Alcohol Consumption Patterns during COVID-19 Lockdown and Their Relationship with Perceived Immune Fitness and Reported COVID-19 Symptoms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
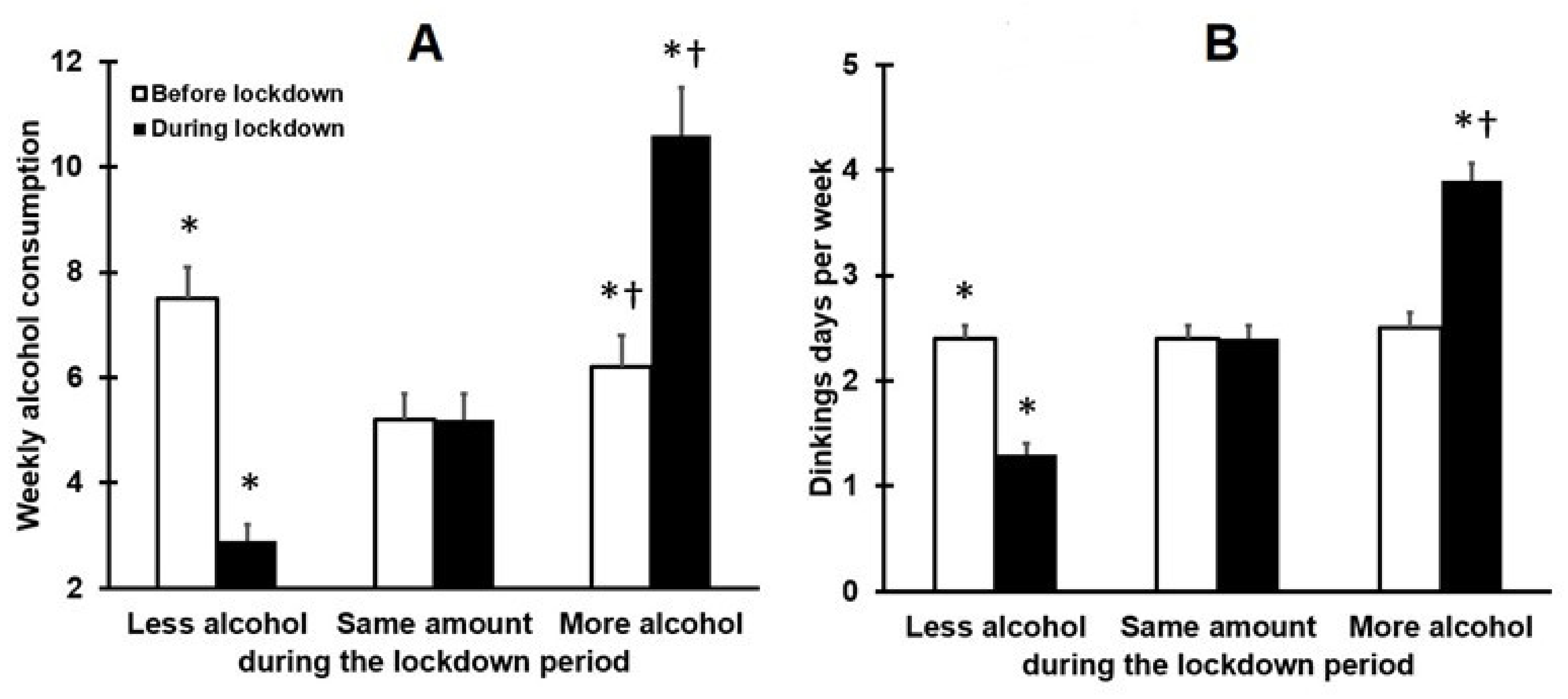
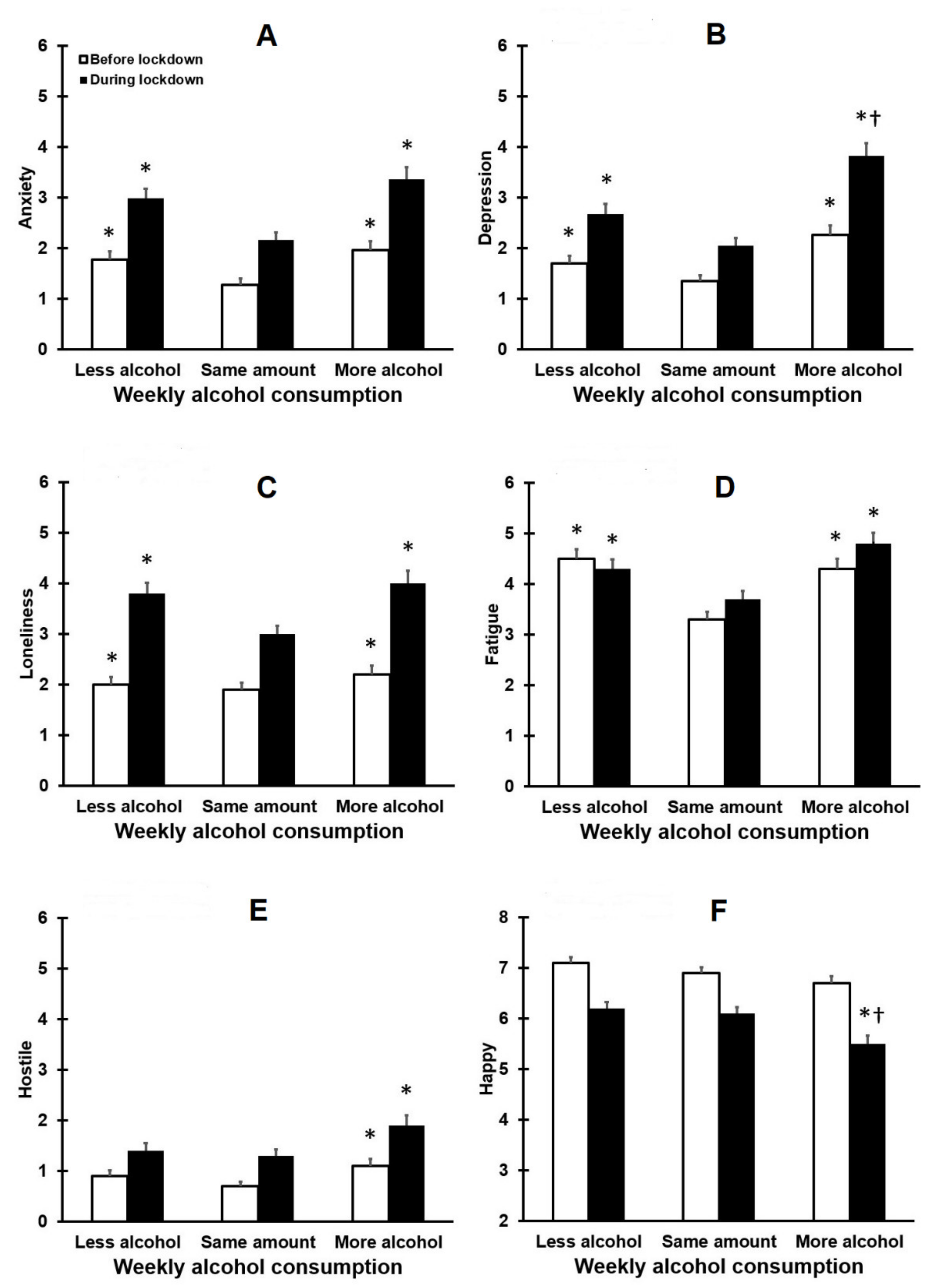
3.1. Drinking Outcomes and Mood
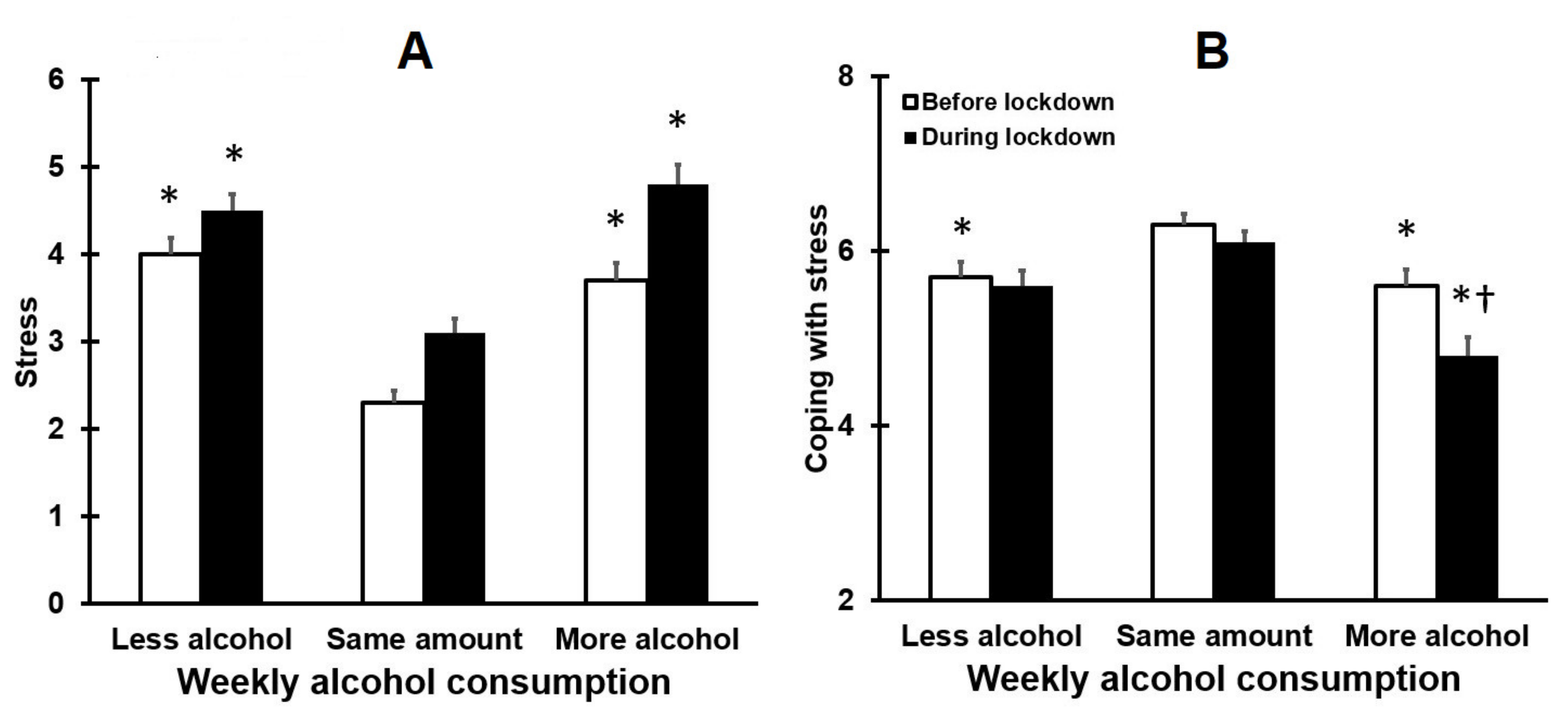
3.2. Stress and Coping
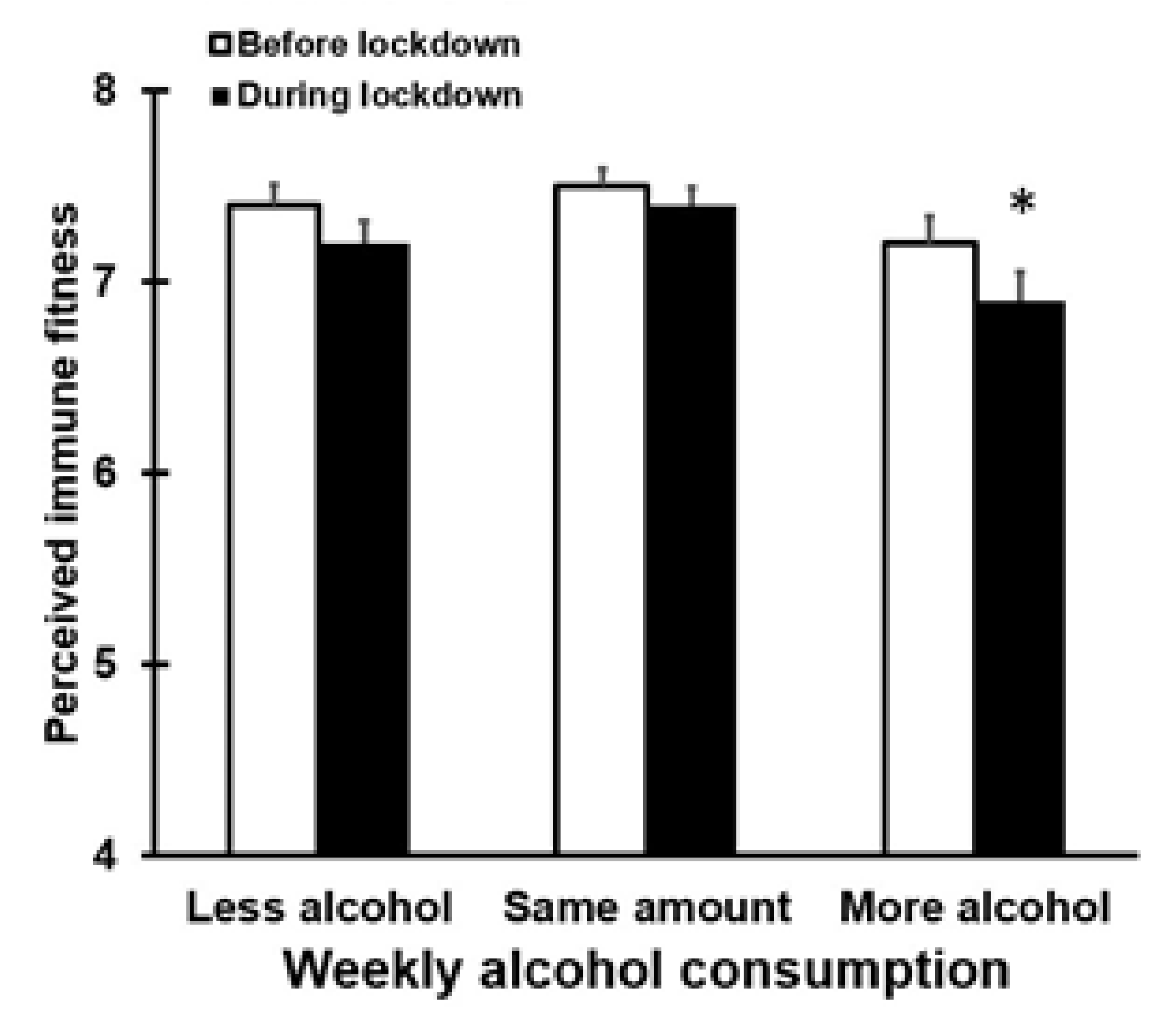
3.3. Alcohol Consumption and Perceived Immune Fitness
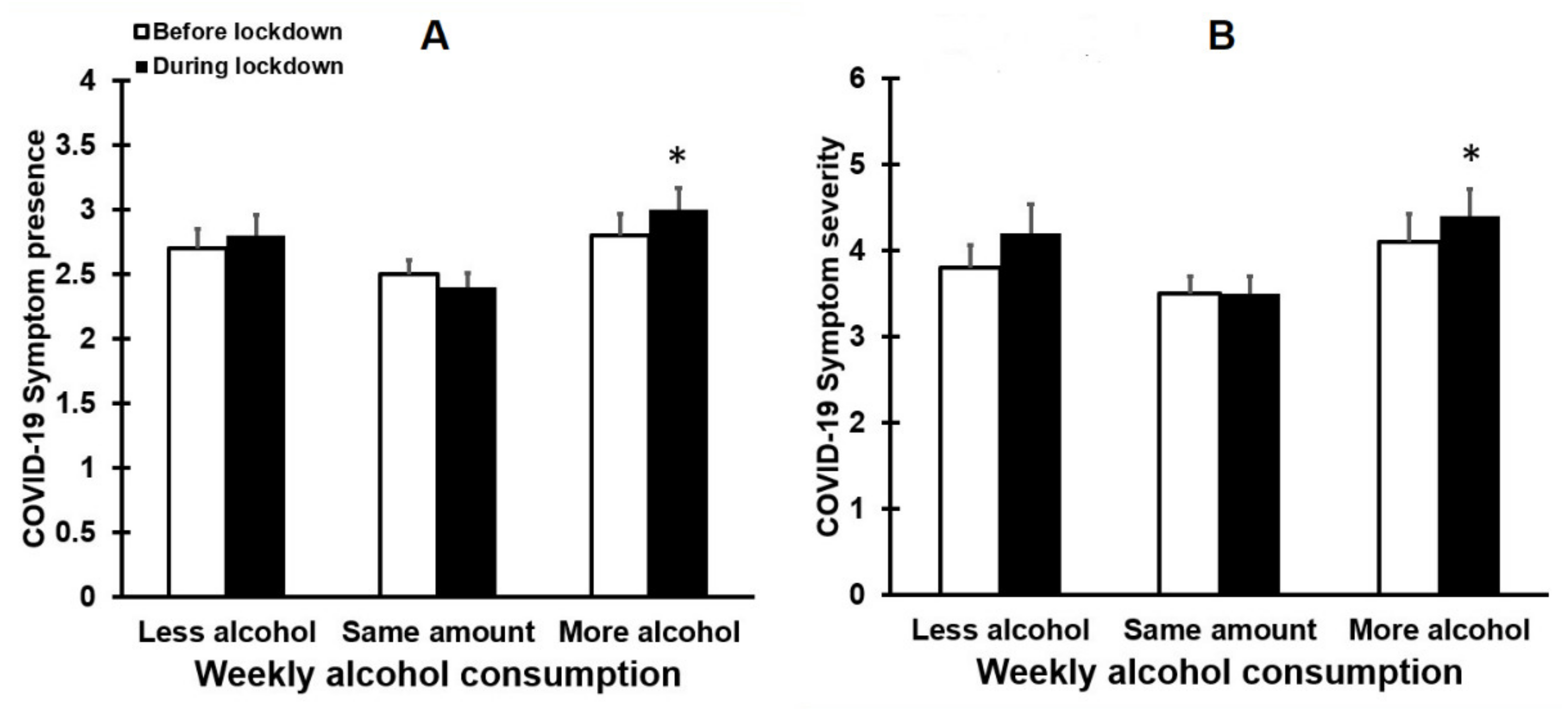
3.4. Alcohol Consumption and the Presence and Severity of COVID-19 Symptoms
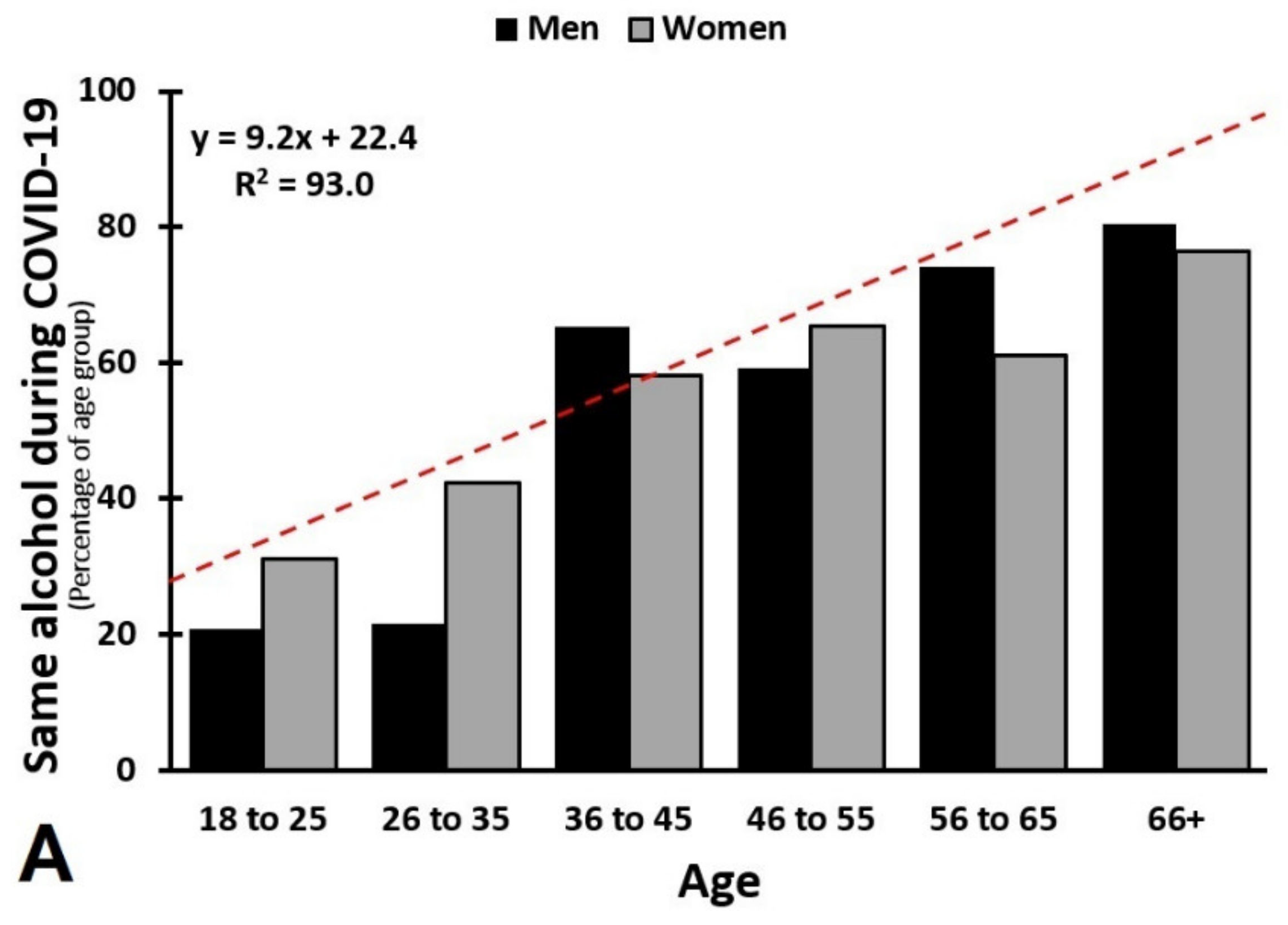
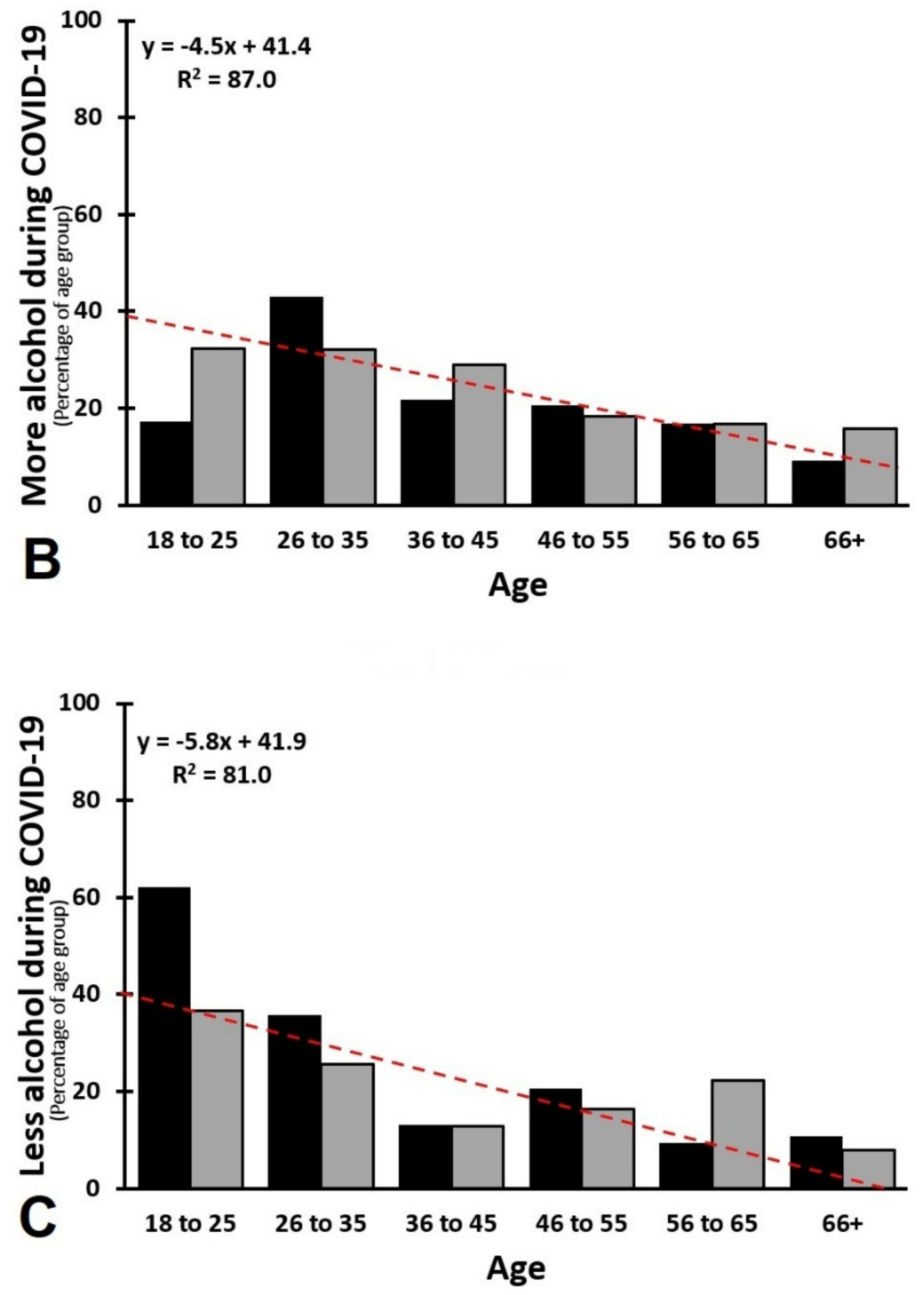
3.5. Age and Sex as Confounding Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arsenault, L.; Ballard, C.; Christensen, H.; Silver, R.C.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- World Health Organization. Virtual Press Conference on COVID-19. Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2 (accessed on 1 February 2021).
- Passavanti, M.; Argentieri, A.; Barbieri, D.M.; Lou, B.; Wijayaratna, K.; Foroutan Mirhosseini, A.S.; Wang, F.; Naseri, S.; Qamhia, I.; Tangerås, M.; et al. The psychological impact of COVID-19 and restrictive measures in the world. J. Affect. Disord. 2021, 283, 36–51. [Google Scholar] [CrossRef]
- Pieh, C.; Budimir, S.; Humer, E.; Probst, T. Comparing Mental Health during the COVID-19 Lockdown and 6 Months after the Lockdown in Austria: A Longitudinal Study. Front. Psychiatry 2021, 12, 625973. [Google Scholar] [CrossRef] [PubMed]
- Elhadi, M.; Alsoufi, A.; Msherghi, A.; Alshareea, E.; Ashini, A.; Nagib, T.; Abuzid, N.; Abodabos, S.; Alrifai, H.; Gresea, E.; et al. Psychological health, sleep quality, behavior, and internet use among people during the COVID-19 pandemic: A cross-sectional study. Front. Psychiatry 2021, 12, 632496. [Google Scholar] [CrossRef]
- Manthey, J.; Kilian, C.; Carr, S.; Bartak, M.; Bloomfield, K.; Braddick, F.; Gual, A.; Neufeld, M.; O’Donnell, A.; Petruzelka, B.; et al. Use of alcohol, tobacco, cannabis, and other substances during the first wave of the SARS-CoV-2 pandemic in Europe: A survey on 36,000 European substance users. Subst. Abus. Treat. Prev. Policy 2021, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, A.; Georgiadou, E.; Reinhard, I.; Müller, A.; Lemenager, T.; Kiefer, F.; Hillemacher, T. The effects of the lockdown during the COVID-19 pandemic on alcohol and tobacco consumption behavior in Germany. Eur. Addict. Res. 2021, 27, 242–256. [Google Scholar] [CrossRef]
- Simou, E.; Leonardi-Bee, J.; Britton, J. The effect of alcohol consumption on the risk of ARDS: A systematic review and meta-analysis. Chest 2018, 154, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of pneumonia: A systematic review and meta-analysis. BMJ Open 2018, 8, e022344. [Google Scholar] [CrossRef] [Green Version]
- Te Velde, A.A.; Bezema, T.; Van Kampen, A.H.; Kraneveld, A.D.; Hart, B.A.; Van Middendorp, H.; Hack, E.C.; Van Montfrans, J.M.; Belzer, C.; Jans-Beken, L.; et al. Embracing complexity beyond systems medicine: A new approach to chronic immune disorders. Front. Immunol. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Yeligar, S.M.; Brown, L.A.S. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. Sci. World J. 2012, 2012, 740308. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.J.; Guidot, D.M. Alcohol and the lung. Alcohol Res. Curr. Rev. 2017, 38, 243–254. [Google Scholar]
- Barbosa, C.; Cowell, A.J.; Dowd, W.N. Alcohol Consumption in Response to the COVID-19 Pandemic in the United States. J. Addict. Med. 2020, 15, 341–344. [Google Scholar] [CrossRef]
- Capasso, A.; Jones, A.M.; Ali, S.H.; Foreman, J.; Tozan, Y.; DiClemente, R.J. Increased alcohol use during the COVID-19 pandemic: The effect of mental health and age in a cross-sectional sample of social media users in the U.S. Prev. Med. 2021, 145, 106422. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Smith, L.; Armstrong, N.C.; Yakkundi, A.; Barnett, Y.; Butler, L.; McDermott, D.T.; Koyanagi, A.; Shin, J.I.; Meyer, J.; et al. Alcohol use and mental health during COVID-19 lockdown: A cross-sectional study in a sample of UK adults. Drug Alcohol Depend. 2021, 219, 108488. [Google Scholar] [CrossRef] [PubMed]
- Lechner, W.V.; Laurene, K.R.; Patel, S.; Anderson, M.; Grega, C.; Kenne, D.R. Changes in alcohol use as a function of psychological distress and social support following COVID-19 related University closings. Addict. Behav. 2020, 110, 106527. [Google Scholar] [CrossRef]
- Boschuetz, N.; Cheng, S.; Mei, L.; Loy, V.M. Changes in alcohol use patterns in the United States during COVID-19 pandemic. WMJ 2020, 119, 171–176. [Google Scholar] [PubMed]
- White, H.R.; Stevens, A.K.; Hayes, K.; Jackson, K.M. Changes in alcohol consumption among college students due to COVID-19: Effects of campus closure and residential change. J. Stud. Alcohol Drugs 2020, 81, 725–730. [Google Scholar] [CrossRef]
- Evans, S.; Alkan, E.; Bhangoo, J.K.; Tenenbaum, H.; Ng-Knight, T. Effects of the COVID-19 lockdown on mental health, wellbeing, sleep, and alcohol use in a UK student sample. Psychiatry Res. 2021, 298, 113819. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, N.C.; Wilson, O.W.A.; Pena, A.; Duffy, M.; Bopp, M. What happens when the party moves home? The effect of the COVID-19 pandemic on U.S. college student alcohol consumption as a function of legal drinking status using longitudinal data. Transl. Behav. Med. 2021, 11, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Garnett, C.; Jackson, S.; Oldham, M.; Brown, J.; Steptoe, A.; Fancourt, D. Factors associated with drinking behaviour during COVID-19 social distancing and lockdown among adults in the UK. Drug Alcohol Depend. 2021, 219, 108461. [Google Scholar] [CrossRef] [PubMed]
- Schmits, E.; Glowacz, F. Changes in alcohol use during the COVID-19 pandemic: Impact of the lockdown conditions and mental health factors. Int. J. Ment. Health Addict. 2021, 1–12. [Google Scholar] [CrossRef]
- Weerakoon, S.M.; Jetelina, K.K.; Knell, G. Longer time spent at home during COVID-19 pandemic is associated with binge drinking among US adults. Am. J. Drug Alcohol Abus. 2021, 47, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Callinan, S.; Smit, K.; Mojica-Perez, Y.; D’Aquino, S.; Moore, D.; Kuntsche, E. Shifts in alcohol consumption during the COVID-19 pandemic: Early indications from Australia. Addiction 2020, 116, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.; To, Q.G.; Khalesi, S.; Williams, S.L.; Alley, S.J.; Thwaite, T.L.; Fenning, A.S.; Vandelanotte, C. Depression, anxiety and stress during COVID-19: Associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int. J. Environ. Res. Public Health 2020, 17, 4065. [Google Scholar] [CrossRef] [PubMed]
- Alpers, S.E.; Skogen, J.C.; Mæland, S.; Pallesen, S.; Rabben, Å.K.; Lunde, L.H.; Fadnes, L.T. Alcohol consumption during a pandemic lockdown period and change in alcohol consumption related to worries and pandemic measures. Int. J. Environ. Res. Public Health 2021, 18, 1220. [Google Scholar] [CrossRef]
- Panagiotidis, P.; Rantis, K.; Holeva, V.; Parlapani, E.; Diakogiannis, I. Changes in alcohol use habits in the general population, during the COVID-19 lockdown in Greece. Alcohol Alcohol. 2020, 55, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Cloonan, S.A.; Taylor, E.C.; Lucas, D.A.; Dailey, N.S. Alcohol dependence during COVID-19 lockdowns. Psychiatry Res. 2021, 296, 113676. [Google Scholar] [CrossRef]
- Chodkiewicz, J.; Talarowska, M.; Miniszewska, J.; Nawrocka, N.; Bilinski, P. Alcohol consumption reported during the COVID-19 pandemic: The initial stage. Int. J. Environ. Res. Public Health 2020, 17, 4677. [Google Scholar] [CrossRef]
- The Nielsen Company. Rebalancing the ‘COVID-19 Effect’ on Alcohol Sales. Available online: https://www.nielsen.com/us/en/insights/article/2020/rebalancing-the-covid-19-effect-on-alcohol-sales/ (accessed on 2 August 2021).
- BMJ. Covid-19 and Alcohol—A Dangerous Cocktail. Available online: https://www.bmj.com/content/369/bmj.m1987 (accessed on 1 February 2021).
- Szajnoga, D.; Klimek-Tulwin, M.; Piekut, A. COVID-19 lockdown leads to changes in alcohol consumption patterns. Results from the Polish national survey. J. Addict. Dis. 2021, 39, 215–225. [Google Scholar] [CrossRef]
- Grossman, E.R.; Benjamin-Neelon, S.E.; Sonnenschein, S. Alcohol Consumption during the COVID-19 Pandemic: A Cross-Sectional Survey of US Adults. Int. J. Environ. Res. Public. Health 2020, 17, 9189. [Google Scholar] [CrossRef]
- Vanderbruggen, N.; Matthys, F.; Van Laere, S.; Zeeuws, D.; Santermans, L.; van den Ameele, S.; Crunelle, C.L. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: Results from a web-based survey. Eur. Addict. Res. 2020, 26, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Hammarberg, K.; Kirkman, M.; Nguyen, H.T.M.; Fisher, J. Alcohol use and mental health status during the first months of COVID-19 pandemic in Australia. J. Affect. Disord. 2020, 277, 810–813. [Google Scholar] [CrossRef]
- Kiani, P.; Merlo, A.; Saeed, H.M.; Benson, S.; Bruce, G.; Hoorn, R.; Kraneveld, A.D.; van de Loo, A.J.; Severeijns, N.R.; Sips, A.S.; et al. Immune fitness and the psychosocial and health consequences of the COVID-19 pandemic lockdown in The Netherlands: Methodology and design of the CLOFIT study. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 16. [Google Scholar] [CrossRef]
- Merlo, A.; Severeijns, N.R.; Benson, S.; Scholey, A.; Garssen, J.; Bruce, G.; Verster, J.C. Mood, changes in alcohol consumption and hangovers in young adults during COVID-19 lockdown: A model explaining associations with perceived immune fitness and experiencing COVID-19 symptoms. submitted.
- Wilson, D.M.C.; Nielsen, E.; Ciliska, D. Lifestyle assessment: Testing the FANTASTIC Instrument. Can. Fam. Physician 1984, 30, 1863–1866. [Google Scholar]
- Sharratt, J.K.; Sharratt, M.T.; Smith, D.M.; Howell, N.J.; Davenport, L. FANTASTIC Lifestyle survey of University of Waterloo employees. Can. Fam. Physician 1984, 30, 1869–1872. [Google Scholar]
- Wilod Versprille, L.J.F.; van de Loo, A.J.A.E.; Mackus, M.; Arnoldy, L.; Sulzer, T.A.L.; Vermeulen, S.A.; Abdula-had, S.; Huls, H.; Baars, T.; Kraneveld, A.D.; et al. Development and validation of the Immune Status Questionnaire (ISQ). Int. J. Environ. Res. Public Health 2019, 16, 4743. [Google Scholar] [CrossRef] [Green Version]
- Van Schrojenstein Lantman, M.; Otten, L.S.; Mackus, M.; de Kruijff, D.; van de Loo, A.J.A.E.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Mental resilience, perceived immune functioning, and health. J. Multidiscip. Healthc. 2017, 10, 107–112. [Google Scholar] [CrossRef] [Green Version]

| Overall | Men | Women | p-Value | |
|---|---|---|---|---|
| N (%) | 761 (100%) | 292 (38.4%) | 469 (61.6%) | - |
| Age (year) | 42.3 (19.0) | 48.0 (19.2) | 38.7 (18.0) * | <0.0001 * |
| Height (m) | 1.74 (0.09) | 1.81 (0.08) | 1.70 (0.07) * | <0.0001 * |
| Weight (kg) | 77.9 (16.8) | 85.5 (14.9) | 73.1 (16.2) * | <0.0001 * |
| BMI (kg/m2) | 25.6 (5.1) | 26.0 (4.4) | 25.4 (5.4) | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlo, A.; Hendriksen, P.A.; Severeijns, N.R.; Garssen, J.; Bruce, G.; Verster, J.C. Alcohol Consumption Patterns during COVID-19 Lockdown and Their Relationship with Perceived Immune Fitness and Reported COVID-19 Symptoms. Healthcare 2021, 9, 1039. https://doi.org/10.3390/healthcare9081039
Merlo A, Hendriksen PA, Severeijns NR, Garssen J, Bruce G, Verster JC. Alcohol Consumption Patterns during COVID-19 Lockdown and Their Relationship with Perceived Immune Fitness and Reported COVID-19 Symptoms. Healthcare. 2021; 9(8):1039. https://doi.org/10.3390/healthcare9081039
Chicago/Turabian StyleMerlo, Agnese, Pauline A. Hendriksen, Noortje R. Severeijns, Johan Garssen, Gillian Bruce, and Joris C. Verster. 2021. "Alcohol Consumption Patterns during COVID-19 Lockdown and Their Relationship with Perceived Immune Fitness and Reported COVID-19 Symptoms" Healthcare 9, no. 8: 1039. https://doi.org/10.3390/healthcare9081039
APA StyleMerlo, A., Hendriksen, P. A., Severeijns, N. R., Garssen, J., Bruce, G., & Verster, J. C. (2021). Alcohol Consumption Patterns during COVID-19 Lockdown and Their Relationship with Perceived Immune Fitness and Reported COVID-19 Symptoms. Healthcare, 9(8), 1039. https://doi.org/10.3390/healthcare9081039







