Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Impact of Virtual Reality (VR)-Based Telerehabilitation on the Functional Condition of Stroke Patients
3.2. Effect of Telerehabilitation on Functional Condition of the Occupied Upper Limb and Level of Stroke Knowledge
3.3. Impact of Social Networks on the Course and Effects of Telerehabilitation
3.4. Effects of Tele Aerobic Training on Cardiorespiratory Fitness of Post-Stroke Patients
3.5. Assessing the Impact of Telerehabilitation Based on a Collaborative Model on the Functional Status of Post-Stroke Patients
3.6. Effect Of Telerehabilitation on Improving Cognitive Function in Post-Stroke Patients
3.7. Level of Acceptance of Telerehabilitation in Stroke Patients
3.8. Effect of Telerehabilitation on Balance Improvement in Post-Stroke Patients
3.9. Feasibility of a Tele Therapeutic Program in Post-Stroke Patients
3.10. The Impact of Telerehabilitation on the Functional Condition of Stroke Survivors in African Countries
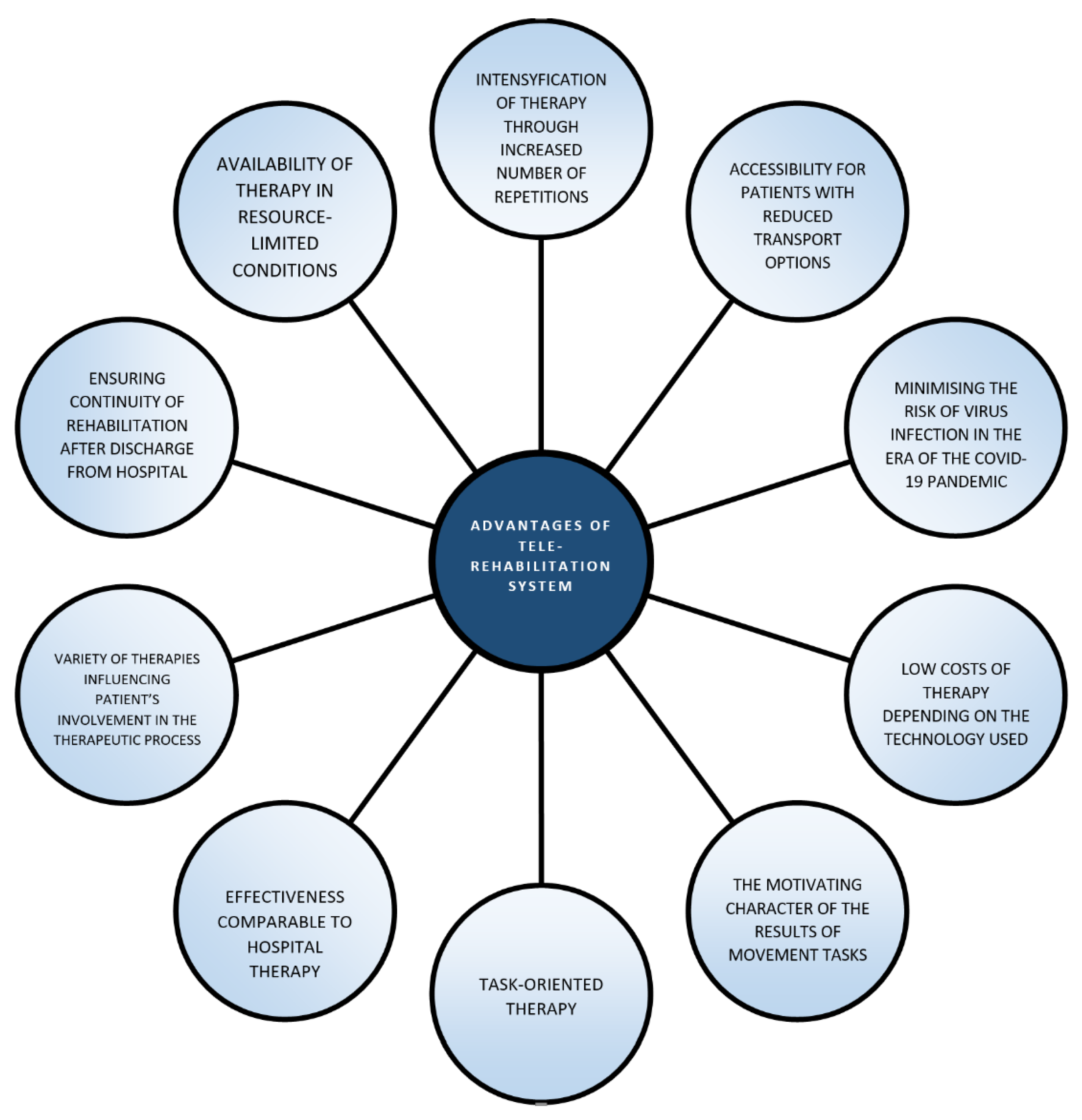
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TR | Telerehabilitation |
| VR | Virtual Reality |
| BBS | Berg Balance Scale |
| TUG | Timed Up-and-Go |
| FTSST | 5 Times Sit-to-Stand |
| CB&M | Community Balance and Mobility Scale |
| SIS | Stroke Impact Scale |
| FMA (UE) | Fugl-Meyer Assessment Upper Extremity |
| FMA (LE) | Fugl-Meyer Assessment Lower Extremity |
References
- Varghese, C.; Onuma, O.; Johnson, W.; Brainin, M.; Hacke, W.; Norrving, B. Organizational update: World Health Organization. Stroke 2017, 48, e341–e342. [Google Scholar] [CrossRef] [PubMed]
- Podury, A.; Raefsky, S.M.; Dodakian, L.; McCafferty, L.; Le, V.; McKenzie, A.; See, J.; Zhou, R.J.; Nguyen, T.; Vanderschelden, B.; et al. Social network structure is related to functional improvement from home-based telerehabilitation after stroke. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Sheehy, L.; Taillon-Hobson, A.; Sveistrup, H.; Bilodeau, M.; Fergusson, D.; Levac, D.; Finestone, H. Home-based virtual reality training after discharge from hospital-based stroke rehabilitation: A parallel randomized feasibility trial. Trials 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berg-Weger, M.; Morley, J.E. Loneliness and social isolation in older adults during the COVID-19 pandemic: Implications for gerontological social work. J Nutr. Health Aging 2020, 14, 1–3. [Google Scholar] [CrossRef]
- Negrini, S.; Grabljevec, K.; Boldrini, P.; Boldrini, C.; Moslavac, S.; Zampolini, M.; Christodoulou, N. Up to 2.2 million people experiencing disability suffer collateral damage each day of Covid-19 lockdown in Europe. Eur. J. Phys. Rehabil. Med. 2020, 56, 361–365. [Google Scholar]
- Dhand, A.; Luke, D.; Lang, C.; Tsiaklides, M.; Feske, S.; Lee, J.M. Social networks and risk of delayed hospital arrival after acute stroke. Nat. Comm. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Markus, H.; Brainin, M. COVID-19 and stroke–a global world stroke organization perspective. Int. J. Stroke 2020, 15, 361–364. [Google Scholar] [CrossRef]
- Tenforde, S.A.; Zafonte, R.; Hefner, J.; Iaccarino, M.A.; Silver, J.; Paganoni, S. Efficacy of home-based telerehabilitation versus in-clinic therapy for adults after stroke. Am. J. Phys. Med. Rehabil. 2020, 99, 764–765. [Google Scholar] [CrossRef]
- Laver, K.E.; Adey-Wakeling, Z.; Crotty, M.; Lannin, N.A.; George, S.; Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020, 1, CD010255. [Google Scholar] [CrossRef]
- Laver, K.E.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane DB Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group; Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Amorim, P.; Santos, B.S.; Dias, P.; Silva, S.; Martins, H. Serious Games for Stroke Telerehabilitation of Upper Limb-A Review for Future Research. Int. J. Telerehabilitation 2020, 12, 65–76. [Google Scholar] [CrossRef]
- Burgos, P.I.; Lara, O.; Lavado, A.; Rojas-Sepúlveda, I.; Delgado, C.; Bravo, E.; Kamisato, C.; Torres, J.; Castañeda, V.; Cerda, M. Exergames and telerehabilitation on smartphones to improve balance in stroke patients. Brain Sci. 2020, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke. A randomized clinical trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Zheng, K.; Dodakian, L.; See, J.; Zhou, R.; Chiu, N.; Augsburger, R.; McKenzie, A.; Cramer, S.C. A qualitative study on user acceptance of a home-based stroke telerehabilitation system. Top. Stroke Rehabil. 2020, 27, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Canadian Stroke Congress 2019 Abstract Supplement. Int. J. Stroke 2019, 14, 3–52. [CrossRef]
- Dodakian, L.; McKenzie, A.L.; Le, V.; See, J.; Pearson-Fuhrhop, K.; Burke Quinlan, E.; Zhou, R.J.; Augsberger, R.; Tran, X.A.; Friedman, N.; et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil. Neural Repair 2017, 31, 923–933. [Google Scholar] [CrossRef]
- Richmond, T.; Peterson, C.; Cason, J.; Billings, M.; Terrell, E.A.; Lee, A.C.W.; Towey, M.; Parmanto, B.; Saptano, A.; Cohn, E.R.; et al. American Telemedicine Association’s principles for delivering telerehabilitation services. Int. J. Telerehabil. 2017, 9, 63–68. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics–2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Dhand, A.; Lang, C.E.; Luke, D.A.; Kim, A.; Li, K.; McCafferty, L.; Mu, Y.; Rosner, B.; Feske, S.K.; Lee, J.-M. Social network mapping and functional recovery within 6 months of ischemic stroke. Neurorehabil. Neural Repair 2019, 33, 922–932. [Google Scholar] [CrossRef]
- Prust, M. Head-to-head comparison of social network assessments in stroke survivors. Neurohospitalist 2020, 11, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zachrison, K.S.; Dhand, A.; Schwamm, L.H.; Onnela, J.-P. A network approach to stroke systems of care. Circ. Cardiovasc. Qual. Outcomes 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Galloway, M.; Marsden, D.L.; Callister, R.; Nilsson, M.; Erickson, K.I.; English, C. The feasibility of a telehealth exercise program aimed at increasing cardiorespiratory fitness for people after stroke. Int. J. Telerehabilitation 2019, 11, 9–28. [Google Scholar] [CrossRef]
- Barengo, N.C.; Antikainen, R.; Borodulin, K.; Harald, K.; Jousilahti, P. Leisure-time physical activity reduces total and cardiovascular mortality and cardiovascular disease incidence in olderz adults. J. Am. Geriatr. Soc. 2017, 65, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Prout, E.C.; Mansfield, A.; McIlroy, W.E.; Brooks, D. Patients’ perspectives on aerobic exercise early after stroke. Disabil. Rehabil. 2017, 39, 684–690. [Google Scholar] [CrossRef]
- Ragupathi, L.; Stribling, J.; Yakunina, Y.; Fuster, V.; McLaughlin, M.A.; Vedanthan, R. Availability, use, and barriers to cardiac rehabilitation in LMIC. Global Heart 2017, 12, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Reinholdsson, M.; Palstam, A.; Sunnerhagen, K.S. Prestroke physical activity could influence acute stroke severity (part of PAPSIGOT). Neurology 2018, 91. [Google Scholar] [CrossRef]
- Galloway, M. An Exploration of Low Doses of Exercise on Cardiorespiratory Fitness in People with Chronic Stroke. Ph.D. Thesis, University of Newcastle, Callaghan, Australia, 2019. (Unpublished). [Google Scholar]
- Jackson, S.; Mercer, C.; Singer, B.J. An exploration of factors influencing physical activity levels amongst a cohort of people living in the community after stroke in the south of England. Disabil. Rehabil. 2018, 40, 414–424. [Google Scholar] [CrossRef]
- Maddison, R.; Rawstorn, J.C.; Stewart, R.A.H.; Benatar, J.; Whittaker, R.; Rolleston, A.; Jiang, Y.; Gao, L.; Moodie, M.; Warren, W.; et al. Effects and costs of real-time cardiac telerehabilitation: Randomised controlled non-inferiority trial. Heart 2018, 105, 122–129. [Google Scholar] [CrossRef]
- Tyagi, S.; Lim, D.; Ho, W.; Koh, Y.; Cai, V.; Koh, G.; Legido-Quigley, H. Acceptance of telerehabilitation by stroke patients: Perceived barriers and facilitators. Arch. Phys. Med. Rehabil. 2018, 99, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Zhishui, W.; Xu, J.; Yue, C.; Li, Y.; Liang, Y. Collaborative care model based telerehabilitation exercise training program for acute stroke patients in China: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2020, 29. [Google Scholar] [CrossRef]
- Chen, J.; Jin, W.; Dong, W.S.; Jin, Y.; Qiao, F.L.; Zhou, Y.F.; Ren, C.C. Effects of home-based telecommunication rehabilitation on physical function for stroke survivors with hemiplegia: A random controlled trial. Am. J. phys. Med. Rehabil. 2017, 96, 152–160. [Google Scholar] [CrossRef]
- Torrisi, M.; Maresca, G.; Cannavò, D.A.; Sciarrone, F.; Gemelli, P.; Silvestri, G.; Bramanti, A.; De Luca, R.; Calabrò, R.S. Using telerehabilitation to improve cognitive function in post-stroke survivors: Is this the time for continuity of care? Int. J. Rehabil. Res. 2019, 42, 344–351. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Leonardi, S.; Spadaro, L.; Russo, M.; Aragona, B.; Torrisi, M.; Maggio, M.G.; BioEng, A.B.; Naro, A.; De Cola MStat, M.C.; et al. Improving cognitive function in patients with stroke: Can computerized training be the future? J. Stroke Cerebrovasc. Dis. 2018, 27, 1055–1060. [Google Scholar] [CrossRef]
- Venkatesh, V.; Thong, J.Y.; Xu, X. Davis User Acceptance of Information Technology: Toward a Unified View. MIS Q. 2003, 27, 425. [Google Scholar] [CrossRef]
- Noh, H.-J.; Lee, S.H.; Bang, D.H. Three-dimensional balance training using visual feedback on balance and walking ability in subacute stroke patients: A single-blinded randomized controlled pilot trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 994–1000. [Google Scholar] [CrossRef]
- Yang, C.M.; Wang, Y.-C.; Lee, C.-H.; Chen, M.-H.; Hsieh, C.-L. A comparison of test–retest reliability and random measurement error of the Barthel Index and modified Barthel Index in patients with chronic stroke. Disabil. Rehabil. 2020, 1–5. [Google Scholar] [CrossRef]
- Rose, D.K.; DeMark, L.; Fox, E.J.; Clark, D.J.; Wludyka, P. A backward walking training program to improve balance and mobility in acute stroke. J. Neurol. Phys. Ther. 2018, 42, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sarfo, F.S.; Ulasavets, U.; Opare-Sem, O.K.; Ovbiagele, B. Telerehabilitation after stroke: An updated systematic review of the literature. J. Stroke Cereb. Dis. 2018, 27, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Tchero, H.; Tabue, M.; Lannuzel, A.; Rusch, E. Telerehabilitation for stroke survivors: Systematic review and meta-analysis. J. Med. Internet. Res. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.D.; Bird, M.L.; English, C.; Gall, S.L.; Breslin, M.; Smith, S.; Schmidt, M.; Callisaya, M.L. Connecting patients and therapists remotely using technology is feasible and facilitates exercise adherence after stroke. Top. stroke Rehabil. 2020, 27, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sousa de, D.G.; Harvey, L.A.; Dorsch, S.; Glinsky, J.V. Interventions involving repetitive practice improve strength after stroke: A systematic review. J. Physiother. 2018, 64, 210–221. [Google Scholar] [CrossRef]
- Ameer, K.; Ali, K. iPad use in stroke neuro-rehabilitation. Geriatrics 2017, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Porter, R.E.; DeBaun-Sprague, E.; Van Puymbroeck, M.; Schmid, A.A. Exercise after stroke: Patient adherence and beliefs after discharge from rehabilitation. Top. Stroke Rehabil. 2017, 24, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Odetunde, O.M.; Binuyo, O.T.; Maruf, F.A.; Ayenowowon, S.O.; Okonji, A.M.; Odetund, N.A.; Mbada, C.E. Development and feasibility testing of video home based telerehabilitation for stroke survivors in resource limited settings. Int. J. Telerehabilitation 2020, 12, 125–135. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Shrestha, P.; Dev, R. Feasibility and effectiveness of telephone-based telephysiotherapy for treatment of pain in low-resource setting: A retrospective prepost design. Pain Res. Manag. 2020, 2020. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the21stCentury: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Ulasavets, U.; Opare-Sem, O.K.; Ovbiagele, B. Pilot trial of a tele-rehab intervention to improve outcomes after stroke in Ghana: A feasibility and user satisfaction study. J. Neurol. Sci. 2018, 387, 94–97. [Google Scholar] [CrossRef]
- Zhao, J.; Rudd, A.; Liu, R. Challenges and potential solutions of stroke care during the coronavirus disease 19 (COVID-19) pandemic. Stroke 2020, 51, 1356–1357. [Google Scholar] [CrossRef]
- Jeffers, M.S.; Karthikeyan, S.; Gomez-Smith, M.; Gasinzigwa, S.; Achenbach, J.; Feiten, A. Does stroke rehabilitation really matter? part b: An algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil. Neural Repair 2018, 32, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, D.; Zhang, S.; Shi, Y.; Qiao, F.; Zhou, Y.; Liu, J.; Ren, C. The effects of home-based telerehabilitation in patients with stroke: A randomized controlled trial. Neurology 2020, 95, e2318–e2330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Abel, K.T.; Janecek, J.T.; Chen, Y.; Zheng, K.; Cramer, S.C. Home-based technologies for stroke rehabilitation: A systematic review. Int. J. Med. Inform. 2019, 123, 11–22. [Google Scholar] [CrossRef] [PubMed]
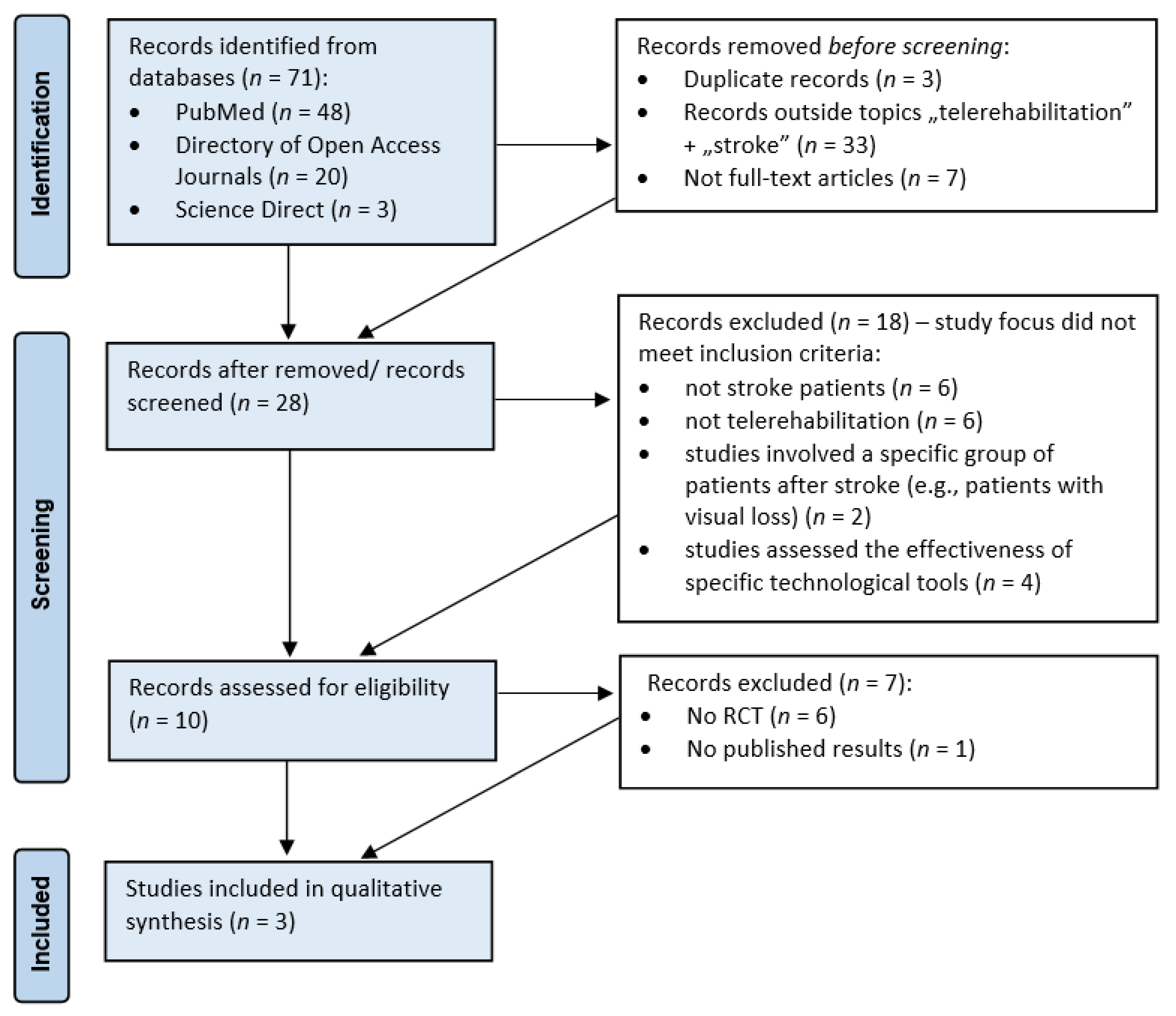
| Article Type | Focus | Reference |
|---|---|---|
| RCT with published results |
| Cramer S. et al., 2019 Wu Z. et al., 2020 Burgos P. et al., 2020 |
| RCT without published results |
| Sheehy L. et al., 2019 |
| Case study |
| Chen Y. et al., 2020 Odetunde M. et al., 2020 |
| Other trials (not randomized and/ or not controlled) |
| Podury A. et al., 2021 Galloway M. et al., 2019 Torrisi M. et al., 2019 Simpson D. et al., 2020 |
Inclusion Criteria:
|
Exclusion Criteria:
|
| Post-Pre Assessment Average (95% Confidence Intervals): | Experimental Group (EG) | Control Group (CG) | p-Values for Change Over Time (EG & CG Combined) |
|---|---|---|---|
| TUG (seconds) | −0.1 (−1.8, 1.6) | −1.4 (−3.7, 1) | 0.326 |
| TUG + cognitive task (seconds) | −1.7 (−4.2, 0.7) | −3.4 (−5.7, −1.1) | 0.004 |
| FTSST (seconds) | −3 (−5.8, −0.2) | −2 (−4.1, 0.2) | 0.006 |
| BBS (/56) | −0.5 (−4.2, 3.3) | 0.6 (−0,5, 1.6) | 0.959 |
| CB&M (/96) | 5.6 (−5, 16.2) | 6.1 (1.6, 10.7) | 0.049 |
| SIS (/295) | 7.7 (−2.1, 17.6) | 13.8 (2.2, 25.3) | 0.006 |
| Intervention Group | Control Group | RM-ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Weeks | 8 Weeks | 12 Weeks | Baseline | 4 Weeks | 8 Weeks | 12 Weeks | F (Time*Group) | p | |
| FM(UE) | 11.93 ± 2.50 | 35.90 ± 2.78 | 49.10 ± 3.00 | 55.33 ± 2.81 | 2.61 ± 1.78 | 29.35 ± 2.36 | 39.35 ± 4.13 | 47.42 ± 3.90 | 42.523 | <0.001 |
| FM(LE) | 13.37 ± 1.38 | 23.87 ± 1.28 | 25.50 ± 1.74 | 28.37 ± 2.51 | 14.13 ± 1.43 | 20.84 ± 1.39 | 24.23 ± 1.86 | 27.87 ± 1.73 | 57.000 | <0.001 |
| BBS | 21.07 ± 3.29 | 30.50 ± 2.84 | 38.13 ± 2.84 | 43.13 ± 2.32 | 20.87 ± 2.33 | 28.06 ± 2.28 | 34.19 ± 2.15 | 38.29 ± 2.70 | 9.205 | <0.001 |
| TUG | 41.93 ± 3.57 | 30.37 ± 3.62 | 22.73 ± 2.49 | 19.50 ± 2.73 | 40.58 ± 4.40 | 34.23 ± 2.86 | 27.13 ± 2.50 | 23.97 ± 3.35 | 16.320 | <0.001 |
| 6MWT | 91.73 ± 7.46 | 111.50 ± 8.12 | 128.90 ± 7.42 | 141.63 ± 8.68 | 92.35 ± 6.15 | 107.94 ± 5.14 | 123.13 ± 5.71 | 129.45 ± 7.06 | 10.530 | <0.001 |
| Authors/Year | Participants | Intervention | Outcomes Measurement | Results |
|---|---|---|---|---|
| Cramer S. et al., 2019 | n = 124 (34 women, 90 men) (n = 62 in experimental group) Mean age (SD) of 61 years Adults ischemic stroke or intracerebral hemorrhage 4 to 36 weeks prior | Experimental and control group received 18 supervised and 18 unsupervised 70-min sessions. The treatment approach was based on an upper-extremity task-specific training manual and Accelerated Skill Acquisition Program All sessions for both groups included at least 15 min per day of arm exercises (the same 88 exercises for both groups) and at least 15 min per day of functional training. The TR system (for experimental group) consisted of an internet-enabled computer with table, chair, and 12 gaming input devices, but no keyboard, as no computer operation was required by patients. | FMA-UE (Fugl-Meyer Assessment upper extremity) Box and Block Test SIS (Stroke Impact Scale) | Both groups showed significant treatment-related motor gains, with a mean (SD) unadjusted FM score change from baseline to 30 days after therapy of 8.36 (7.04) points in the control group (p < 001) and 7.86 (6.68) points in the experimental group (p < 001). The adjusted mean change in FM score was 0.06 points larger in the experimental group (95% CI, −2.14 to 2.26; p = 96). The noninferiority margin (30% of the mean FM score change in the control group) was 2.47, which fell outside of this 95% CI, indicating that TR was not inferior to standard therapy on the primary end point [14]. Box and Block Test scores increased by 9.5 (p < 001) in the experimental group and by 8.8 (p < 001) in the control group and indicated noninferiority of TR therapy. Stroke Impact Scale hand motor domain scores increased by 23.7 (p < 001) in the experimental group and by 29.2 (p < 001) in the control group, although noninferiority was not demonstrated with this outcome [14]. |
| Wu Z. et al., 2020 | n = 61 (25 women, 36 men) (n = 30 in experimental group) Mean age (SD) of 58 years Adults ischemic or hemorrhagic stroke No information about time since stroke | Patients in the intervention group received home remote rehabilitation based on a collaborative care model. A collaborative care team consisting of neurologists, nurses, rehabilitation therapists, counselors and caregivers was established. Rehabilitation therapists assess the extent of patient dysfunction and work with family caregivers to develop rehabilitation plans and goals. The home remote rehabilitation guidance uses the Internet-based TCMeeting v6.0 video conferencing system. Patients in the control group received only routine rehabilitation and nursing measures, including dietary guidance, medication guidance and rehabilitation guidance, which were conducted by telephone follow-up once a week. | FMA-total (Fugl-Meyer Assessment total) FMA-UE (Fugl-Meyer Assessment upper extremity) FMA-LE (Fugl-Meyer Assessment lower extremity) BBS (Berg Balance Scale) TUG (Timed “Up&Go” Test) 6 MWT (6-min Walk Test) MBI (Modified Barthel Index) | See Table 4. |
| Burgos P. et al., 2020 | n = 10 (4 women, 6 men) (n = 6 in experimental group) Mean age (SD) of 61 years. Adults ischemic or hemorrhagic stroke in subacute (6–8 weeks after stroke) | Both groups received their standard rehabilitation treatment at the hospital site (3 sessions of 40 min per week of physical therapy for 4 weeks). In addition, the intervention group received 9 sessions of 30 min per week for 4 weeks. In each session, participants trained in balance tasks using smartphone-based exergames controlled by body motions. | BBS (Berg Balance Scale). MBT (Mini-BESTest). BI (Barthel Index) SUS (System Usability Scale) | Balance results improved in the BBS, with mean values of PRE = 35 ± 4.42 (62.50% ± 7.91), POST = 46.33 ± 3.01 (82.67% ± 5.37), and MBT PRE = 10.33 ± 2.87 (36.89% ± 10.26), and POST = 18.67 ± 2.81 (66.67% ± 10.01) with a statistically significant variation within PRE and POST (F(1/5) = 60.84, p < 0.001 and F(1/5) = 45.96, p = 0.001, respectively). Functional independence, measured by BI, also improved in the study group with PRE = 65.00 ± 4.47, and POST = 82.50 ± 8.80. There was also a statistically significant variation within PRE and POST times (F(1/5) = 18.85, p = 0.007) [13]. In comparison with the control participants, BBS variation PRE–POST for the study group was higher, with 20.20% ± 6.36 vs. 12.50% ± 8.63, with a statistically significant difference in the variation between groups (F(1/7) = 9.15, p = 0.019; Cohen-d = 2.98). For MBT PRE–POST variation, it was 29.7% ± 10.75 in the telerehabilitation group and 16.96% ± 9.39 in the control group, without significant differences between groups (F(1/7) = 1.61, p = 0.245; Cohen-d = 2.94). Functional independence (BI) in participants trained with our telerehabilitation system was higher compared to the controls: 17.50 ± 9.87 vs. 3.75 ± 8.53, with a significant difference between groups (F(1/7) = 7.97, p = 0.025; Cohen-d = 2.50) [13]. The average SUS score was higher than 80 (87.5 ± 11.61), which can be interpreted as an excellent system user usability level [13]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, P.M.; Śliwiński, M.; Studnicki, R.; Hansdorfer-Korzon, R. Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic. Healthcare 2021, 9, 654. https://doi.org/10.3390/healthcare9060654
Ostrowska PM, Śliwiński M, Studnicki R, Hansdorfer-Korzon R. Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic. Healthcare. 2021; 9(6):654. https://doi.org/10.3390/healthcare9060654
Chicago/Turabian StyleOstrowska, Paulina Magdalena, Maciej Śliwiński, Rafał Studnicki, and Rita Hansdorfer-Korzon. 2021. "Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic" Healthcare 9, no. 6: 654. https://doi.org/10.3390/healthcare9060654
APA StyleOstrowska, P. M., Śliwiński, M., Studnicki, R., & Hansdorfer-Korzon, R. (2021). Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic. Healthcare, 9(6), 654. https://doi.org/10.3390/healthcare9060654






