Compounding Parenteral Products in Pediatric Wards—Effect of Environment and Aseptic Technique on Product Sterility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Compounding of Parenteral Product Samples
2.3. Study Methods
2.4. Sterility Test and Identification of Contaminants
2.5. Analysis of Data
2.6. Study Ethics
3. Results
3.1. Observation during Compunding
3.2. Environmental Monitoring during Compounding
3.3. Monitoring of Personnel after Compounding
3.4. Sterility Test for Compounded Samples
3.5. Contaminants
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council of Europe. Parenteral preparations. In European Pharmacopoeia, 9th ed.; Counsil of Europe: Stardburg, France, 2016; pp. 871–873. [Google Scholar]
- Staes, C.; Jacobs, J.; Mayer, J.; Allen, J. Description of outbreaks of healt-care-associated infections related to compounding pharmacies, 2000–2012. Am. J. Health-Syst. Pharm. 2013, 70, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habsah, H.; Zeehaida, M.; Van Rostenberghe, H.; Noraida, R.; Wan Pauzi, W.I.; Fatimah, I.; Rosliza, A.R.; Nik Sharimah, N.Y.; Maimunah, H. An outbreak of Pantoea spp. in a neonatal intensive care unit secondary to contaminated parenteral nutrition. J. Hosp. Infect. 2005, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Arslan, U.; Erayman, I.; Kirdar, S.; Yukekkaya, S.; Cimen, O.; Tuncer, I.; Bozdogan, B. Serratia marcescens sepsis outbreak in neonatal intensive care unit. Pediatr. Int. 2010, 52, 208–212. [Google Scholar] [CrossRef]
- Paul, L.; Hegde, A.; Pai, T.; Shetty, S.; Baliga, S.; Shenoy, S. An outbreak of Brunkholderia cepacia Bacteremia in a Neonatal Intensive Care Unit. Indian J. Pediatr. 2016, 83, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, B.; Sriram, A.; Shetty, S.; Doshi, R.; Varior, R. An unusual source of Brunkholderia capacia outbreak in a neonatal intensive care unit. J. Hosp. Infect. 2016, 94, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, B. Drug delivery and formulation. In Pediatric Clinical Pharmacology; Rane, A., Schwab, M., Hannsjörg, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 91–105. [Google Scholar]
- Cousins, D.; Sabatier, B.; Begue, D.; Schmitt, C.; Hoppe-Tichy, T. Medication errors in intravenous drug preparation and administration: A multicentre audit in the UK, Germany and France. Qual. Saf. Health Care 2005, 14, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Mendes, J.R.; Lopes, M.C.B.T.; Vancini-Campanharo, C.R.; Okuno, M.F.P.; Batista, R.E.A. Types and frequency of errors in the preparation and administration of drugs. Einstein 2018, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.; Subasyini, S. Medication Errors in Intravenous Drug Preparation and Administration. Med. J. Malays. 2013, 68, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Suvikas-Peltonen, E.; Hakoinen, S.; Celikkayalar, E.; Laaksonen, R.; Airaksinen, M. Incorrect aseptic techniques in medicine preparation and recommendations for safer practices: A systematic review. Eur. J. Hosp. Pharm. 2017, 24, 175–181. [Google Scholar] [CrossRef]
- Turpin, R.; Solem, C.; Pontes-Arruda, A.; Sanon, M.; Mehta, S.; Xiaoqing Liu, F.; Botteman, M. The impact of parenteral nutrition preparation on bloodstream infections risk and cost. Eur. J. Clin. Nutr. 2014, 68, 953–958. [Google Scholar] [CrossRef]
- Thomas, M.; Sanborn, M.; Couldry, R.I.V. Admixture contamination rates: Traditional practice site versus a class 1000 cleanroom. Am. J. Health Syst. Pharm. 2005, 62, 2386–2392. [Google Scholar] [CrossRef] [Green Version]
- Stucki, C.; Sautter, A.-M.; Favet, J.; Bonnabry, P. Microbial contamination of syringes during preparation: The direct influence of environmental cleanliness and risk manipulations on end-product quality. Am. J. Health-Syst. Pharm. 2009, 66, 2032–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.D.; Hand, K.S.; Elia, M. Systematic review and meta-analysis of the risk of microbial contamination of parenteral doses prepared under aseptic techniques in clinical and pharmaceutical environments: An update. J. Hosp. Infect. 2015, 91, 306–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larmené-Beld, K.; Frijlink, H.; Taxis, K. A systematic review and meta-analysis of microbial contamination of parenteral medication prepared in a clinical versus pharmacy environment. Eur. J. Clin. Pharmacol. 2019, 75, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.; Elia, M. Improved aseptic technique can reduce variable contamination rates of ward-prepared parenteral doses. J. Hosp. Infect. 2013, 83, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Finnish Medicines Agency (Fimea): Lääkealan Turvallisuus ja Kehittämiskeskuksen Määräys 6/2012: Sairaala-Apteekin ja Lääkekeskuksen Toiminta. 2012. Available online: https://www.fimea.fi/documents/160140/764653/22690_Maarays_6_2012.pdf (accessed on 16 June 2021).
- Suvikas-Peltonen, E.; Palmgren, J.; Häggman, V.; Celikkayalar, E.; Manninen, R.; Airaksinen, M. Auditing safety of compounding and reconstituting of intravenous medicines on hospital wards in Finland. Int. J. Pharm. Compd. 2017, 21, 518–529. [Google Scholar]
- EudraLex The Rules Governing Medicinal Products in the European Union Volume 4–EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Annex 1: Manufacture of Sterile Medicinal Products (corrected version). European Comission: Brussels, Belgium. 2008. Available online: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-4/2008_11_25_gmp-an1_en.pdf (accessed on 27 May 2021).
- European Pharmacopoeia Commission. European Pharmacopoeia: 5.1.9. Guidelines for using the test for sterility. In European Pharmacopoeia, 9th ed.; Council of Europe: Stardburg, France, 2016; pp. 592–593. Available online: https://www.edqm.eu/ (accessed on 24 May 2021).
- Sandle, T.; Skinner, K.; Sandle, J.; Gebala, B.; Kothandaraman, P. Evaluation of the Gen III Omnilog® ID System microbial identification system for the profiling of cleanroom bacteria. Eur. J. Pharm. Sci. 2013, 18, 44–50. [Google Scholar]
- A Working Group of the Scottish Quality Assurance Specialist Interest Group. Guidelines on Test Methods for Environmental Monitoring for Aseptic Dispensing Facilities, 2nd ed. 2004. Available online: https://elsmar.com/pdf_files/Environmental%20Monitoring%20For%20Aseptic%20Dispensing%20Facilities.pdf (accessed on 24 May 2021).
- Reyes, G.; Navarro, J.-L.; Gamallo, C.; dela Cuevas, M.-C. Case report—Cardiac general: Type A aortic dissection associated with Dietzia maris. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 666–668. [Google Scholar] [CrossRef]
- Pidoux, O.; Argenson, J.-N.; Jacomo, V.; Drancourt, M. Molecular identification of Dietzia maris hip prothesis Infection Isolate. J. Clin. Microbiol. 2001, 39, 2634–2636. [Google Scholar] [CrossRef] [Green Version]
- Bemer-Melchior, P.; Haloun, A.; Riegel, P.; Drugeon, H.B. Bacteremia due to Dietzia maris in an immunocompromised patient. Clin. Infect. Dis. 1999, 29, 1338–1340. [Google Scholar] [CrossRef] [Green Version]
- Valverde, A.; Peix, A.; Rivas, R.; Velazquez, E.; Salazar, S.; Santa-Regina, I.; Rodriguez-Barrueco, C.; Igual, J.M. Paenibacillus castaneae sp. nov., isolated from the phyllosphere of Castanea sativa Miller. Int. J. Syst. Evol. Microbiol. 2008, 58, 2560–2564. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.C.; Chow, V.C.Y.; Lee, C.H.; Ling, J.M.L.; Wong, H.L.; Chan, R.C.Y. Persistent Staphylococcus captis septicemia in a preterm infant. Pediatr. Infect. Dis. J. 2006, 25, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Guen, C.G.-L.; Fournier, S.; Andre-Richet, B.; Caillon, J.; Espaze, E.; Richet, H.; Roze, J.C.; Lepelletier, D. Almond oil implicated in a Stephylococcus captis outbreak in a neonatal intensive care unit. J. Perinatol. 2007, 27, 713–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, C.; Tseng, H.; Yang, Y.J.; Lin, C.H.; Huang, A.H.; Wu, Y.H. Staphylococcus captis basteremia of very low birth weight premature infants at neonatal intensive care units: Clinical significance and antimicrobial susceptibility. J. Microbil. Immunol. Infect. 1999, 32, 26–32. [Google Scholar]

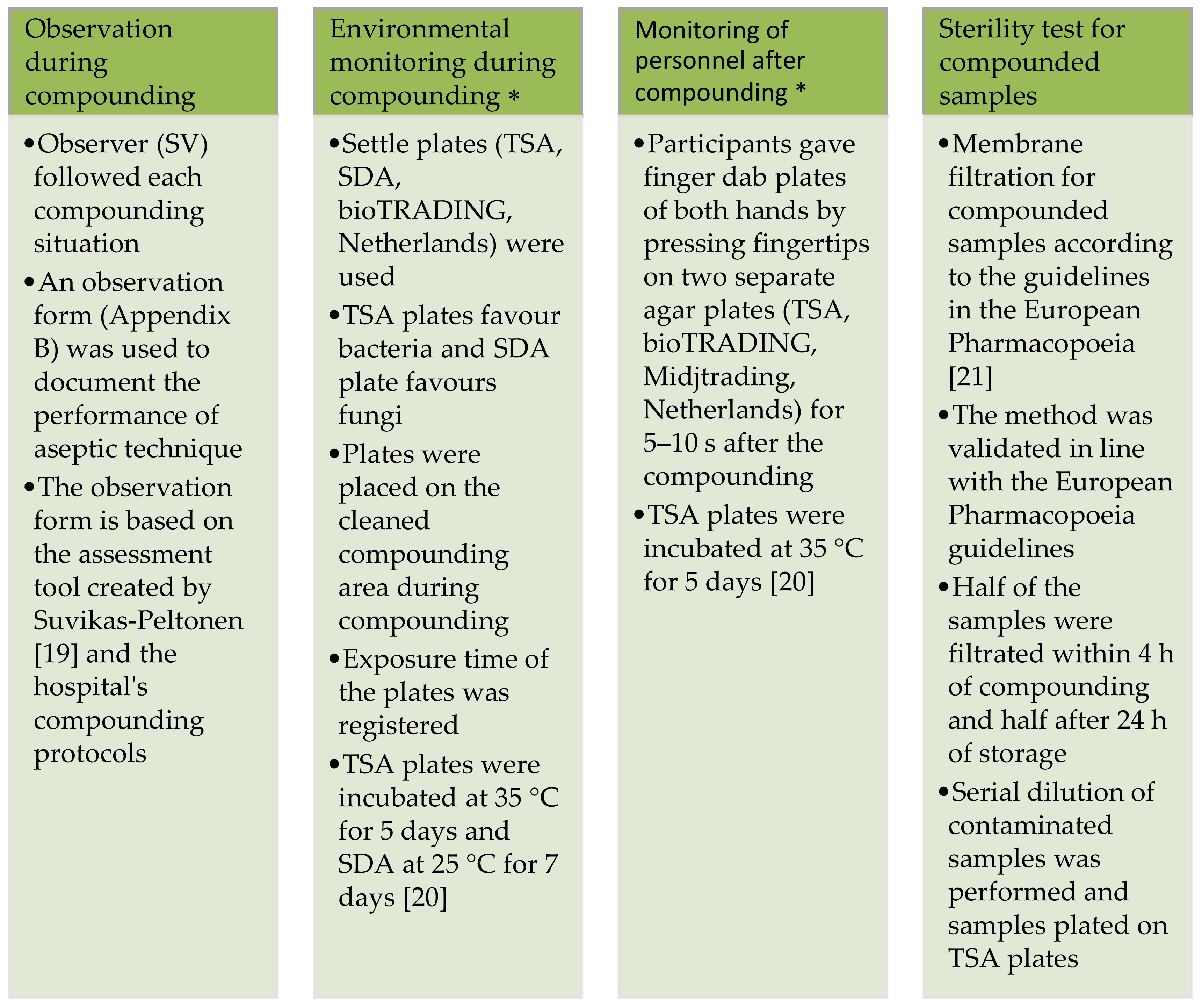
| Aseptic Working Techniques Used While Compounding: | PR, Step Followed (%) | MR, Step Followed (%) | BSC, Step Followed (%) |
|---|---|---|---|
| Participant does not have infectious disease | 100 | 100 | 100 |
| Participant is not wearing jewellery or watches on his/her hands or wrists | 100 | 100 | 100 |
| Participant cleans the table with an alkaline cleanser | 56 | 72 | - |
| Participant cleans the table with 80% denaturized ethanol | 78 | 75 | - |
| BSC is kept on for 15 min before starting the compounding | - | - | 92 |
| Airflow of the BSC is at maximum speed | - | - | 100 |
| Participant washes hands with soap before compounding | 44 | 56 | 67 |
| Participant disinfects hands before compounding | 100 | 100 | 100 |
| Participant uses a disposable non-sterile protective gown | - | - | 50 |
| Participant uses a hair cover | - | - | 0 |
| Participant puts on a surgical face mask | 11 | 11 | 0 |
| Participant puts on non-sterile gloves before disinfecting the equipment to be used in the compounding and cleaning the compounding area | 36 | 31 | 67 |
| Participant cleans the cabinet with an alcoholic disinfectant | - | - | 100 |
| Participant places a sterile drape cover onto the benchtop in the BSC | - | - | 50 |
| Participant disinfects the equipment to be used in the compounding prior to use or when transferring it into the BSC | 11 | 14 | 47 |
| Participant disinfects his/her hands before changing the gloves | - | - | 100 |
| Participant changes the gloves before starting the compounding | 100 | 100 | 100 |
| Participant disinfects the septum of the vial | 100 | 100 | 100 |
| Front glass of the BSC is kept in working position | - | - | 100 |
| Participant opens sterile packages inside the BSC | - | - | 92 |
| Participant does not touch the connecting part of the syringe or the needle | 100 | 100 | 97 |
| Participant disinfects the septum of the vial before piercing it again | 63 (n = 19) | 20 (n = 14) | 13 (n = 14) |
| Participant mixes the product by turning back and forth, not shaking | 97 | 97 | 94 |
| No spatters | - | - | 69 |
| Participant wipes possible spatters from the BSC immediately (if outside the drape cover) | - | - | 0 (n = 11) |
| Participant cleans the BSC with an alcoholic disinfectant after compounding | - | - | 50 |
| Participant leaves the BSC on maximum or half speed after finishing the compounding and cleaning | - | - | 83 |
| Ward 1 | Plate | PR1 | PR2 | PR3 | MR1 | MR2 | MR3 | BSC1 | BSC2 | BSC3 |
| Participant 1 | TSA | 24 | 11.4 | 24 | 42.4 | 14.1 | 0 | 0 | 0 | 0 |
| SDA | 0 | 24.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Participant 2 | TSA | 80 | 68.6 | 40 | 0 | 0 | 0 | 0 | 0 | 0 |
| SDA | 0 | 34.3 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Participant 3 | TSA | 60 | 0 | 30 | 180 | 26.7 | 0 | 0 | 0 | 0 |
| SDA | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | |
| Participant 4 | TSA | 26.7 | 26.7 | 0 | 0 | 21.8 | 30 | 0 | 0 | 0 |
| SDA | 26.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Participant 5 | TSA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SDA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Participant 6 | TSA | 186.7 | 48 | 40 | 64.5 | 20 | 0 | 0 | 0 | 0 |
| SDA | 53.3 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | |
| Mean | TSA | 37 CFU/4 h | 22.25 CFU/4 h | 0 CFU/4 h | ||||||
| SDA | 12.17 CFU/4 h | 6.11 CFU/4 h | 0 CFU/4 h | |||||||
| Combined | 49.17 CFU/4 h | 28.36 CFU/4 h | 0 CFU/4 h | |||||||
| Ward 2 | Plate | PR1 | PR2 | PR3 | MR1 | MR2 | MR3 | BSC1 | BSC2 | BSC3 |
| Participant 7 | TSA | 0 | 0 | 72 | 0 | 30 | 0 | 0 | 0 | 0 |
| SDA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Participant 8 | TSA | 0 | 0 | 13.3 | 30.9 | 17.1 | 0 | 0 | 0 | 0 |
| SDA | 0 | 0 | 0 | 15.5 | 0 | 0 | 0 | 0 | 0 | |
| Participant 9 | TSA | 0 | 0 | 0 | 72 | 0 | 0 | 0 | 0 | 0 |
| SDA | 0 | 0 | 0 | 0 | 48 | 40 | 0 | 0 | 0 | |
| Participant 10 | TSA | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SDA | 16 | 0 | 0 | 24 | 0 | 0 | 20 | 0 | 0 | |
| Participant 11 | TSA | 45.7 * | 0 | 68.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| SDA | 11.4 * | 0 | 0 | 40 | 0 | 0 | 0 | 0 | 0 | |
| Participant 12 | TSA | 34.3 | 48 | 96 | 0 | 0 | 68.6 | 21.8 | 0 | 0 |
| SDA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean | TSA | 22.66 CFU/4 h | 12.15 CFU/4 h | 1.12 CFU/4 h | ||||||
| SDA | 1.52 CFU/4 h | 9.30 CFU/4 h | 1.11 CFU/4 h | |||||||
| Combined | 24.18 CFU/4 h | 21.45 CFU/4 h | 2.23 CFU/4 h | |||||||
| Ward 1 | Plate | PR1 | PR2 | PR3 | MR1 | MR2 | MR3 | BSC1 | BSC2 | BSC3 |
| Participant 1 | Right | 0 | 0 | 1 | 7 | 1 | 0 | 0 | 0 | 1 |
| Left | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | |
| Participant 2 | Right | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Left | 1 | 3 | 0 | 0 | 0 | 1 | 7 | 0 | 1 | |
| Participant 3 | Right | 2 | 3 | 2 | 16 | 2 | 0 | 0 | 5 | 0 |
| Left | 8 | 1 | 6 | 12 | 1 | 1 | 0 | 2 | 0 | |
| Participant 4 | Right | 0 | 0 | 1 | 1 | 0 | 3 | 2 | 0 | 0 |
| Left | 1 | 2 | 2 | 3 | 0 | 2 | 0 | 0 | 0 | |
| Participant 5 | Right | 1 | 0 | 4 | 4 | 0 | 4 | 3 | 0 | 2 |
| Left | 1 | 1 | 23 | 13 | 0 | 8 | 5 | 1 | 0 | |
| Participant 6 | Right | 1 | 0 | 0 | 0 | 1 | 5 | 0 | 2 | 0 |
| Left | 2 | 1 | 2 | 5 | 3 | 1 | 0 | 2 | 0 | |
| Mean | Right | 0.9 | 2.4 | 0.9 | ||||||
| Left | 3.1 | 2.9 | 1.0 | |||||||
| Ward 2 | Plate | PR1 | PR2 | PR3 | MR1 | MR2 | MR3 | BSC1 | BSC2 | BSC3 |
| Participant 7 | Right | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Left | 0 | 1 | 3 | 0 | 1 | 0 | 1 | 0 | 1 | |
| Participant 8 | Right | 0 | 0 | 4 | 13 | 0 | 3 | 1 | 0 | 0 |
| Left | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 2 | 0 | |
| Participant 9 | Right | 1 | 0 | 0 | 1 | 3 | 2 | 0 | 0 | 3 |
| Left | 1 | 0 | 1 | 0 | 1 | 4 | 0 | 0 | 1 | |
| Participant 10 | Right | 0 | 0 | 0 | 6 | 9 | 4 | 1 | 1 | 2 |
| Left | 0 | 1 | 1 | 6 | 10 | 0 | 2 | 0 | 0 | |
| Participant 11 | Right | 2 * | 1 | 0 | 11 | 0 | 1 | 0 | 10 | 10 |
| Left | 0 * | 3 | 6 | 2 | 0 | 0 | 0 | 8 | 24 | |
| Participant 12 | Right | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Left | 4 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Mean | Right | 0.6 | 2.9 | 1.6 | ||||||
| Left | 1.3 | 1.7 | 2.2 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtanen, S.; Kapp, K.; Rautamo, M.; Schepel, L.; Lindén-Lahti, C.; Cruz, C.D.; Tammela, P. Compounding Parenteral Products in Pediatric Wards—Effect of Environment and Aseptic Technique on Product Sterility. Healthcare 2021, 9, 1025. https://doi.org/10.3390/healthcare9081025
Virtanen S, Kapp K, Rautamo M, Schepel L, Lindén-Lahti C, Cruz CD, Tammela P. Compounding Parenteral Products in Pediatric Wards—Effect of Environment and Aseptic Technique on Product Sterility. Healthcare. 2021; 9(8):1025. https://doi.org/10.3390/healthcare9081025
Chicago/Turabian StyleVirtanen, Sonja, Karmen Kapp, Maria Rautamo, Lotta Schepel, Carita Lindén-Lahti, Cristina D. Cruz, and Päivi Tammela. 2021. "Compounding Parenteral Products in Pediatric Wards—Effect of Environment and Aseptic Technique on Product Sterility" Healthcare 9, no. 8: 1025. https://doi.org/10.3390/healthcare9081025
APA StyleVirtanen, S., Kapp, K., Rautamo, M., Schepel, L., Lindén-Lahti, C., Cruz, C. D., & Tammela, P. (2021). Compounding Parenteral Products in Pediatric Wards—Effect of Environment and Aseptic Technique on Product Sterility. Healthcare, 9(8), 1025. https://doi.org/10.3390/healthcare9081025