaux, C.; Mayor, B.; Hurot, J.-M.; Deleaval, P.; Chazot, C. Increased Levels of Serum Parathyroid Hormone and Fibroblast Growth Factor-23 Are the Main Factors Associated with the Progression of Vascular Calcification in Long-Hour Hemodialysis Patients. Nephron 2012, 120, c132–c138. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Villodres, M.; García Gutiérrez, G.; García Frías, P.; Rioja Villodres, J.; Martín Velázquez, M.; Sánchez Chaparro, M.Á.; López, C.P.; Valdivielso, P. Fractional excretion of phosphorus and vascular calcification in stage 3 chronic kidney disease. J. Investig. Med. 2019, 67, 674. [Google Scholar] [CrossRef]
- Kahn, J.; Ram, L.M.; Eberhard, K.; Müller, H.; Groselj-Strele, A.; Obermayer-Pietsch, B. Calcification score evaluation in patients listed for renal transplantation. Clin. Transplant. 2017, 31, e12888. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; De Borst, M.; Berg, E.V.D.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, I.M.; Kim, D.; Lee, J.P.; So, Y.H. Effect of Inflow Arterial Calcification on Arteriovenous Fistula Maturation. Ann. Vasc. Surg. 2019, 58, 331–337. [Google Scholar] [CrossRef]
- Kim, H.G.; Song, S.W.; Kim, T.Y.; Kim, Y.O. Risk factors for progression of aortic arch calcification in patients on maintenance hemodialysis and peritoneal dialysis. Hemodial. Int. 2011, 15, 460–467. [Google Scholar] [CrossRef]
- Kimura, K.; Saika, Y.; Otani, H.; Fujii, R.; Mune, M.; Yukawa, S. Factors associated with calcification of the abdominal aorta in hemodialysis patients. Kidney Int. 1999, 56, S238–S241. [Google Scholar] [CrossRef]
- Komatsu, M.; Okazaki, M.; Tsuchiya, K.; Kawaguchi, H.; Nitta, K. Aortic Arch Calcification Predicts Cardiovascular and All-Cause Mortality in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2014, 39, 658–667. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, C.-C.; Hsu, C.-Y.; Chiou, T.T.-Y.; Ng, H.-Y.; Wu, C.-H.; Kuo, W.-H.; Lee, Y.-T. Calcification of the Aortic Arch Predicts Cardiovascular and All-Cause Mortality in Chronic Hemodialysis Patients. Cardiorenal Med. 2014, 4, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Lee, Y.-T.; Tain, Y.-L.; Ng, H.-Y.; Kuo, W.-H. Circulating microRNAs and vascular calcification in hemodialysis patients. J. Int. Med. Res. 2019, 47, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.T.; Park, K.S.; Kwon, Y.E.; Han, S.H.; Kang, S.-W.; Choi, K.H.; Oh, K.-H.; Park, S.K.; Chae, D.W.; et al. Normal body mass index with central obesity has increased risk of coronary artery calcification in Korean patients with chronic kidney disease. Kidney Int. 2016, 90, 1368–1376. [Google Scholar] [CrossRef]
- Lioufas, N.M.; Pedagogos, E.; Hawley, C.M.; Pascoe, E.M.; Elder, G.J.; Badve, S.; Valks, A.; Toussaint, N.D.; on behalf of the IMPROVE-CKD Investigators. Aortic Calcification and Arterial Stiffness Burden in a Chronic Kidney Disease Cohort with High Cardiovascular Risk: Baseline Characteristics of the Impact of Phosphate Reduction On Vascular End-Points in Chronic Kidney Disease Trial. Am. J. Nephrol. 2020, 51, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Zhou, Y.; Zhu, D.; Wang, Q.; Hao, L. Aberrant activation of Wnt pathways in arteries associates with vascular calcification in chronic kidney disease. Int. Urol. Nephrol. 2016, 48, 1313–1319. [Google Scholar] [CrossRef]
- Lockhart, M.E.; Robbin, M.L.; McNamara, M.M.; Allon, M. Association of pelvic arterial calcification with arteriovenous thigh graft failure in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 2564–2569. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Marchais, S.J. Vascular Calcifications, Arterial Aging and Arterial Remodeling in ESRD. Blood Purif. 2013, 35, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Maharem, D.A.; Gomaa, S.H.; El Ghandor, M.K.; Mohamed, E.I.; Matrawy, K.A.; Zaytoun, S.S.; Nomeir, H.M. Association of serum fetuin-A and fetuin-A gene polymorphism in relation to mineral and bone disorders in patients with chronic kidney disease. Egypt. J. Med. Hum. Genet. 2013, 14, 337–352. [Google Scholar] [CrossRef]
- Mazzaferro, S.; Pasquali, M.; Pugliese, F.; Barresi, G.; Carbone, I.; Francone, M.; Sardella, D.; Taggi, F. Serum Levels of Calcification Inhibition Proteins and Coronary Artery Calcium Score: Comparison between Transplantation and Dialysis. Am. J. Nephrol. 2007, 27, 75–83. [Google Scholar] [CrossRef]
- Merjanian, R.; Budoff, M.; Adler, S.; Berman, N.; Mehrotra, R. Coronary artery, aortic wall, and valvular calcification in nondialyzed individuals with type 2 diabetes and renal disease. Kidney Int. 2003, 64, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Adachi, H.; Nomura-Nakayama, K.; Okada, K.; Okino, K.; Hayashi, N.; Fujimoto, K.; Furuichi, K.; Yokoyama, H. Circulating CTRP9 correlates with the prevention of aortic calcification in renal allograft recipients. PLoS ONE 2020, 15, e0226526. [Google Scholar] [CrossRef]
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Mizuno, K.; Ishine, M.; Doi, S.; Masaki, T.; Shigemoto, K. Coronary artery calcification score and common iliac artery calcification score in non-dialysis CKD patients. Nephrology 2018, 23, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Dupuy, A.-M.; Jaussent, I.; Vernhet, H.; Gahide, G.; Klouche, K.; Bargnoux, A.-S.; Delcourt, C.; Canaud, B.; Cristol, J.-P. A cut-off value of plasma osteoprotegerin level may predict the presence of coronary artery calcifications in chronic kidney disease patients. Nephrol. Dial. Transplant. 2009, 24, 3389–3397. [Google Scholar] [CrossRef][Green Version]
- Munguía, P.; Caramelo, R.; Rubio, M.; Sahdalá, L.; Arnaudas, L.; Paul, J.; Blasco, A.; Lou, L.; Aladren, M.; Sanjuan, A.; et al. Pre-Transplant Assessment of Vascular Calcification as a Risk Factor of Mortality, Graft Loss, and Cardiovascular Events in Renal Transplant Recipients. Transplant. Proc. 2015, 47, 2368–2370. [Google Scholar] [CrossRef]
- Nitta, K.; Hanafusa, N.; Okazaki, M.; Komatsu, M.; Kawaguchi, H.; Tsuchiya, K. Association Between Risk Factors Including Bone-Derived Biomarkers and Aortic Arch Calcification in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhao, H.; Wu, B.; Tsai, S.; Wu, J.; Zhang, M.; Lu, L.; Qiao, J.; Men, C.; Zuo, L.; et al. Abdominal aortic calcification is superior to other arteries calcification in predicting the mortality in peritoneal dialysis patients—A 8 years cohort study. BMC Nephrol. 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Yang, S.; Gan, L.; Zhao, H.; Zuo, L. Different type and dosage of heparin were not associated with the progression of coronary artery calcification in haemodialysis patients. Nephrol. 2020, 25, 551–558. [Google Scholar] [CrossRef]
- Okamoto, T.; Hatakeyama, S.; Kodama, H.; Horiguchi, H.; Kubota, Y.; Kido, K.; Momota, M.; Hosogoe, S.; Tanaka, Y.; Takashima, T.; et al. The relationship between poor nutritional status and progression of aortic calcification in patients on maintenance hemodialysis. BMC Nephrol. 2018, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Baralic, M.; Brkovic, V.; Arsenovic, A.; Stojanov, V.; Lalic, N.; Stanisavljevic, D.; Jankovic, A.; Radivojevic, N.; Pejanovic, S.; et al. Significance of acPWV for Survival of Hemodialysis Patients. Medicina 2020, 56, 435. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.L.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Söderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Bellasi, A.; Gamboa, C.; Ferramosca, E.; Ratti, C.; Block, G.A.; Muntner, P. All-cause Mortality in Hemodialysis Patients with Heart Valve Calcification. Clin. J. Am. Soc. Nephrol. 2011, 6, 1990–1995. [Google Scholar] [CrossRef]
- Renaud, H.; Atik, A.; Hervé, M.; Morinière, P.; Hocine, C.; Belbrik, S.; Fournier, A. Evaluation of Vascular Calcinosis Risk Factors in Patients on Chronic Hemodialysis: Lack of Influence of Calcium Carbonate. Nephron 1988, 48, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Ramos, A.; Brandão, A.; Rebelo, J.R.; Guerra, A.; Resina, C.; Vila-Lobos, A.; Carvalho, F.; Remédio, F.; Ribeiro, F. Cardiac valve calcification in haemodialysis patients: Role of calcium-phosphate metabolism. Nephrol. Dial. Transplant. 1998, 13, 2037–2040. [Google Scholar] [CrossRef] [PubMed]
- Roca-Tey, R.; Páez, R.; Rivas, A.; Samon, R.; Ibrik, O.; Giménez, I.; Viladoms, J. Prevalence and functional effect of arteriovenous fistula calcifications, evaluated by spiral CT in chronic haemodialysis patients. Nefrología 2009, 29, 214–221. [Google Scholar] [CrossRef]
- Schlieper, G.; Krüger, T.; Djuric, Z.; Damjanovic, T.; Markovic, N.; Schurgers, L.; Brandenburg, V.M.; Westenfeld, R.; Dimkovic, S.; Ketteler, M.; et al. Vascular access calcification predicts mortality in hemodialysis patients. Kidney Int. 2008, 74, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.-H.; Tsai, I.-C.; Ho, H.-C.; Wu, M.-J.; Chen, C.-H.; Yu, T.-M.; Chuang, Y.-W.; Huang, S.-T. Coronary Artery Calcification in Kidney Transplant Recipients with Long-term Follow-up. Transplant. Proc. 2012, 44, 687–690. [Google Scholar] [CrossRef]
- Sigrist, M.K.; Taal, M.W.; Bungay, P.; McIntyre, C.W. Progressive Vascular Calcification over 2 Years Is Associated with Arterial Stiffening and Increased Mortality in Patients with Stages 4 and 5 Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 1241–1248. [Google Scholar] [CrossRef]
- Stróżecki, P.; Odrowąż-Sypniewska, G.; Manitius, J. Cardiac Valve Calcifications and Left Ventricular Hypertrophy in Hemodialysis Patients. Renal Fail. 2005, 27, 733–738. [Google Scholar] [CrossRef]
- Tangvoraphonkchai, K.; Davenport, A. Reduction in Aortic Pulse Wave Velocity Is Associated with a Short-Term Reduction in Dual-Energy X-Ray Absorptiometry Lumbar Spine Bone Mineral Density T Score. Blood Purif. 2019, 48, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, C.; Carvalho, A.B.; Higa, A.; Jorgetti, V.; Draibe, S.A.; Canziani, M.E.F. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Miner. Res. 2010, 25, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.N.; Kircelli, F.; Yaprak, M.; Sisman, A.R.; Gungor, O.; Bayraktaroğlu, S.; Ozkahya, M.; Asci, G.; Floege, J.; Ok, E. FGF-23 levels are associated with vascular calcification, but not with atherosclerosis, in hemodialysis patients. Int. Urol. Nephrol. 2016, 48, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Wong, C.-K.; Yau, Y.-Y.; Wong, S.; Chan, I.H.-S.; Lam, C.W.-K. Skin Autofluorescence Associates with Vascular Calcification in Chronic Kidney Disease. Arter. Thromb. Vasc. Biol. 2014, 34, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Wang, M.; Woo, J.; Lam, C.W.-K.; Li, P.K.-T.; Lui, S.-F.; Sanderson, J.E. Cardiac Valve Calcification as an Important Predictor for All-Cause Mortality and Cardiovascular Mortality in Long-Term Peritoneal Dialysis Patients: A Prospective Study. J. Am. Soc. Nephrol. 2003, 14, 159–168. [Google Scholar] [CrossRef]
- Wu, C.-F.; Lee, Y.-F.; Lee, W.-J.; Su, C.-T.; Lee, L.J.-H.; Wu, K.-D.; Chen, P.-C.; Kao, T.-W. Severe aortic arch calcification predicts mortality in patients undergoing peritoneal dialysis. J. Formos. Med. Assoc. 2017, 116, 366–372. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Abe, H.; Tominaga, T.; Nakamura, M.; Kishi, S.; Matsuura, M.; Nagai, K.; Tsuchida, K.; Minakuchi, J.; Doi, T. Polymorphism in the human matrix Gla protein gene is associated with the progression of vascular calcification in maintenance hemodialysis patients. Clin. Exp. Nephrol. 2013, 17, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hellberg, M.; Kouidi, E.; Deligiannis, A.; Höglund, P.; Clyne, N. Relationships between abdominal aortic calcification, glomerular filtration rate, and cardiovascular risk factors in patients with non-dialysis dependent chronic kidney disease. Clin. Nephrol. 2018, 90, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Abd alamir, M.; Radulescu, V.; Goyfman, M.; Mohler, E.R., III; Gao, Y.L.; Budoff, M.J. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: Results from CRIC study. Atherosclerosis 2015, 242, 117–122. [Google Scholar] [CrossRef]
- Adragao, T.; Pires, A.; Lucas, C.; Birne, R.; Magalhaes, L.; Gonçalves, M.; Negrao, A.P. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1480–1488. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.; Dobre, M.A.; Chen, J.; Hsu, C.-Y.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum Calcification Propensity and Coronary Artery Calcification Among Patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Chung, W.; Kim, Y.H.; Oh, Y.K.; Lee, J.; Choi, K.H.; Ahn, C.; Kim, Y.-S. The Correlation of Serum Osteoprotegerin with Non-Traditional Cardiovascular Risk Factors and Arterial Stiffness in Patients with Pre-Dialysis Chronic Kidney Disease: Results from the KNOW-CKD Study. J. Korean Med. Sci. 2018, 33, e322. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Debowska, M.; Lukaszuk, T.; Bobrowski, L.; Barany, P.; Söderberg, M.; Thiagarajan, D.; Frostegård, J.; Wennberg, L.; Lindholm, B.; et al. Phenotypic features of vascular calcification in chronic kidney disease. J. Intern. Med. 2020, 287, 422–434. [Google Scholar] [CrossRef]
- Floege, J.; Raggi, P.; Block, G.A.; Torres, P.U.; Csiky, B.; Naso, A.; Nossuli, K.; Moustafa, M.; Goodman, W.G.; Lopez, N.; et al. Study design and subject baseline characteristics in the ADVANCE Study: Effects of cinacalcet on vascular calcification in haemodialysis patients. Nephrol. Dial. Transplant. 2010, 25, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Gelev, S.; Spasovski, G.; Trajkovski, Z.; Damjanovski, G.; Amitov, V.; Selim, G.; Dzekova, P.; Sikole, A. Factors associated with various arterial calcifications in haemodialysis patients. Prilozi 2008, 29, 185–199. [Google Scholar]
- Kestenbaum, B.R.; Adeney, K.L.; de Boer, I.H.; Ix, J.H.; Shlipak, M.G.; Siscovick, D.S. Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009, 76, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.R.L.; Medeiros, A.M.C.; Pereira, H.S.; Lima, K.C.; Oliveira, P.T. Presence and associated factors of carotid artery calcification detected by digital panoramic radiography in patients with chronic kidney disease undergoing hemodialysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 198–204. [Google Scholar] [CrossRef]
- Maréchal, C.; Coche, E.; Goffin, E.; Dragean, A.; Schlieper, G.; Nguyen, P.; Floege, J.; Kanaan, N.; Devuyst, O.; Jadoul, M. Progression of Coronary Artery Calcification and Thoracic Aorta Calcification in Kidney Transplant Recipients. Am. J. Kidney Dis. 2012, 59, 258–269. [Google Scholar] [CrossRef]
- Moldovan, D.; Moldovan, I.; Rusu, C.; Racasan, S.; Patiu, I.M.; Brumboiu, A.; Bondor, C.I.; Parvu, L.; Kacso, I.; Orasan, R.; et al. Vascular calcifications and renal osteodystrophy in chronic hemodialysis patients: What is the relationship between them? Int. Urol. Nephrol. 2011, 43, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Ferramosca, E.; Bellasi, A.; Block, G.A.; Raggi, P. Development of a cardiovascular calcification index using simple imaging tools in haemodialysis patients. Nephrol. Dial. Transplant. 2007, 22, 508–514. [Google Scholar] [CrossRef][Green Version]
- Porter, C.J.; Stavroulopoulos, A.; Roe, S.D.; Pointon, K.; Cassidy, M.J. Detection of coronary and peripheral artery calcification in patients with chronic kidney disease stages 3 and 4, with and without diabetes. Nephrol. Dial. Transplant. 2007, 22, 3208–3213. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Gong, K.; Cheng, X.; Chen, Y.; Zhao, M.-H. Plasma Complement Protein C3a Level Was Associated with Abdominal Aortic Calcification in Patients on Hemodialysis. J. Cardiovasc. Transl. Res. 2019, 12, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; You, L.; Yu, X.; Zhang, M.; Zhu, R.; Hao, C.; Zhang, Z.; Chen, J. Association of Serum Phosphorus Variability with Coronary Artery Calcification among Hemodialysis Patients. PLoS ONE 2014, 9, e93360. [Google Scholar] [CrossRef]
- Bellasi, A.; Veledar, E.; Ferramosca, E.; Ratti, C.; Block, G.; Raggi, P. Markers of vascular disease do not differ in black and white hemodialysis patients despite a different risk profile. Atheroscler. 2008, 197, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Rader, D.J.; Reilly, M.; Mohler, E.R.; Lash, J.; Yang, W.; Rosen, L.; Glenn, M.; Teal, V.; Feldman, H.I. Relationship of Estimated GFR and Coronary Artery Calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2011, 58, 519–526. [Google Scholar] [CrossRef]
- Cai, H.; Lu, R.; Zhang, M.; Pang, H.; Zhu, M.; Zhang, W.; Ni, Z.; Qian, J.; Yan, Y. Serum Soluble Klotho Level Is Associated with Abdominal Aortic Calcification in Patients on Maintenance Hemodialysis. Blood Purif. 2015, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Goffin, E.; Meijers, B.; Kanaan, N.; Bammens, B.; Coche, E.; Claes, K.; Jadoul, M. Sclerostin Serum Levels and Vascular Calcification Progression in Prevalent Renal Transplant Recipients. J. Clin. Endocrinol. Metab. 2015, 100, 4669–4676. [Google Scholar] [CrossRef]
- Fayed, A.; Elnokeety, M.M.; Attia, K.; Din, U.A.S.E. Calcification of abdominal aorta in patients recently starting hemodialysis: A single-center experience from Egypt. Saudi J. Kidney Dis. Transplant. 2019, 30, 819–824. [Google Scholar] [CrossRef]
- Filgueira, A.; Carvalho, A.B.; Tomiyama, C.; Higa, A.; Rochitte, C.E.; Santos, R.D.; Canziani, M.E.F. Is Coronary Artery Calcification Associated with Vertebral Bone Density in Nondialyzed Chronic Kidney Disease Patients? Clin. J. Am. Soc. Nephrol. 2011, 6, 1456–1462. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rebora, P.; Rizzo, M.A.; Luise, M.C.; Riva, H.; Bertoli, S.; Conte, F.; Stella, A.; Ondei, P.; et al. Atrial fibrillation and low vitamin D levels are associated with severe vascular calcifications in hemodialysis patients. J. Nephrol. 2016, 29, 419–426. [Google Scholar] [CrossRef]
- Golembiewska, E.; Qureshi, A.R.; Dai, L.; Lindholm, B.; Heimbürger, O.; Söderberg, M.; Brismar, T.B.; Ripsweden, J.; Barany, P.; Johnson, R.J.; et al. Copeptin is independently associated with vascular calcification in chronic kidney disease stage 5. BMC Nephrol. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Gruppen, M.P.; Groothoff, J.W.; Prins, M.; van der Wouw, P.; Offringa, M.; Bos, W.J.W.; Davin, J.C.; Heymans, H.S. Cardiac disease in young adult patients with end-stage renal disease since childhood: A Dutch cohort study. Kidney Int. 2003, 63, 1058–1065. [Google Scholar] [CrossRef][Green Version]
- Ho, T.-Y.; Chen, N.-C.; Hsu, C.-Y.; Huang, C.-W.; Lee, P.-T.; Chou, K.-J.; Fang, H.-C.; Chen, C.-L. Evaluation of the association of Wnt signaling with coronary artery calcification in patients on dialysis with severe secondary hyperparathyroidism. BMC Nephrol. 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Okuno, S.; Kitatani, K.; Maekawa, K.; Izumotani, T.; Yamakawa, T.; Jono, S.; Shoji, T.; Shioi, A.; Inaba, M.; et al. C-reactive protein is a significant predictor of vascular calcification of both aorta and hand arteries. Semin. Nephrol. 2004, 24, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Okuno, S.; Kitatani, K.; Kim, M.; Shoji, T.; Nakatani, T.; Inaba, M.; Nishizawa, Y. Different risk factors for peripheral vascular calcification between diabetic and non-diabetic haemodialysis patients—Importance of glycaemic control. Diabetologia 2002, 45, 1446–1448. [Google Scholar] [PubMed]
- Manghat, P.; Souleimanova, I.; Cheung, J.; Wierzbicki, A.; Harrington, D.; Shearer, M.; Chowiencki, P.; Fogelman, I.; Nerlander, M.; Goldsmith, D.; et al. Association of bone turnover markers and arterial stiffness in pre-dialysis chronic kidney disease (CKD). Bone 2011, 48, 1127–1132. [Google Scholar] [CrossRef]
- Wada, J.; Nakayama, K.; Nakao, K.; Takatori, Y.; Inoue, J.; Kojo, S.; Akagi, S.; Fukushima, M.; Makino, H. Long-term effect of cinacalcet hydrochloride on abdominal aortic calcification in patients on hemodialysis with secondary hyperparathyroidism. Int. J. Nephrol. Renov. Dis. 2013, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Mizuiri, S.; Yorioka, N.; Hamada, C.; Tomino, Y. Determinants of coronary artery calcification in maintenance hemodialysis patients. J. Artif. Organs 2015, 18, 251–256. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Jono, S.; Ishimura, E.; Shioi, A. Hyperphosphatemia and vascular calcification in end-stage renal disease. J. Ren. Nutr. 2005, 15, 178–182. [Google Scholar] [CrossRef]
- Oprisiu, R.; Bunea, D.; Tarek, S.; Hedi, B.; Fournier, A. Progression of vascular calcification and dyslipidemia in patients on chronic hemodialysis. Am. J. Kidney Dis. 2002, 39, 209. [Google Scholar] [CrossRef] [PubMed]
- Pateinakis, P.; Papagianni, A.; Douma, S.; Efstratiadis, G.; Memmos, D. Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients. BMC Nephrol. 2013, 14, 122. [Google Scholar] [CrossRef][Green Version]
- Raggi, P.; Boulay, A.; Chasan-Taber, S.; Amin, N.; Dillon, M.; Burke, S.K.; Chertow, G.M. Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002, 39, 695–701. [Google Scholar] [CrossRef]
- Schlieper, G.; Brandenburg, V.; Djuric, Z.; Damjanovic, T.; Markovic, N.; Schurgers, L.; Krüger, T.; Westenfeld, R.; Ackermann, D.; Haselhuhn, A.; et al. Risk Factors for Cardiovascular Calcifications in Non-Diabetic Caucasian Haemodialysis Patients. Kidney Blood Press. Res. 2009, 32, 161–168. [Google Scholar] [CrossRef]
- Sharma, R.; Pellerin, D.; Gaze, D.C.; Mehta, R.L.; Gregson, H.; Streather, C.P.; Collinson, P.O.; Brecker, S.J. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis 2007, 191, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.; Bungay, P.; Taal, M.; McIntyre, C.W. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 707–714. [Google Scholar] [CrossRef]
- Stavroulopoulos, A.; Porter, C.J.; Pointon, K.; Monaghan, J.M.; Roe, S.D.; Cassidy, M.J. Evolution of coronary artery calcification in patients with chronic kidney disease Stages 3 and 4, with and without diabetes. Nephrol. Dial. Transplant. 2011, 26, 2582–2589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumida, Y.; Nakayama, M.; Nagata, M.; Nakashita, S.; Suehiro, T.; Kaizu, Y.; Ikeda, H.; Izumaru, K. Carotid artery calcification and atherosclerosis at the initiation of hemodialysis in patients with end-stage renal disease. Clin. Nephrol. 2010, 73, 360–369. [Google Scholar] [CrossRef]
- Tamei, N.; Ogawa, T.; Ishida, H.; Ando, Y.; Nitta, K. Serum fibroblast growth factor-23 levels and progression of aortic arch calcification in non-diabetic patients on chronic hemodialysis. J. Atheroscler. Thromb. 2011, 18, 217–223. [Google Scholar] [CrossRef]
- Turan, M.N.; Gungor, O.; Asci, G.; Kircelli, F.; Acar, T.; Yaprak, M.; Ceylan, N.; Demirci, M.S.; Bayraktaroglu, S.; Töz, H.; et al. Epicardial adipose tissue volume and cardiovascular disease in hemodialysis patients. Atherosclerosis 2013, 226, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Vipattawat, K.; Kitiyakara, C.; Phakdeekitcharoen, B.; Kantachuvesiri, S.; Sumethkul, V.; Jirasiritham, S.; Stitchantrakul, W.; Disthabanchong, S. Vascular calcification in long-term kidney transplantation. Nephrology 2014, 19, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Inaba, M.; Shidara, K.; Okada, S.; Emoto, M.; Ishimura, E.; Nishizawa, Y. Association of glycated albumin, but not glycated hemoglobin, with peripheral vascular calcification in hemodialysis patients with type 2 diabetes. Life Sci. 2008, 83, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Kim, S.-W.; Han, H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1915–1920. [Google Scholar] [CrossRef][Green Version]
- Aoun, M.; Makki, M.; Azar, H.; Matta, H.; Chelala, D.N. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Axelsson, J.; Wang, X.; Ketteler, M.; Qureshi, A.R.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Nordfors, L.; Stenvinkel, P. Is Fetuin-A/α2-Heremans-Schmid Glycoprotein Associated with the Metabolic Syndrome in Patients with Chronic Kidney Disease? Am. J. Nephrol. 2008, 28, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Hulbert-Shearon, E.T.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Buiten, M.S.; De Bie, M.K.; Krijger, A.B.-D.; Van Dam, B.; Dekker, F.; Jukema, J.W.; Rabelink, T.J.; Rotmans, J.I. Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol. 2014, 15, 197. [Google Scholar] [CrossRef]
- Chao, C.; Yuan, T.; Yeh, H.; Chen, H.; Huang, J.; Chen, H. Risk Factors Associated with Altered Circulating MicroRNA-125b and Their Influences on Uremic Vascular Calcification Among Patients with End-Stage Renal Disease. J. Am. Hear. Assoc. 2019, 8, e010805. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chiu, Y.-L.; Hsu, S.-P.; Pai, M.-F.; Yang, J.-Y.; Peng, Y.-S. Low serum fetuin A levels and incident stroke in patients with maintenance haemodialysis. Eur. J. Clin. Investig. 2013, 43, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Claes, K.J.; Viaene, L.; Heye, S.; Meijers, B.; D’Haese, P.; Evenepoel, P. Sclerostin: Another Vascular Calcification Inhibitor? J. Clin. Endocrinol. Metab. 2013, 98, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.L.C.; Elias, R.M.; Dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moysés, R.M.A. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014, 15, 1–7. [Google Scholar] [CrossRef]
- González-Parra, E.; Álvaro, A.; Lorenzo, O.; Tarín, N.; González-Casaus, M.L.; Cristóbal, C.; Huelmos, A.; Fernandez, J.L.T.; Pello, A.M.; Carda, R.; et al. Important abnormalities of bone mineral metabolism are present in patients with coronary artery disease with a mild decrease of the estimated glomerular filtration rate. J. Bone Miner. Metab. 2016, 34, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ekundayo, O.; Nemeth, Z.K.; Yang, Y.; Covic, A.; Mathe, Z.; Kovesdy, C.P.; Molnar, M.Z.; Mucsi, I. Association between serum osteoprotegerin level and mortality in kidney transplant recipients—A prospective observational cohort study. Transpl. Int. 2021, 34, 844–854. [Google Scholar] [CrossRef]
- Holden, R.M.; Booth, S.L.; Tuttle, A.; James, P.D.; Morton, A.R.; Hopman, W.M.; Nolan, R.; Garland, J.S. Sequence Variation in Vitamin K Epoxide Reductase Gene Is Associated with Survival and Progressive Coronary Calcification in Chronic Kidney Disease. Arter. Thromb. Vasc. Biol. 2014, 34, 1591–1596. [Google Scholar] [CrossRef]
- Ikee, R.; Toyoyama, T.; Endo, T.; Tsunoda, M.; Hashimoto, N. Impact of sevelamer hydrochloride on serum magnesium concentrations in hemodialysis patients. Magnes Res. 2016, 29, 184–190. [Google Scholar] [CrossRef]
- Jean, G.; Charra, B.; Chazot, C. Vitamin D Deficiency and Associated Factors in Hemodialysis Patients. J. Ren. Nutr. 2008, 18, 395–399. [Google Scholar] [CrossRef]
- Jean, G.; Lataillade, D.; Genet, L.; Legrand, E.; Kuentz, F.; Moreau-Gaudry, X.; Fouque, D. Association between Very Low PTH Levels and Poor Survival Rates in Haemodialysis Patients: Results from the French ARNOS Cohort. Nephron Clin. Pract. 2011, 118, c211–c216. [Google Scholar] [CrossRef]
- Karsli Ceppioğlu, S.; Yurdun, T.; Canbakan, M. Assessment of Matrix Gla Protein, Klotho Gene Polymorphisms, and Oxidative Stress in Chronic Kidney Disease. Renal Fail. 2011, 33, 866–874. [Google Scholar] [CrossRef]
- Kato, A.; Odamaki, M.; Ishida, J.; Hishida, A. Relationship between Serum Pre-B Cell Colony-Enhancing Factor/Visfatin and Atherosclerotic Parameters in Chronic Hemodialysis Patients. Am. J. Nephrol. 2009, 29, 31–35. [Google Scholar] [CrossRef]
- Kuo, T.-H.; Lin, W.-H.; Chao, J.-Y.; Wu, A.-B.; Tseng, C.-C.; Chang, Y.-T.; Liou, H.-H.; Wang, M.-C. Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Liabeuf, S.; Glorieux, G.; Lenglet, A.; Diouf, M.; Schepers, E.; Desjardins, L.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; on behalf of the European Uremic Toxin (EUTox) Work Group. Does P-Cresylglucuronide Have the Same Impact on Mortality as Other Protein-Bound Uremic Toxins? PLoS ONE 2013, 8, e67168. [Google Scholar] [CrossRef]
- Metry, G.; Stenvinkel, P.; Qureshi, A.R.T.; Carrero, J.J.; Yilmaz, M.I.; Bárány, P.; Snaedal, S.; Heimbürger, O.; Lindholm, B.; Suliman, M.E. Low serum fetuin-A concentration predicts poor outcome only in the presence of inflammation in prevalent haemodialysis patients. Eur. J. Clin. Investig. 2008, 38, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Carrero, J.J.; Qureshi, A.R.T.; Hirai, T.; Takasugi, N.; Ueno, T.; Taniguchi, Y.; Lindholm, B.; Yorioka, N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos. Int. 2011, 22, 1695–1701. [Google Scholar] [CrossRef]
- Nemeth, Z.K.; Mardare, N.G.; Czira, M.E.; Deak, G.; Kiss, I.; Mathe, Z.; Remport, A.; Ujszaszi, A.; Covic, A.; Molnar, M.Z.; et al. Serum osteoprotegerin is associated with pulse pressure in kidney transplant recipients. Sci. Rep. 2015, 5, 14518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishiura, R.; Fujimoto, S.; Sato, Y.; Yamada, K.; Hisanaga, S.; Hara, S.; Nakao, H.; Kitamura, K. Elevated Osteoprotegerin Levels Predict Cardiovascular Events in New Hemodialysis Patients. Am. J. Nephrol. 2009, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Tsutaya, C.; Hatakeyama, S.; Konishi, S.; Okita, K.; Tanaka, Y.; Imanishi, K.; Takashima, T.; Saitoh, F.; Suzuki, T.; et al. Low serum butyrylcholinesterase is independently related to low fetuin-A in patients on hemodialysis: A cross-sectional study. Int. Urol. Nephrol. 2018, 50, 1713–1720. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.J.; Jhee, J.H.; Yun, H.; Kim, H.; Jung, S.; Kee, Y.K.; Yoon, C.; Park, J.T.; Kim, H.C.; et al. Extracellular Fluid Excess Is Significantly Associated with Coronary Artery Calcification in Patients with Chronic Kidney Disease. J. Am. Hear. Assoc. 2018, 7, e008935. [Google Scholar] [CrossRef]
- Ramalho, J.; Petrillo, E.M.; Takeichi, A.P.M.; Moyses, R.M.A.; Titan, S.M. Calcitriol and FGF-23, but neither PTH nor sclerostin, are associated with calciuria in CKD. Int. Urol. Nephrol. 2019, 51, 1823–1829. [Google Scholar] [CrossRef]
- Scialla, J.J.; Leonard, M.B.; Townsend, R.R.; Appel, L.; Wolf, M.; Budoff, M.J.; Chen, J.; Lustigova, E.; Gadegbeku, C.A.; Glenn, M.; et al. Correlates of Osteoprotegerin and Association with Aortic Pulse Wave Velocity in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.R.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.M.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating Nonphosphorylated Carboxylated Matrix Gla Protein Predicts Survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef]
- Sigrist, M.K.; Levin, A.; Er, L.; McIntyre, C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. 2009, 24, 3157–3162. [Google Scholar] [CrossRef][Green Version]
- Stenvinkel, P.; Wang, K.; Qureshi, A.R.; Axelsson, J.; Pecoits-Filho, R.; Gao, P.; Barany, P.; Lindholm, B.; Jogestrand, T.; Heimberger, O.; et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int. 2005, 67, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Stolic, R.V.; Jovanovic, A.; Trajkovic, G.Z.; Kostić, M.M.; Odalovic, A.M.; Sovtic, S.R.; Sipic, M.V.; Pajovic, S.D.; Sojevic-Timotijevic, Z.N. Is low magnesium a clue to arteriovenous fistula complications in hemodialysis? Int. Urol. Nephrol. 2016, 48, 773–779. [Google Scholar] [CrossRef]
- Thambiah, S.C.; Roplekar, R.; Manghat, P.; Fogelman, I.; Fraser, W.D.; Goldsmith, D.; Hampson, G. Circulating Sclerostin and Dickkopf-1 (DKK1) in Predialysis Chronic Kidney Disease (CKD): Relationship with Bone Density and Arterial Stiffness. Calcif. Tissue Int. 2012, 90, 473–480. [Google Scholar] [CrossRef]
- Ulusoy, S.; Özkan, G.; Mentese, A.; Yavuz, A.; Karahan, S.C.; Sümer, A.U. Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) level in hemodialysis patients and parameters affecting that level. Clin. Biochem. 2012, 45, 1444–1449. [Google Scholar] [CrossRef]
- Viaene, L.; Behets, G.; Claes, K.; Meijers, B.; Blocki, F.; Brandenburg, V.; Evenepoel, P.; D’Haese, P.C. Sclerostin: Another bone-related protein related to all-cause mortality in haemodialysis? Nephrol. Dial. Transplant. 2013, 28, 3024–3030. [Google Scholar] [CrossRef]
- Zhang, A.-H.; Guo, W.-K.; Yu, L.; Liu, W.-H. Relationship of Serum Soluble Klotho Levels and Echocardiographic Parameters in Patients on Maintenance Hemodialysis. Kidney Blood Press. Res. 2019, 44, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, L.; Jin, L. Association Between Angiotensin-Converting Enzyme 2 and Coronary Artery Calcification in Patients on Maintenance Hemodialysis Therapy. Ther. Apher. Dial. 2015, 19, 466–470. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of sclerostin with cardiovascular events and mortality in dialysis patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yu, Y.; Wu, P.; Lin, F.-J.; Yao, Y.; Xie, Y.; Jiang, G.-R. Serum phosphorus is related to left ventricular remodeling independent of renal function in hospitalized patients with chronic kidney disease. Int. J. Cardiol. 2016, 221, 134–140. [Google Scholar] [CrossRef]
- Chao, C.-T.; Lin, S.-H. Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins 2020, 12, 812. [Google Scholar] [CrossRef]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Amann, K. Media Calcification and Intima Calcification Are Distinct Entities in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1599–1605. [Google Scholar] [CrossRef]
- Lee, J.J.; Pedley, A.; Hoffmann, U.; Massaro, J.M.; O’Donnell, C.J.; Benjamin, E.J.; Long, M.T. Longitudinal Associations of Pericardial and Intrathoracic Fat with Progression of Coronary Artery Calcium (from the Framingham Heart Study). Am. J. Cardiol. 2018, 121, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.-B.; Chang, J.-R.; Chen, W.-J.; Zhao, L.; Zhang, B.-H.; Yu, Y.-R.; Tang, C.-S.; Yin, X.-H.; Qi, Y.-F. Angiotensin-(1-7) inhibits vascular calcification in rats. Peptides 2013, 42, 25–34. [Google Scholar] [CrossRef]
- McCabe, K.M.; Zelt, J.G.; Kaufmann, M.; Laverty, K.; Ward, E.; Barron, H.; Jones, G.; Adams, M.A.; Holden, R.M. Calcitriol Accelerates Vascular Calcification Irrespective of Vitamin K Status in a Rat Model of Chronic Kidney Disease with Hyperphosphatemia and Secondary Hyperparathyroidism. J. Pharmacol. Exp. Ther. 2018, 366, 433–445. [Google Scholar] [CrossRef]
- Almeida, Y.E.; Fessel, M.R.; Carmo, L.S.D.; Jorgetti, V.; Farias-Silva, E.; Pescatore, L.A.; Gamarra, L.; Andrade, M.C.; Simplicio-Filho, A.; Mangueira, C.L.P.; et al. Excessive cholecalciferol supplementation increases kidney dysfunction associated with intrarenal artery calcification in obese insulin-resistant mice. Sci. Rep. 2020, 10, 87. [Google Scholar] [CrossRef]
- De Maré, A.; Maudsley, S.; Azmi, A.; Hendrickx, J.O.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin as Regulatory Molecule in Vascular Media Calcification and the Bone-Vascular Axis. Toxins 2019, 11, 428. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Vascular Calcification in Chronic Kidney Disease: The Role of Vitamin K- Dependent Matrix Gla Protein. Front. Med. (Lausanne) 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ceponiene, I.; Li, D.; El Khoudary, S.R.; Nakanishi, R.; Stein, J.H.; Wong, N.D.; Nezarat, N.; Kanisawa, M.; Rahmani, S.; Osawa, K.; et al. Association of Coronary Calcium, Carotid Wall Thickness, and Carotid Plaque Progression with Low-Density Lipoprotein and High-Density Lipoprotein Particle Concentration Measured by Ion Mobility (From Multiethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2021, 142, 52–58. [Google Scholar] [CrossRef]
- Parhami, F.; Basseri, B.; Hwang, J.; Tintut, Y.; Demer, L.L. High-density lipoprotein regulates calcification of vascular cells. Circ. Res. 2002, 91, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Osako, M.K.; Nakagami, H.; Koibuchi, N.; Shimizu, H.; Nakagami, F.; Koriyama, H.; Shimamura, M.; Miyake, T.; Rakugi, H.; Morishita, R. Estrogen inhibits vascular calcification via vascular RANKL system: Common mechanism of osteoporosis and vascular calcification. Circ. Res. 2010, 107, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Balica, M.; Boström, K.; Shin, V.; Tillisch, K.; Demer, L.L. Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17 beta-estradiol. Circulation. 1997, 95, 1954–1960. [Google Scholar] [CrossRef]
- Woodward, H.J.; Zhu, D.; Hadoke, P.W.F.; MacRae, V.E. Regulatory Role of Sex Hormones in Cardiovascular Calcification. Int. J. Mol. Sci. 2021, 22, 4620. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Jovanovich, A. Mineral Bone Abnormalities and Vascular Calcifications. Adv. Chronic Kidney Dis. 2019, 26, 409–416. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, H.; Zeng, X.; Feng, S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol. Med. Rep. 2017, 15, 1539–1546. [Google Scholar] [CrossRef]

| Authors | Country | Time | CKD Stages | Sample Size | Gender Effect | Data | Potential Modifiers | Ref. |
|---|---|---|---|---|---|---|---|---|
| Genetic susceptibility | ||||||||
| Holden et al. | Canada | 2014 | 3–5 | 167 | Neutral | CC vs. CG/GG, male 68% vs. 55%, p = 0.11 | VKORC1 (vitamin K epoxide reductase complex 1) polymorphism | [166] |
| Yoshikawa et al. | Japan | 2013 | 5D (HD) | 134 | Neutral | CC vs. CT vs. TT genotype, male 14% vs. 48% vs. 33%, p = 0.53 | MGP Genotype T-138C | [110] |
| Body composition and inflammatory status | ||||||||
| Axelsson et al. | Sweden | 2008 | 5 | 198 | Male lower | Male β = −1.68, p < 0.001 | Total fat mass | [157] |
| Park et al. | Korean | 2018 | 1–5 | 1741 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, male 80.6% vs. 59.0% vs. 39.7% vs. 39.6, p < 0.001 Male β = 0.095 (−0.421~0.231), p = 0.566 | ECF excess | [179] |
| Choi et al. | Korea | 2019 | 5D (HD) | 97 | Neutral | Marker count 2 vs. 1 vs. 0, male 61.5% vs. 41.9% vs. 34.1%, p = 0.216 | Nutrition (albumin) and inflammation (CRP) markers | [43] |
| Golembiewska et al. | Sweden | 2020 | 5 | 149 | Neutral | High vs. middle vs. low levels, male 71% vs. 67% vs. 65%, p = 0.84 Regression model, male β −0.08, p = 0.31 | Copeptin | [134] |
| Harada et al. | Brazil | 2014 | 2–5 | 117 | Male more severe | Area in males vs. females, 220.4 ± 110.5 vs. 135.4 ± 76.0, p < 0.001 | Pericardial fat | [58] |
| Turan et al. | Turkey | 2013 | 5D (HD) | 191 | Neutral | Low vs. middle vs. high, female 46% vs. 39% vs. 58%, p = 0.1 | Epicardial fat | [152] |
| Miyatake et al. | Japan | 2020 | 5T | 50 | Female higher HDL, adiponectin but lower LDL | For LDL-C, male vs. female 113.0 (97.0–132.5) vs. 90.0 (76.5–98.3), p < 0.01 For HDL-C, male vs. female 57.0 (51.0–67.0) vs. 78.0 (66.8–96.5), p < 0.01 For HMW-ADPN, male vs. female 2.48 (1.62–3.33) vs. 4.52 (3.02–6.79), p < 0.01 For LMW-ADPN, male vs. female 1.67 (1.14–1.89) vs. 2.26 (1.85–2.83), p < 0.01 For CTRP9, male vs. female 2.08 (2.01–2.13) vs. 2.03 (2.00–2.07), p > 0.05 | Lipid profile, circulating C1q/TNF-a related protein-9 (CTRP9), and adiponectin | [86] |
| Zhang et al. | China | 2015 | 5D (HD) | 90 | Male higher | ≤30 vs. 30–60 vs. ≥ 60 U/L, male 29.4% vs. 46.1% vs. 85.7%, p < 0.001 Male correlation coefficient 0.362, p < 0.001 | Serum ACE2 | [190] |
| Medications | ||||||||
| Chen et al. | Sweden | 2017 | 5D, 5T | 240 | Neutral | Users vs. non-users, male 65% vs. 62%, p = 0.678 | Statin use | [41] |
| Epigenetic mediators | ||||||||
| Chao et al. | Taiwan | 2019 | 5D | 223 | Neutral | High vs. low levels, female 63% vs. 51%, p = 0.14 | microRNA-125b | [160] |
| Divalent ion/electrolyte balance | ||||||||
| Block et al. | United States | 1998 | 5D (HD) | 6407 | Female higher | Male OR 0.774, p = 0.0001 | Serum phosphorus > 6.5 mg/dL | [158] |
| Zou et al. | China | 2016 | 1–5 | 296 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, male 60.2% vs. 50.0% vs. 61.2%, p = 0.21 | Serum phosphorus | [192] |
| Karsli Ceppioğlu et al. | Turkey | 2011 | 3–5 | 84 | Female higher | Female vs. male, higher in the former, p = 0.002 | Serum copper | [170] |
| Ikee et al. | Japan | 2016 | 5D (HD) | 86 | Neutral | Male vs. female, 2.51 ± 0.38 vs. 2.42 ± 0.33, p = 0.52 | Serum magnesium | [167] |
| Stolic et al. | Serbia | 2016 | 5D (HD) | 88 | Neutral | Male vs. female, mean 1.2 (0.7–1.6) vs. 1.2 (0.8–1.5) mmol/L, p = 0.896 | Serum magnesium | [185] |
| Ramalho et al. | Brazil | 2019 | 3–4 | 356 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, 59% vs. 64% vs.66%, p = 0.5 Male β = 0.22 (−0.02~0.45), p = 0.07 | Urinary calcium excretion | [180] |
| Osteogenesis/mineralization regulators | ||||||||
| Aoun et al. | Lebanon | 2017 | 5D (HD) | 50 | Male lower | <5000 vs. >5000 pmol/L, male 68.3% vs. 22.2%, p = 0.02 | Dp-ucMGP | [156] |
| Schlieper et al. | Serbia | 2011 | 5 | 188 | Neutral | <6139 vs. >6139 pmol/L, female OR 0.62 (0.35–1.11), p = 0.11 | dp-cMGP | [182] |
| Okamoto et al. | Japan | 2018 | 5D (HD) | 230 | Neutral | <0.213 vs. ≥0.213 g/L, male 61% vs. 68%, p = 0.400 Male OR 0.48 (0.22–1.02), p = 0.056 | Fetuin-A | [178] |
| Chen et al. | Taiwan | 2013 | 5D (HD) | 238 | Neutral | Tertile 1 (lowest) vs. 2 vs. 3, female 55% vs. 48% vs. 57%, p = 0.5 | Fetuin-A | [161] |
| Stenvinkel et al. | Sweden | 2005 | 5 | 258 | Neutral | Male vs. female, median 0.225 vs. 0.223 g/L, p > 0.05 | Fetuin-A | [184] |
| Metry et al. | Sweden | 2008 | 5D (HD) | 222 | Neutral | High Fetuin-A/Low CRP vs. High Fetuin-A/High CRP vs. Low Fetuin-A/Low CRP vs. Low Fetuin-A/High CRP, 60.9% vs. 52.5% vs. 44.4% vs. 58.8%, p > 0.05 | Fetuin-A and CRP | [174] |
| Turan et al. | Turkey | 2016 | 5D (HD) | 224 | Neutral | <35.5 vs. 35.5–123.4 vs. >123.4 pg/mL, male 56.6% vs. 46.5% vs. 50.0%, p = 0.38 | FGF-23 | [106] |
| Nishiura et al. | Japan | 2009 | 5D (HD) | 99 | Male higher | Low vs. high, male 56% vs. 75.5%, p = 0.057 Male HR 3.034 (1.028–8.948), p = 0.044 | Osteoprotegerin | [177] |
| Hou et al. | Taiwan | 2019 | 5D (HD) | 120 | Female lower | 72.97–237.13 vs. 237.36–323.54 vs. 329.61–835.78 pg/L, female 62.5% vs. 55.0% vs. 32.5%, p = 0.008 | Osteoprotegerin | [61] |
| Sigrist et al. | United Kingdom | 2009 | 3–4 | 134 | Neutral | ≤25 vs. >25 pmol/L, male 63% vs. 70%, p = 0.47 | Osteoprotegerin | [183] |
| Nakashima et al. | Japan | 2011 | 5D (HD) | 151 | Neutral | Male β = 0.531, p = 0.51 | Osteoprotegerin | [175] |
| Scialla et al. | United States | 2011 | 1–5 | 351 | Female higher | 1.21–5.03 vs. 5.05–7.45 vs. 7.46–22.31 pmol/L, female 24.8% vs. 26.5% vs. 40.2%, p = 0.02 Female related OPG % difference 10.2%, p = 0.05 | Osteoprotegerin | [181] |
| Gupta et al. | Hungary | 2021 | 5D (HD) | 982 | Female higher | <3.2 vs. 3.2–4.39 vs. >4.39 pmol/L, male 63% vs. 57% vs. 52%, p = 0.015 | Osteoprotegerin | [165] |
| Nemeth et al. | Hungary | 2015 | 5T | 993 | Male lower | <3.2 vs. 3.2–4.39 vs. >4.39 pmol/L, male 63% vs. 58% vs. 52%, p = 0.02 | Osteoprotegerin | [176] |
| Chae et al. | Korea | 2018 | 1–5 | 1832 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, female 36.2% vs. 43.4% vs. 42.5% vs. 38.6%, p = 0.517 (mean, 6.76 pmol/L) | Osteoprotegerin | [115] |
| Jean et al. | France | 2011 | 5D (HD) | 1138 | Neutral | <50 vs. ≥50 pg/mL, female 43% vs. 40%, p > 0.05 | Parathyroid hormone | [169] |
| González-Parra E et al. | Spain | 2015 | 1–5 | 704 | Female higher in both | For PTH, male r = −0.084 (−0.155~−0.012), p = 0.0215 For FGF-23, male r = −0.191 (−0.301~−0.080), p = 0.0007 | Parathyroid hormone and FGF-23 | [164] |
| Thambiah et al. | United Kingdom | 2012 | 3B-4 | 77 | Male higher | Male vs. female, mean 50.5 vs. 34 pmol/L, p = 0.015 Male β = 0.23, p = 0.024 | Sclerostin | [186] |
| Kuo et al. | Taiwan | 2019 | 5D (PD) | 89 | Male higher | <80.2 vs. ≥80.2 pmol/L, male 36.4% vs. 62.2%, p = 0.015 Male OR 2.882 (1.219–6.815), p = 0.016 | Sclerostin | [172] |
| Goncalves et al. | Brazil | 2014 | 5D (HD) | 91 | Neutral | High vs. low levels, male 68.9% vs. 52.2%, p = 0.103 Regression model, male OR 0.82 (0.39–1.75), p = 0.62 | Sclerostin | [163] |
| Viaene et al. | Belgium | 2013 | 5D (HD) | 100 | Neutral | Low vs. high, female 47% vs. 35%, p = 0.2 | Sclerostin | [188] |
| Claes et al. | Belgium | 2013 | 1–5 | 154 | Male higher | Male gender significantly associated with higher levels (p = 0.006) | Sclerostin | [162] |
| Zou et al. | China | 2020 | 5D | 165 | Male higher | Among PD patients, male β = 0.259 (0.052–0.416), p = 0.012 | Sclerostin | [191] |
| Jean et al. | France | 2016 | 5D (HD) | 227 | Female lower | Female OR 0.16 (0.075−0.362) | Sclerostin | [66] |
| Evenpoel et al. | Belgium | 2015 | 5T | 268 | Male higher | Male gender significantly associated with higher levels (p = 0.002) | Sclerostin | [130] |
| Zhang et al. | China | 2019 | 5D (HD) | 105 | Neutral | Quartile 1 (lowest) vs. 2 vs. 3 vs. 4, male 57.7% vs.48.1% vs. 46.2% vs. 65.4%, p = 0.473 | Soluble Klotho | [189] |
| Buiten MS | Netherlands | 2014 | 5D | 127 | Female higher | <460 vs. >460 pg/mL, female 16% vs. 31%, p < 0.05 | Soluble Klotho | [159] |
| Cai et al. | China | 2015 | 5D (HD) | 129 | Neutral | ≤379 vs. 379–613 vs. 613–817 vs. >817 pg/mL, male 59.4% vs. 54.5% vs. 56.3% vs. 53.1%, p > 0.05 | Soluble Klotho | [129] |
| Jean et al. | France | 2008 | 5D (HD) | 253 | Female lower levels | Deficient vs. sufficient, female 53% vs. 28%, p < 0.05 | Vitamin D (25-OH-D) deficiency | [168] |
| Chang et al. | Republic of Korea | 2012 | 5D (HD) | 289 | Female lower levels | Female OR 3.892 (1.678–9.025), p = 0.002 | Vitamin D (25-OH-D) deficiency (<15 ng/mL) | [36] |
| Miscellaneous | ||||||||
| Kato et al. | Japan | 2009 | 5D (HD) | 68 | Neutral | No correlation with gender | Serum pre-B cell colony-enhancing factor/visfatin | [171] |
| He et al. | China | 2018 | 5D (HD) | 150 | Neutral | Male correlation coefficient 0.106, p = 0.114 | Serum irisin | [59] |
| Liabeuf et al. | France | 2013 | 2–5, 5D | 139 | Neutral | ≤0.041 vs. >0.041 mg/dL, male 63% vs. 57%, p = 0.5 | Free p-cresylglucuronide | [173] |
| Ulusoy et al. | Turkey | 2012 | 5D (HD) | 103 | Male higher | Pre-dialysis male vs. female levels, 314.7 ± 157.1 vs. 210.4 ± 115.4 ng/mL, p < 0.001 | Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) | [187] |
| Wang et al. | Hong Kong | 2014 | 3–5 | 300 | Male lower | Male correlation coefficient −0.14, p = 0.02 | Tissue AGEs (surrogated by skin autofluorescence) | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.Y.; Lee, S.-Y.; Chang, K.-V.; Chao, C.-T.; Huang, J.-W. Gender-Related Differences in Chronic Kidney Disease-Associated Vascular Calcification Risk and Potential Risk Mediators: A Scoping Review. Healthcare 2021, 9, 979. https://doi.org/10.3390/healthcare9080979
Wu PY, Lee S-Y, Chang K-V, Chao C-T, Huang J-W. Gender-Related Differences in Chronic Kidney Disease-Associated Vascular Calcification Risk and Potential Risk Mediators: A Scoping Review. Healthcare. 2021; 9(8):979. https://doi.org/10.3390/healthcare9080979
Chicago/Turabian StyleWu, Patrick Yihong, Szu-Ying Lee, Ke-Vin Chang, Chia-Ter Chao, and Jenq-Wen Huang. 2021. "Gender-Related Differences in Chronic Kidney Disease-Associated Vascular Calcification Risk and Potential Risk Mediators: A Scoping Review" Healthcare 9, no. 8: 979. https://doi.org/10.3390/healthcare9080979
APA StyleWu, P. Y., Lee, S.-Y., Chang, K.-V., Chao, C.-T., & Huang, J.-W. (2021). Gender-Related Differences in Chronic Kidney Disease-Associated Vascular Calcification Risk and Potential Risk Mediators: A Scoping Review. Healthcare, 9(8), 979. https://doi.org/10.3390/healthcare9080979







