Care Strategies for Reducing Hospital Readmissions Using Stochastic Programming
Abstract
:1. Introduction
2. Literature Review
2.1. Statistical Approaches
2.2. Predictive Approaches
2.3. Optimization Approaches
3. Methodology
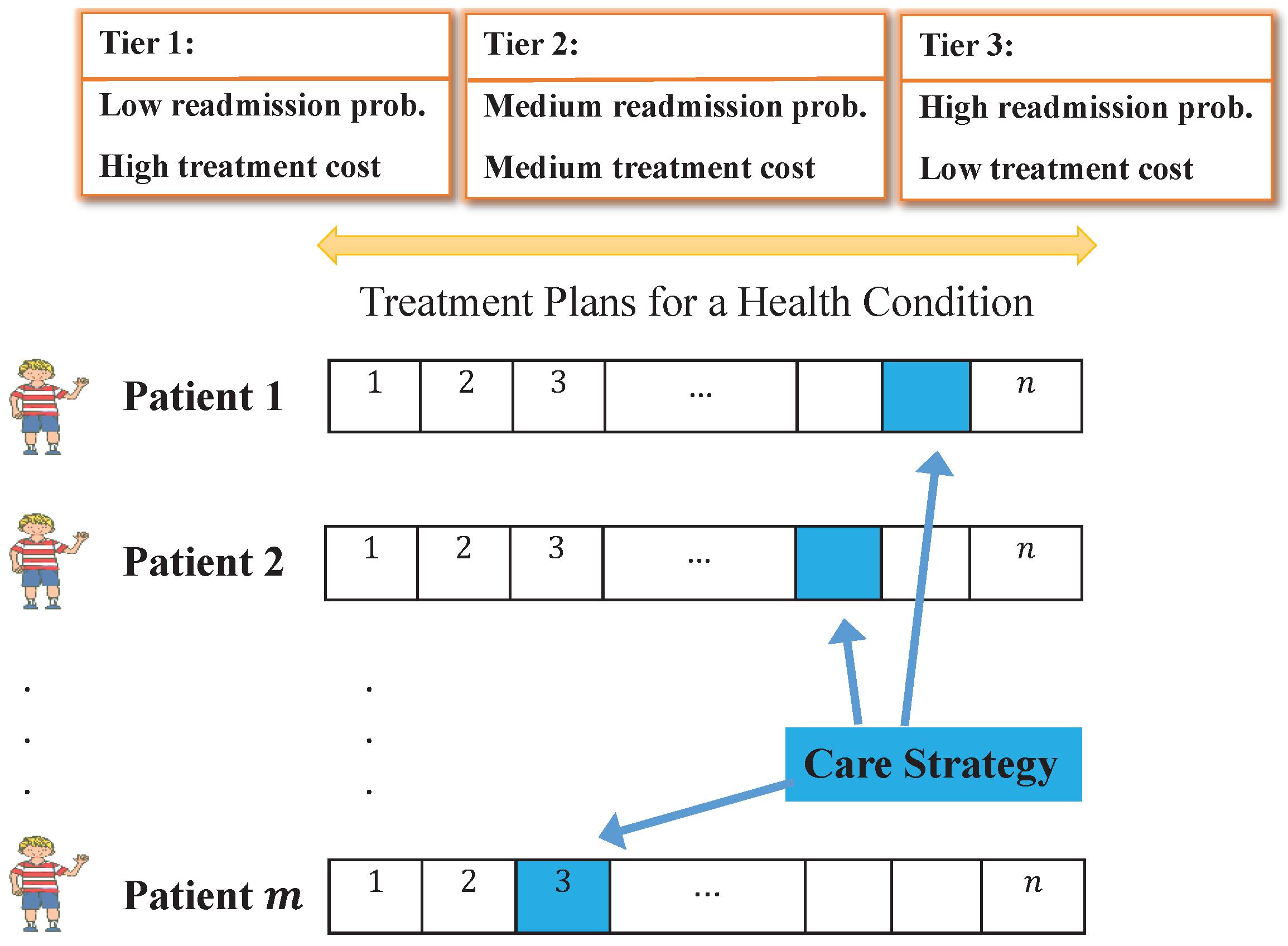
3.1. Problem Statement
3.2. Model Assumptions
- Selecting a care strategy for a given time period: the proposed model is a strategic decision-making policy model that would require periodic (e.g., monthly or quarterly) decisions based on changing goals, treatment plans, budgets, etc.
- The probability of readmission is uncertain: historical data provides the probability of readmission based on condition, but some treatment plans have better success rates than others and some patients are more at-risk for readmission due to behavioral and health reasons.
- Only one treatment plan with a known cost must be chosen for each patient visit: the modeling approach assumes that the cost of each treatment plan is fixed at the time of the decision and that exactly one treatment plan must be chosen for each patient.
- The percentage of hospitals penalized using the mean readmission probability as a target is a reasonable estimator of the percentage of hospitals penalized using the median ERR: recall that CMS penalizes hospitals based on ERR which is a riskadjusted ratio of the number of predicted readmissions at a hospital to the expected the number of readmissions at an average hospital over a three-year performance period. Under HRRP, if a given condition has 25 or more eligible discharges and the ERR is greater than the peer group median ERR, the hospital is subject to a penalty. Otherwise, the hospital avoids the penalty for that condition. Unfortunately, ERR scores are for hospitals over three years and are not directly associated with a treatment plan or care strategy. Therefore, we needed a direct measure to set as a target for the hospital. To solve this dilemma, we explored using readmission probabilities as an estimator. One motivation for doing so is that high ERR scores commonly correspond to high readmission probabilities. ERR measures include a risk-adjustment factor whereas the mean readmission probability does not, thus we next provide evidence of the validity of the assumption.
3.3. Scenario-Based Stochastic Model
3.4. Deterministic Equivalent Model
4. Model Demonstration
4.1. Design of Experiments
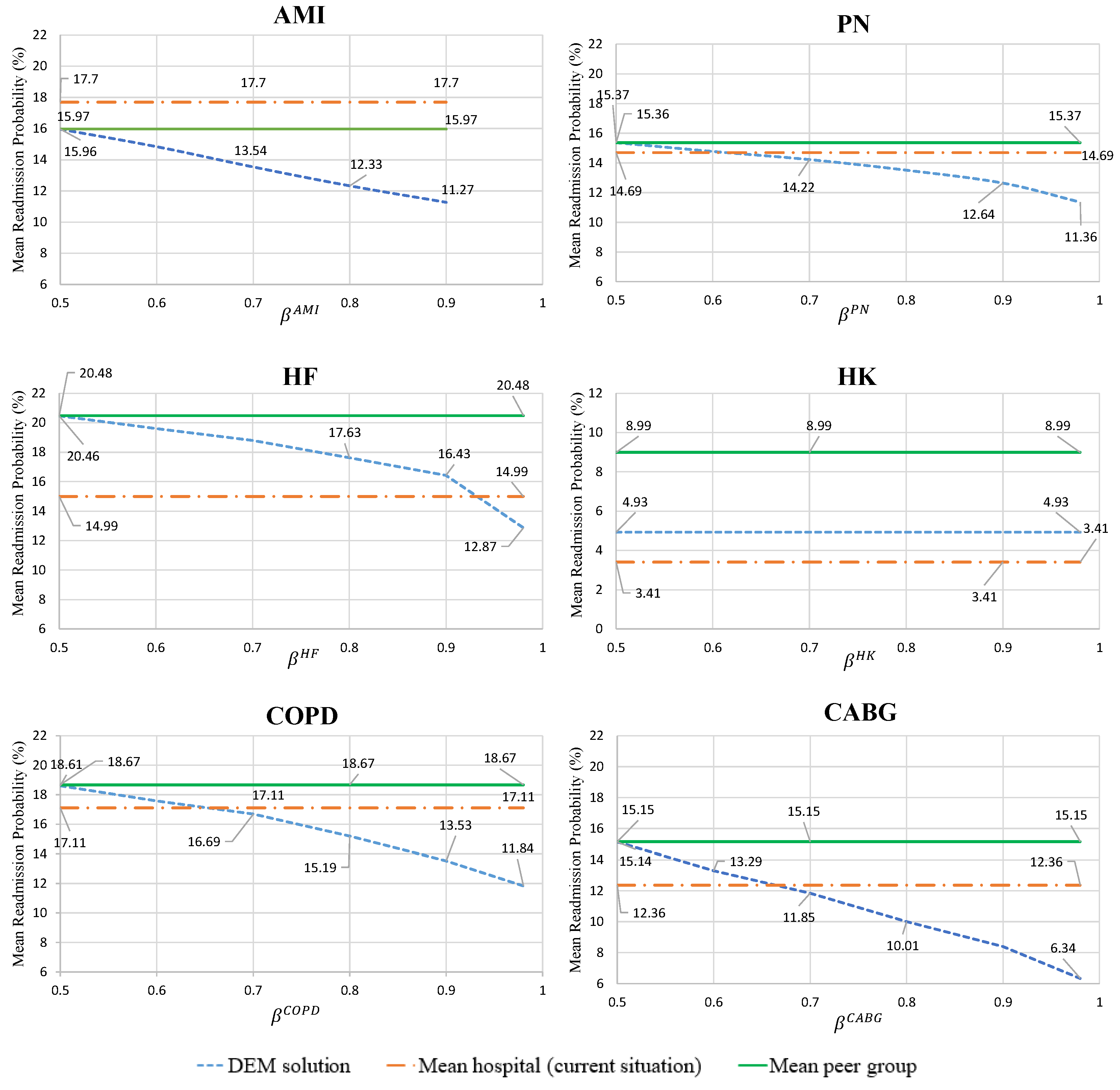
4.2. Computational Results
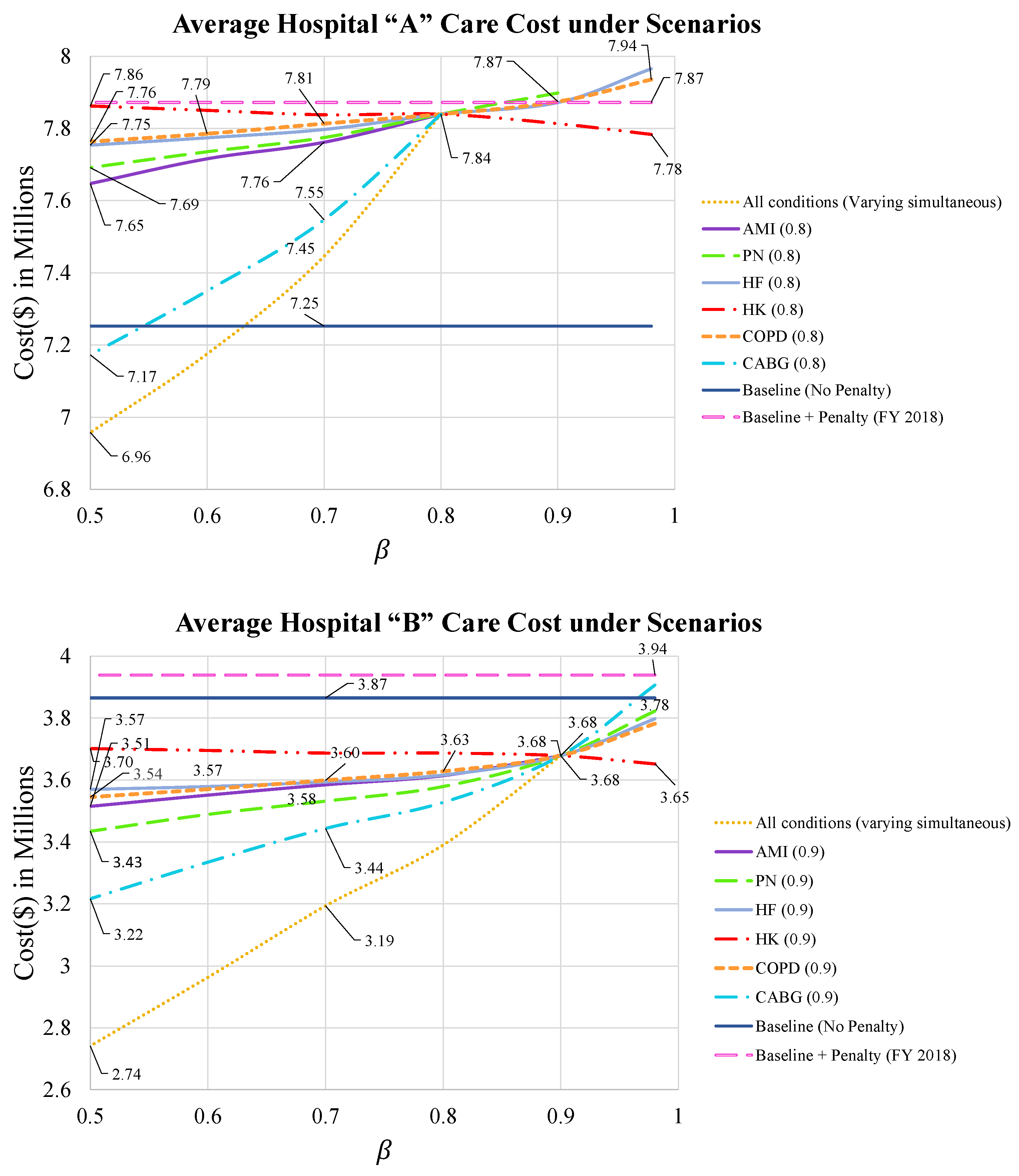
- Cost of optimal care strategies for varying levels of
- 2.
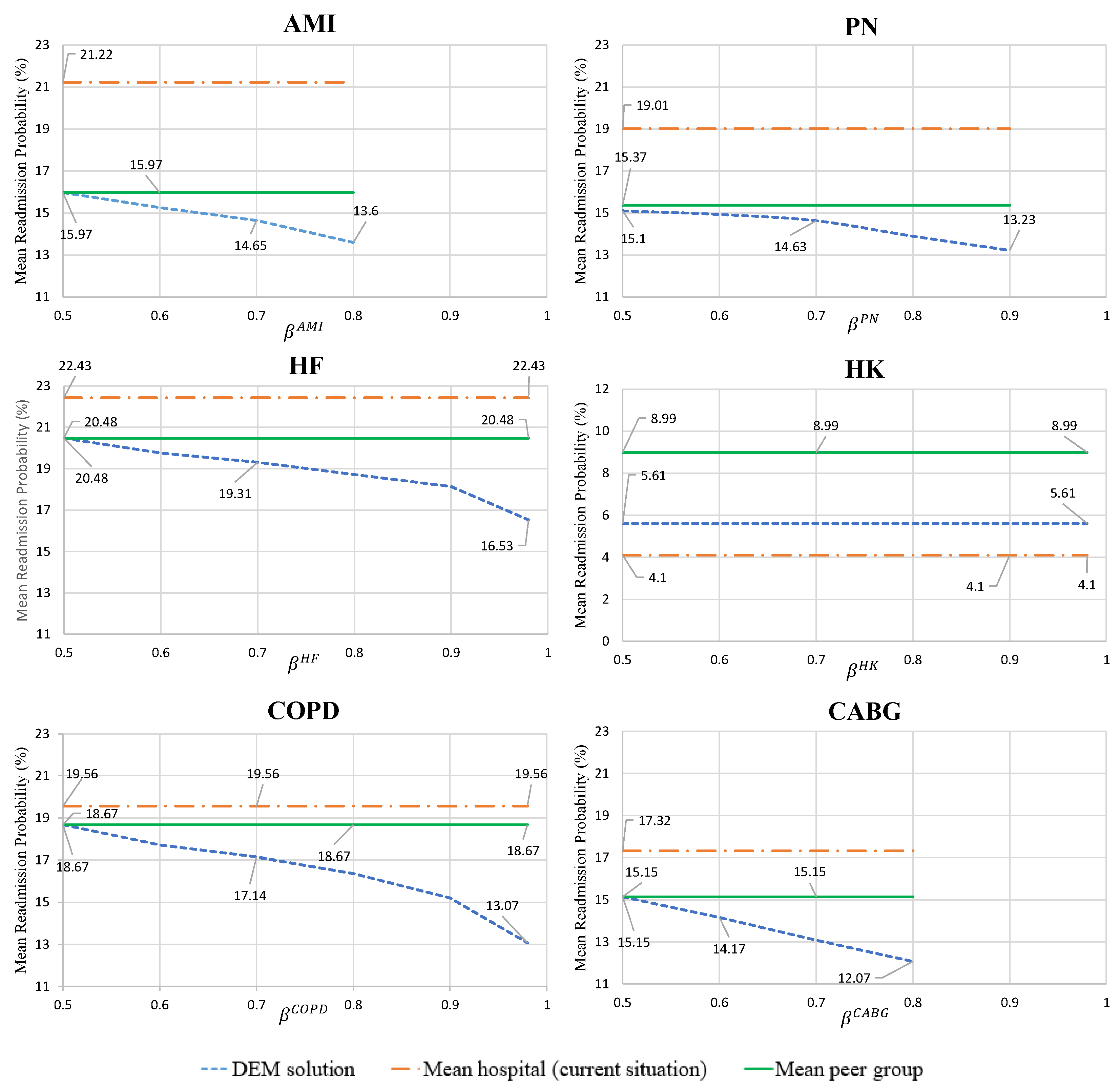
- Mean hospital readmission probability for the optimal care strategies, given by condition
- 3.
- Analysis of the decisions for the optimal care strategies, given by condition
5. Discussion
6. Summary and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.S.; Horwitz, L.I.; Barreto-Filho, J.A.; Kim, N.; Bernheim, S.M.; Suter, L.G.; et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013, 309, 355–363. [Google Scholar] [CrossRef]
- Berenson, R.A.; Paulus, R.A.; Kalman, N.S. Medicare’s readmissions-reduction program—A positive alternative. N. Engl. J. Med. 2012, 366, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.R.; Bech, M.; Quentin, W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy 2015, 119, 264–273. [Google Scholar] [CrossRef]
- Friebel, R.; Hauck, K.; Aylin, P.; Steventon, A. National trends in emergency readmission rates: A longitudinal analysis of administrative data for England between 2006 and 2016. BMJ Open 2018, 8, e020325. [Google Scholar] [CrossRef] [Green Version]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef]
- Zhang, D.J.; Gurvich, I.; Van Mieghem, J.A.; Park, E.; Young, R.S.; Williams, M.V. Hospital readmissions reduction program: An economic and operational analysis. Manag. Sci. 2016, 62, 3351–3371. [Google Scholar] [CrossRef] [Green Version]
- Kripalani, S.; Theobald, C.N.; Anctil, B.; Vasilevskis, E.E. Reducing hospital readmission rates: Current strategies and future directions. Annu. Rev. Med. 2013, 65, 471–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.; Zhang, L.; Witz, S.; Musselman, K.J.; Yi, F.; Mullen, C.J.; Benneyan, J.C.; Zayas-Castro, J.L.; Rico, F.; Cure, L.N.; et al. A literature review of preventable hospital readmissions: Preceding the Readmissions Reduction Act. IIE Trans. Healthc. Syst. Eng. 2016, 6, 193–211. [Google Scholar] [CrossRef]
- MedPAC. Report to Congress: Medicare and the Healthcare Delivery System. Chapter 4: Refining the Hospital Readmissions Reduction Program. 2013. Available online: http://www.medpac.gov (accessed on 10 March 2021).
- CMS.gov. U.S. Centers for Medicare and Medicaid Services. Available online: https://www.cms.gov (accessed on 24 March 2021).
- Shulan, M.; Gao, K.; Moore, C.D. Predicting 30-day all-cause hospital readmissions. Health Care Manag. Sci. 2013, 16, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Rau, J. Under Trump, Hospitals Face Same Penalties Embraced by Obama. 2017. Available online: https://khn.org/news/undertrump-hospitals-face-same-penalties-embraced-by-obama/ (accessed on 25 January 2021).
- Ashton, C.M.; Del Junco, D.J.; Souchek, J.; Wray, N.P.; Mansyur, C.L. The association between the quality of inpatient care and early readmission: A meta-analysis of the evidence. Med. Care 1997, 35, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Gwadry-Sridhar, F.H.; Flintoft, V.; Lee, D.S.; Lee, H.; Guyatt, G.H. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch. Intern. Med. 2004, 164, 2315–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccuti, C.; Casillas, G. Aiming for Fewer Hospital U-Turns: The Medicare Hospital Readmission Reduction Program. 2017. Available online: https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmissionreduction-program (accessed on 22 June 2018).
- Van Walraven, C.; Bennett, C.; Jennings, A.; Austin, P.C.; Forster, A.J. Proportion of hospital readmissions deemed avoidable: A systematic review. Can. Med. Assoc. J. 2011, 183, E391–E402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.P.; Kaplan, C.M.; Cao, Y.; Bazzoli, G.J.; Waters, T.M. Reliability of 30-Day Readmission Measures Used in the Hospital Readmission Reduction Program. Health Serv. Res. 2016, 51, 2095–2114. [Google Scholar] [CrossRef] [Green Version]
- Merton, R.K.; Reader, G.; Kendall, P.L. The Student Physician: Introductory Studies in the Sociology of Medical Education; Harvard Univer. Press: Cambridge, CA, USA, 1957. [Google Scholar]
- Fox, R.C. The evolution of medical uncertainty. In The Milbank Memorial Fund Quarterly. Health and Society; JSTOR: New York, NY, USA, 1980; pp. 1–49. [Google Scholar]
- Lahijanian, B.; Zarandi, M.F.; Farahani, F.V. Double coverage ambulance location modeling using fuzzy traveling time. In Proceedings of the 2016 Annual Conference of the North American Fuzzy Information Processing Society (NAFIPS), El Paso, TX, USA, 31 October–4 November 2016; pp. 1–6. [Google Scholar]
- Lahijanian, B.; Zarandi, M.F.; Farahani, F.V. Proposing a model for operating room scheduling based on fuzzy surgical duration. In Proceedings of the 2016 Annual Conference of the North American Fuzzy Information Processing Society (NAFIPS), El Paso, TX, USA, 31 October–4 November; 2016; pp. 1–5. [Google Scholar]
- Lahijanian, B.; Alvarado, M. A Statistical Analysis of Penalty vs. Incentive Designs for Hospital Readmission. In Proceedings of the IISE Annual Conference and Expo, Orlando, FL, USA, 18–21 May 2019. [Google Scholar]
- Ferro, E.G.; Secemsky, E.A.; Wadhera, R.K.; Choi, E.; Strom, J.B.; Wasfy, J.H.; Wang, Y.; Shen, C.; Yeh, R.W. Patient readmission rates for all insurance types after implementation of the hospital readmissions reduction program. Health Aff. 2019, 38, 585–593. [Google Scholar] [CrossRef]
- Artetxe, A.; Beristain, A.; Grana, M. Predictive models for hospital readmission risk: A systematic review of methods. Comput. Methods Programs Biomed. 2018, 164, 49–64. [Google Scholar] [CrossRef]
- Zhou, H.; Della, P.R.; Roberts, P.; Goh, L.; Dhaliwal, S.S. Utility of models to predict 28-day or 30-day unplanned hospital readmissions: An updated systematic review. BMJ Open 2016, 6, e011060. [Google Scholar] [CrossRef]
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk prediction models for hospital readmission: A systematic review. JAMA 2011, 306, 1688–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Lee, S.; Zhao, C.; Lee, H.K.; Bain, P.A.; Kundinger, T.; Sommers, C.; Baker, C.; Li, J. Reducing COPD readmissions through predictive modeling and incentive-based interventions. Health Care Manag. Sci. 2019, 22, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kong, N.; Sun, X.; Meng, H.; Li, M. Claims data-driven modeling of hospital time-to-readmission risk with latent heterogeneity. Health Care Manag. Sci. 2019, 22, 156–179. [Google Scholar] [CrossRef]
- Shams, I.; Ajorlou, S.; Yang, K. A predictive analytics approach to reducing 30-day avoidable readmissions among patients with heart failure, acute myocardial infarction, pneumonia, or COPD. Health Care Manag. Sci. 2015, 18, 19–34. [Google Scholar] [CrossRef]
- Horwitz, L.; Partovian, C.; Lin, Z.; Herrin, J.; Grady, J.; Conover, M.; Montague, J.; Dillaway, C.; Bartczak, K.; Ross, J.; et al. Hospital-Wide (All-Condition) 30-Day Risk-Standardized Readmission Measure; Technical Report; Walter de Gruyter: Berlin, Germany, 2011; Available online: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf (accessed on 20 September 2020).
- Baechle, C.; Huang, C.D.; Agarwal, A.; Behara, R.S.; Goo, J. Latent topic ensemble learning for hospital readmission cost optimization. Eur. J. Oper. Res. 2020, 281, 517–531. [Google Scholar] [CrossRef]
- Alaeddini, A.; Helm, J.E.; Shi, P.; Faruqui, S.H.A. An integrated framework for reducing hospital readmissions using risk trajectories characterization and discharge timing optimization. IISE Trans. Healthc. Syst. Eng. 2019, 9, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.E.; Alaeddini, A.; Stauffer, J.M.; Bretthauer, K.M.; Skolarus, T.A. Reducing hospital readmissions by integrating empirical prediction with resource optimization. Prod. Oper. Manag. 2016, 25, 233–257. [Google Scholar] [CrossRef]
- Alvarado, M.; Zhang, Y.; Lawley, M. Hospital Readmission Reduction Strategies Using a Penalty-Incentive Model. In Proceedings of the IISE Annual Conference and Expo, Anaheim, CA, USA, 21–24 May 2016. [Google Scholar]
- Bastani, H.; Bayati, M.; Braverman, M.; Gummadi, R.; Johari, R. Analysis of Medicare Pay-for-Performance Contracts. 2016. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2839143 (accessed on 22 June 2020).
- Andritsos, D.A.; Tang, C.S. Incentive programs for reducing readmissions when patient care is co-produced. Prod. Oper. Manag. 2018, 27, 999–1020. [Google Scholar] [CrossRef]
- Arifoğlu, K.; Ren, H.; Tezcan, T. Hospital readmissions reduction program does not provide the right incentives: Issues and remedies. Manag. Sci. 2021, 67, 2191–2210. [Google Scholar] [CrossRef]
- Alvarado, M.; Lahijanian, B.; Zhang, Y.; Lawley, M. Rational Penalty and Incentive Mechanisms for Hospital Readmissions. Manuscript submitted (under review).
- Murphy, F.; Sen, S.; Soyster, A. Electric utility capacity expansion planning with uncertain load forecasts. IIE Trans. 1982, 14, 52–59. [Google Scholar] [CrossRef]
- Föllmer, H.; Schied, A. Stochastic Finance: An Introduction in Discrete Time; De Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Nicholson, L.; Vakharia, A.J.; Erenguc, S.S. Outsourcing inventory management decisions in healthcare: Models and application. Eur. J. Oper. Res. 2004, 154, 271–290. [Google Scholar] [CrossRef]
- Charnes, A.; Cooper, W.W. Chance-constrained programming. Manag. Sci. 1959, 6, 73–79. [Google Scholar] [CrossRef]
- Shylo, O.V.; Prokopyev, O.A.; Schaefer, A.J. Stochastic operating room scheduling for high-volume specialties under block booking. INFORMS J. Comput. 2012, 25, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Beraldi, P.; Bruni, M.E. A probabilistic model applied to emergency service vehicle location. Eur. J. Oper. Res. 2009, 196, 323–331. [Google Scholar] [CrossRef]
- Khodaparasti, S.; Bruni, M.; Beraldi, P.; Maleki, H.; Jahedi, S. A multi-period location-allocation model for nursing home network planning under uncertainty. Oper. Res. Health Care 2018, 18, 4–15. [Google Scholar] [CrossRef]
- Fortz, B.; Poss, M. Easy distributions for combinatorial optimization problems with probabilistic constraints. Oper. Res. Lett. 2010, 38, 545–549. [Google Scholar] [CrossRef]
- Prekopa, A. Stochastic Programming. 1995. Available online: https://books.google.com.hk/books/about/Stochastic_Programming.html?id=SH7vCAAAQBAJ&redir_esc=y (accessed on 24 July 2021).
- HealthcareBluebook.com. Find Your Fair Price. Available online: https://www.healthcarebluebook.com (accessed on 15 April 2021).
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample size determination. ILAR J. 2002, 43, 207–213. [Google Scholar] [CrossRef]
- Rice, R.D.; Han, X.; Wang, X.; Al-Jaghbeer, M.J. COPD overdiagnosis and its effect on 30-day hospital readmission rates. Respir. Care 2021, 66, 11–17. [Google Scholar] [CrossRef]
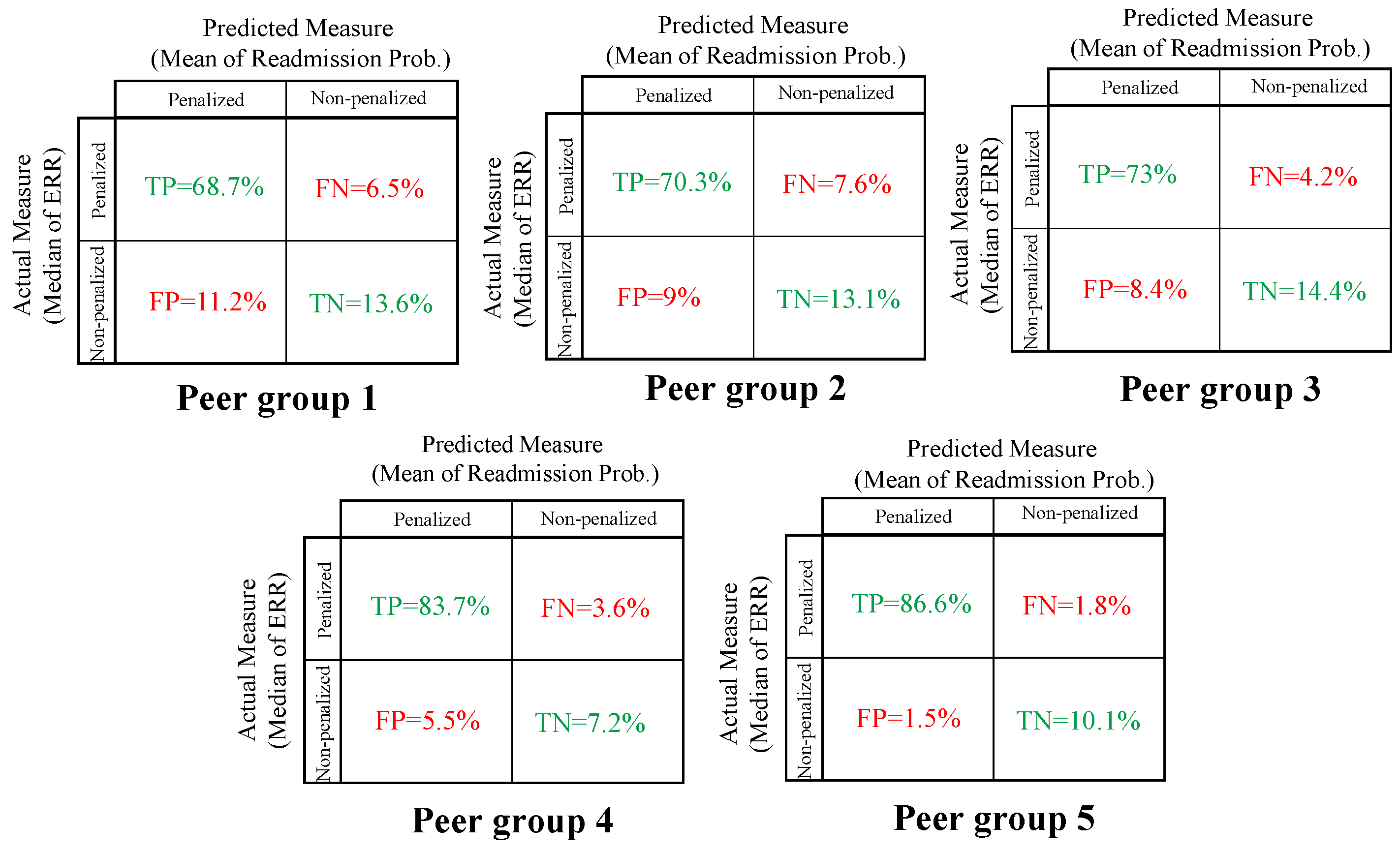


| Peer Group | Accuracy | Misclassification Rate |
|---|---|---|
| 1 | 82.3% | 17.7% |
| 2 | 83.4% | 16.6% |
| 3 | 87.4% | 12.6% |
| 4 | 90.9% | 9.1% |
| 5 | 96.7% | 3.3% |
| Sets | |
|---|---|
| C: | Set of conditions, indexed by c |
| : | Set of scenarios, indexed by |
| : | Set of treatment plans with condition , indexed by k |
| : | Set of patients with condition , indexed by p |
| Parameters | |
| : | Probability of scenario |
| : | Target readmission rate for condition , given as a probability between 0 and 1 |
| : | Confidence level to satisfy the probability constraint for condition |
| : | Confidence level vector |
| : | Cost of treatment plan for patient with condition under scenario |
| : | Mean readmission probability of treatment plan for patient with condition under scenario |
| : | Expected penalty for confidence level vector |
| : | Proportion of baseline cost contributed by condition |
| : | Mean cost of treatment plans for condition |
| Penalty: | One-year penalty cost for the hospital |
| Decision variable | |
| : | if treatment plan for patient with condition under scenario is selected, otherwise |
| Additional Notations | |
|---|---|
| : | Coefficient of variance for the Gaussian random variable for condition |
| : | |
| : | Unique root of Equation (10) |
| : | Cumulative distribution of the standard normal distribution |
| Hospital “A” | Hospital “B” | ||||
|---|---|---|---|---|---|
| Cond. | Peer Group 2 Mean Readmission Prob. | Mean Readmission Prob. | P | Mean Readmission Prob. | P |
| AMI | 15.97% | 21.21% | × | 17.69% | × |
| PN | 15.37% | 19.01% | × | 14.69% | |
| HF | 20.48% | 22.43% | × | 14.99% | |
| HK | 8.01% | 4.21% | 3.41% | ||
| COPD | 18.67% | 19.56% | × | 17.11% | |
| CABG | 15.15% | 17.33% | × | 12.36% | |
| Hospital “A” | Hospital “B” | |||
|---|---|---|---|---|
| Cond. | No. Patients | Cost($) Distributions (TRIA) | No. Patients | Cost($) Distributions (TRIA) |
| AMI | 52 | (6745, 8431, 21,078) | 18 | (8439, 10,548, 26,370) |
| PN | 132 | (6312, 7890, 19,725) | 69 | (7897, 9871, 24,678) |
| HF | 126 | (5990,7487, 18,718) | 44 | (7494, 9367, 23,418) |
| HK | 96 | (3585, 9359, 31,814) | 76 | (4196, 9001, 25,542) |
| COPD | 47 | (6460, 8075, 20,188) | 22 | (8082, 10,102, 25,255) |
| CABG | 21 | (39,864, 56,297, 161,385) | 7 | (47,382, 67,664, 196,103) |
| Current Readmission Reduction Policy | VS | Scenario-Based Stochastic Programming Model |
|---|---|---|
| Decentralized focus on health conditions | Decision-making approach | Centralized focus on each health condition |
| Scattered decisions for patients | Treatment plan selection | Optimal care strategy based on holistic approach for multiple patients and conditions |
| Not formally considered | Treatment plans cost | Selecting treatment with minimum cost |
| Not formally considered | Stochastic data | Included via probabilistic constraints |
| High readmission probability, then more hospital visits and higher out-of-pocket expenses | Patient experience | Lower readmission probability, then less hospital visits and lower out-of-pocket expenses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahijanian, B.; Alvarado, M. Care Strategies for Reducing Hospital Readmissions Using Stochastic Programming. Healthcare 2021, 9, 940. https://doi.org/10.3390/healthcare9080940
Lahijanian B, Alvarado M. Care Strategies for Reducing Hospital Readmissions Using Stochastic Programming. Healthcare. 2021; 9(8):940. https://doi.org/10.3390/healthcare9080940
Chicago/Turabian StyleLahijanian, Behshad, and Michelle Alvarado. 2021. "Care Strategies for Reducing Hospital Readmissions Using Stochastic Programming" Healthcare 9, no. 8: 940. https://doi.org/10.3390/healthcare9080940
APA StyleLahijanian, B., & Alvarado, M. (2021). Care Strategies for Reducing Hospital Readmissions Using Stochastic Programming. Healthcare, 9(8), 940. https://doi.org/10.3390/healthcare9080940






