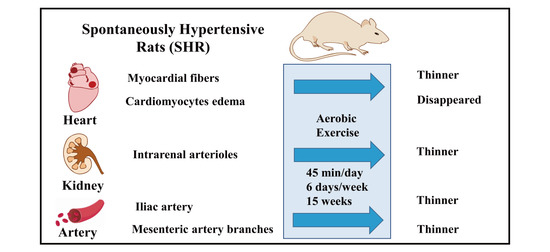
Morphological Study of the Effect of Aerobic Exercise on Organs and Arteries in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Exercise Protocols
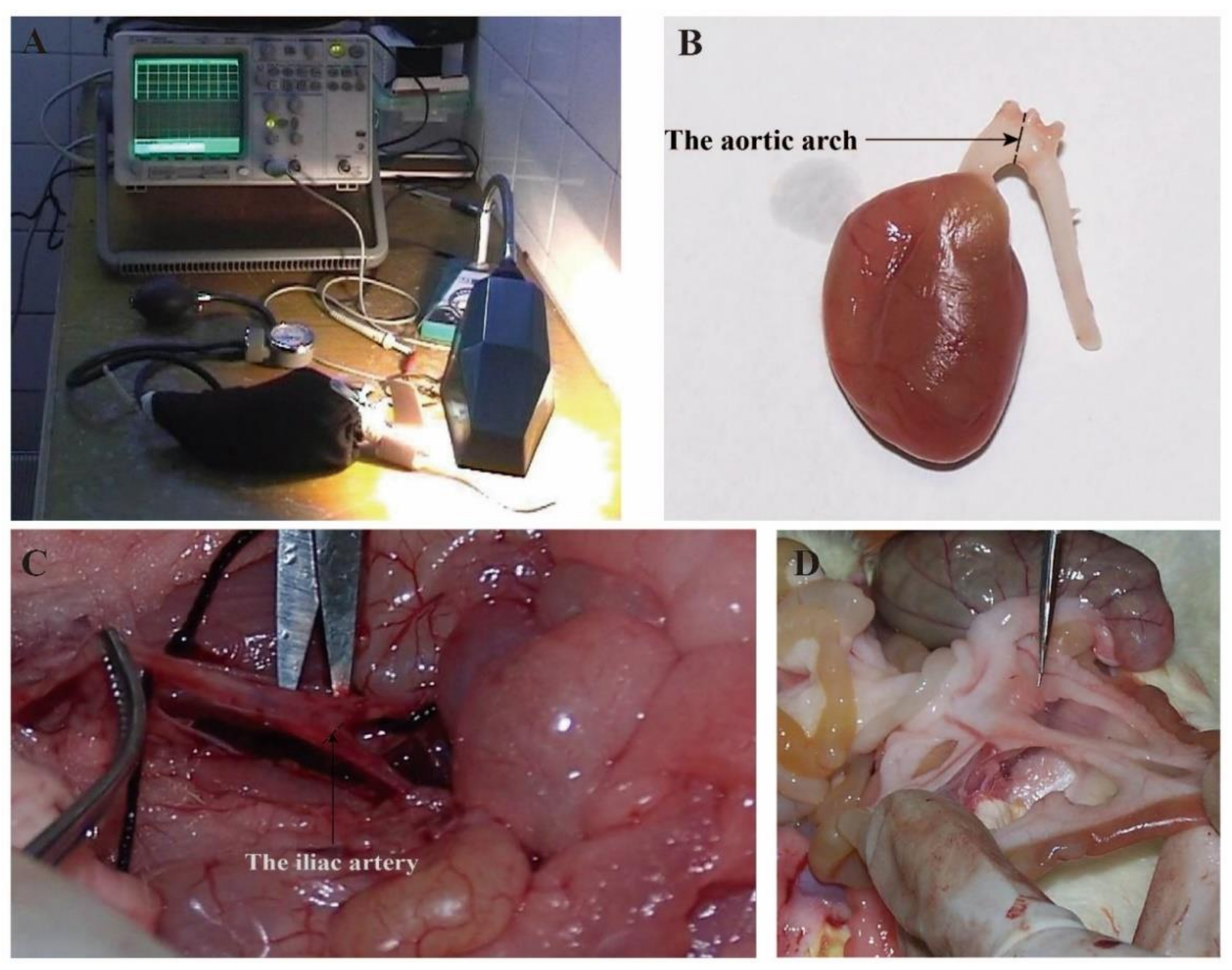
2.3. Experiment Design and Sample Collection
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Statistical Analysis
3. Results
3.1. Aerobic Exercise Improved Body Weight and Systolic Blood Pressure
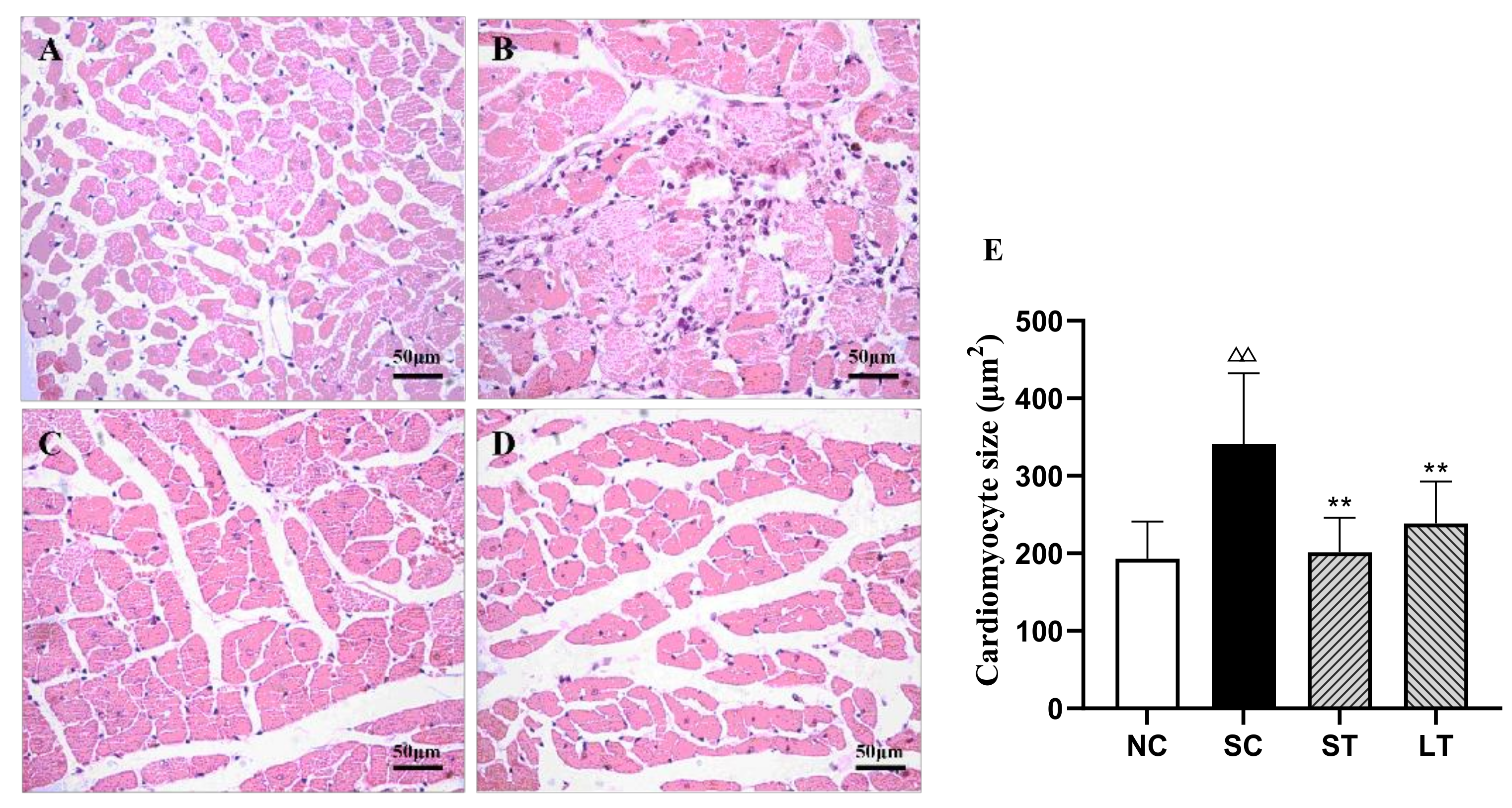
3.2. Aerobic Exercise Improved Heart Morphology and Structure
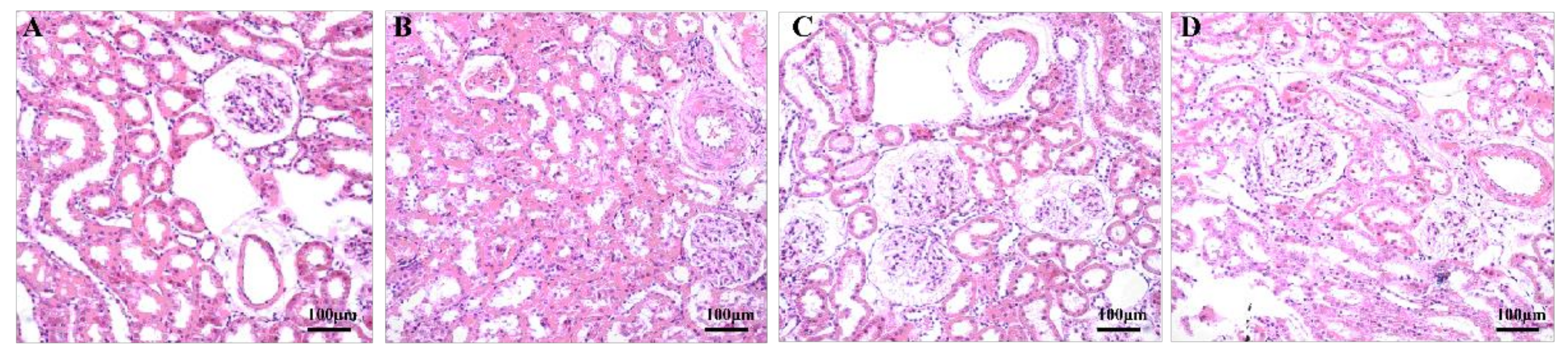
3.3. Aerobic Exercise Improved Kidney Morphology and Structure
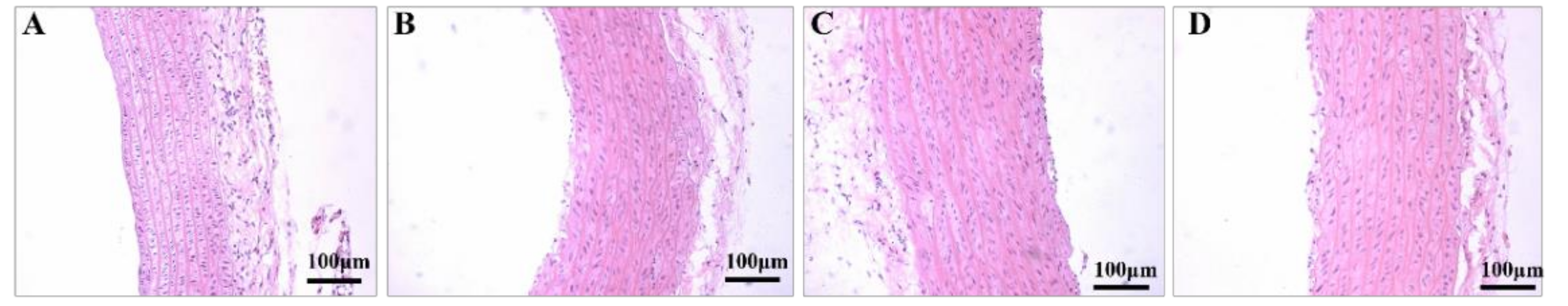
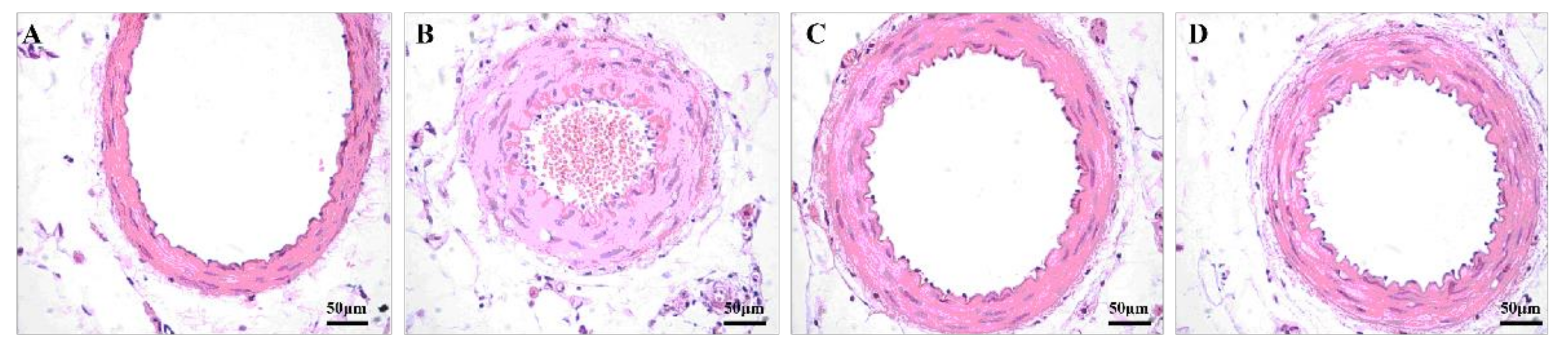
3.4. Aerobic Exercise Improved Artery Morphology and Structure
4. Discussion
5. Limitations and Future Directions
5.1. Possible Mechanism of Aerobic Exercise to Improve Hypertension
5.2. Exercise Prescription for Hypertension
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzoni, D.; Agabiti-Rosei, C.; Agabiti-Rosei, E. Hemodynamic Consequences of Changes in Microvascular Structure. Am. J. Hypertens. 2017, 30, 939–946. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Stanton, T.; Dunn, F.G. Hypertension, Left Ventricular Hypertrophy, and Myocardial Ischemia. Med. Clin. N. Am. 2017, 101, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, M.T.; Vasan, R.S. A Contemporary Approach to Hypertensive Cardiomyopathy: Reversing Left Ventricular Hypertrophy. Curr. Hypertens. Rep. 2020, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.; Farrar, F.C. Kidney Influence on Fluid and Electrolyte Balance. Nurs. Clin. N. Am. 2018, 53, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Sievers, L.K.; Eckardt, K. Molecular Mechanisms of Kidney Injury and Repair in Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Zhang, Y.; Lacolley, P.; Protogerou, A.D.; Safar, M.E. Arterial Stiffness in Hypertension and Function of Large Arteries. Am. J. Hypertens. 2020, 33, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Hypertension, systolic blood pressure, and large arteries. Med. Clin. N. Am. 2009, 93, 605–619. [Google Scholar] [CrossRef]
- Harris, S.S.; Caspersen, C.J.; DeFriese, G.H.; Estes, E.H. Physical activity counseling for healthy adults as a primary preventive intervention in the clinical setting: Report for the US Preventive Services Task Force. JAMA 1989, 261, 3588–3598. [Google Scholar] [CrossRef]
- Lobelo, F.; Stoutenberg, M.; Hutber, A. The Exercise is Medicine Global Health Initiative: A 2014 update. Br. J. Sport Med. 2014, 48, U1627–U1668. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Shan, M.; Zhou, Y.; Huang, Y.; Shi, L. Aerobic exercise-induced inhibition of PKCα/Ca1.2 pathway enhances the vasodilation of mesenteric arteries in hypertension. Arch. Biochem. Biophys. 2019, 678, 108191. [Google Scholar] [CrossRef]
- Duan, Y.; Shi, L.; Jin, Z.; Hu, M.; Huang, H.; Yan, T.; Zhang, K. Swimming Exercise Ameliorates Hypertension-Induced Kidney Dysfunction via Alleviating Renal Interstitial Fibrosis and Apoptosis. Kidney Blood Press. Res. 2021, 46, 219–228. [Google Scholar] [CrossRef]
- Noordzij, M.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Sample size calculations. Nephron Clin. Pract. 2011, 118, c319–c323. [Google Scholar] [CrossRef]
- Shenasa, M.; Shenasa, H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int. J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Textor, S.C. Renovascular Hypertension. Endocrinol. Metab. Clin. N. Am. 2019, 48, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Dajnowiec, D.; Langille, B.L. Arterial adaptations to chronic changes in haemodynamic function: Coupling vasomotor tone to structural remodelling. Clin. Sci. 2007, 113, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Lelbach, A.; Koller, A. Mechanisms underlying exercise-induced modulation of hypertension. J. Hypertens. Res. 2017, 3, 35–43. [Google Scholar]
- Black, J.M.; Stohr, E.J.; Shave, R.; Esformes, J.I. Influence of exercise training mode on arterial diameter: A systematic review and meta-analysis. J. Sci. Med. Sport 2016, 19, 74–80. [Google Scholar] [CrossRef]
- Cuspidi, C.; Rescaldani, M.; Sala, C.; Negri, F.; Grassi, G.; Mancia, G. Prevalence of electrocardiographic left ventricular hypertrophy in human hypertension: An updated review. J. Hypertens. 2012, 30, 2066–2073. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Soliman, E.Z. Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: A critical appraisal. J. Electrocardiol. 2014, 47, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.N.; Devereux, R.B.; Okin, P.M. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—A LIFE review. J. Electrocardiol. 2014, 47, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, L.; Wei, X. Prognostic value of left ventricular hypertrophy in hypertensive patients: A meta-analysis of electrocardiographic studies. J. Clin. Hypertens. 2020, 22, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, J.; Rhee, J.; Chaudhari, V.; Rosenzweig, A. The role of exercise in cardiac aging: From physiology to molecular mechanisms. Circ. Res. 2016, 118, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Leosco, D.; Parisi, V.; Femminella, G.D.; Formisano, R.; Petraglia, L.; Allocca, E.; Bonaduce, D. Effects of exercise training on cardiovascular adrenergic system. Front. Physiol. 2013, 4, 348. [Google Scholar] [CrossRef] [Green Version]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 2003, 17, 1–10. [Google Scholar] [CrossRef]
- Sommer, M.; von der Lippe, N.; Klow, N.E.; Hoieggen, A. Renal fibromuscular dysplasia and hypertension. Tidsskr. Nor. Laegeforen. 2019, 139. [Google Scholar] [CrossRef]
- Van Twist, D.J.L.; de Leeuw, P.W.; Kroon, A.A. Renal artery fibromuscular dysplasia and its effect on the kidney. Hypertens. Res. 2018, 41, 639–648. [Google Scholar] [CrossRef]
- Melo, R.M.; Martinho, E., Jr.; Michelini, L.C. Training-induced, pressure-lowering effect in SHR: Wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension 2003, 42, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Schlader, Z.J.; Chapman, C.L.; Benati, J.M.; Gideon, E.A.; Vargas, N.T.; Lema, P.C.; Johnson, B.D. Renal Hemodynamics During Sympathetic Activation Following Aerobic and Anaerobic Exercise. Front. Physiol. 2018, 9, 1928. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.R.; Gomes, M.J.; Rosa, C.M.; Pagan, L.U.; Zanati, S.G.; Damatto, R.L.; Rodrigues, E.A.; Carvalho, R.F.; Fernandes, A.A.; Martinez, P.F.; et al. Exercise during transition from compensated left ventricular hypertrophy to heart failure in aortic stenosis rats. J. Cell. Mol. Med. 2019, 23, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Bovée, D.M.; Cuevas, C.A.; Zietse, R.; Danser, A.H.; Mirabito, C.K.; Hoorn, E.J. Salt-sensitive hypertension in chronic kidney disease: Distal tubular mechanisms. Am. J. Physiol. Renal Physiol. 2020, 319, F729–F745. [Google Scholar] [CrossRef]
- Guo, X.; Chen, H.; Han, L.; Haulon, S.; Kassab, G.S. Chronic ETA antagonist reverses hypertension and impairment of structure and function of peripheral small arteries in aortic stiffening. Sci. Rep. 2018, 8, 3076. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Martinez-Quinones, P.; McCarthy, C.G.; Watts, S.W.; Klee, N.S.; Komic, A.; Calmasini, F.B.; Priviero, F.; Warner, A.; Chenghao, Y.; Wenceslau, C.F. Hypertension Induced Morphological and Physiological Changes in Cells of the Arterial Wall. Am. J. Hypertens. 2018, 31, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Cable, N.T.; Green, D.J. Impact of exercise training on arterial wall thickness in humans. Clin. Sci. 2012, 122, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, K.; Mikami, Y.; Murayama, T.; Yokode, M.; Fujita, M.; Kita, T.; Kishimoto, C. Atherosclerotic plaques induced by marble-burying behavior are stabilized by exercise training in experimental atherosclerosis. Int. J. Cardiol. 2011, 151, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | Body Weight (g) | Systolic Blood Pressure (mmHg) | ||
|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | |
| NC | 429.60 ± 26.93 | 542.60 ± 43.85 | 126.17 ± 12.51 | 107.40 ± 5.37 |
| SC | 258.50 ± 10.52 Δ | 353.00 ± 17.09 ΔΔ | 181.73 ± 16.26 ΔΔ | 187.50 ± 17.62 ΔΔ |
| ST | 257.63 ± 10.14 | 301.63 ± 17.01 ** | 176.17 ± 16.19 | 159.88 ± 15.02 ** |
| LT | 252.38 ± 14.69 | 288.00 ± 23.03 ** | 181.29 ± 16.59 | 168.94 ± 18.77 * |
| Group | MT (μm) |
|---|---|
| NC | 101.241 ± 22.493 |
| SC | 142.312 ± 18.581 ΔΔ |
| ST | 166.147 ± 40.119 |
| LT | 161.022 ± 33.056 |
| Group | MT/LD | MA/LD | MA/LA |
|---|---|---|---|
| NC | 0.211 ± 0.033 | 48.970 ± 10.316 | 1.027 ± 0.186 |
| SC | 0.391 ± 0.092 ΔΔ | 105.399 ± 27.825 ΔΔ | 2.207 ± 0.673 ΔΔ |
| ST | 0.143 ± 0.054 ** | 36.915 ± 16.286 ** | 0.665 ± 0.324 ** |
| LT | 0.197 ± 0.051 **# | 44.046 ± 9.988 ** | 0.953 ± 0.299 **# |
| Group | MT/LD | MA/LD | MA/LA |
|---|---|---|---|
| NC | 0.217 ± 0.056 | 368.075 ± 64.693 | 1.069 ± 0.317 |
| SC | 0.227 ± 0.026 | 411.249 ± 72.320 Δ | 1.119 ± 0.154 |
| ST | 0.189 ± 0.038 * | 342.216 ± 47.191 * | 0.904 ± 0.216 * |
| LT | 0.265 ± 0.034 ## | 382.047 ± 33.168 # | 1.342 ± 0.207 ## |
| Group | MT/LD | MA/LD | MA/LA |
|---|---|---|---|
| NC | 0.193 ± 0.120 | 149.974 ± 108.107 | 1.046 ± 0.731 |
| SC | 0.383 ± 0.117 ΔΔ | 161.956 ± 49.207 | 2.170 ± 0.863 ΔΔ |
| ST | 0.242 ± 0.113 * | 130.511 ± 42.309 | 1.251 ± 0.723 * |
| LT | 0.267 ± 0.100 * | 141.565 ± 43.618 | 1.386 ± 0.686 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Luan, Y.; Zhang, B.; Zhang, S.; Wang, X. Morphological Study of the Effect of Aerobic Exercise on Organs and Arteries in Spontaneously Hypertensive Rats. Healthcare 2021, 9, 1066. https://doi.org/10.3390/healthcare9081066
Xiong Y, Luan Y, Zhang B, Zhang S, Wang X. Morphological Study of the Effect of Aerobic Exercise on Organs and Arteries in Spontaneously Hypertensive Rats. Healthcare. 2021; 9(8):1066. https://doi.org/10.3390/healthcare9081066
Chicago/Turabian StyleXiong, Yingzhe, Yisheng Luan, Bing Zhang, Shu Zhang, and Xiaofei Wang. 2021. "Morphological Study of the Effect of Aerobic Exercise on Organs and Arteries in Spontaneously Hypertensive Rats" Healthcare 9, no. 8: 1066. https://doi.org/10.3390/healthcare9081066
APA StyleXiong, Y., Luan, Y., Zhang, B., Zhang, S., & Wang, X. (2021). Morphological Study of the Effect of Aerobic Exercise on Organs and Arteries in Spontaneously Hypertensive Rats. Healthcare, 9(8), 1066. https://doi.org/10.3390/healthcare9081066