An Evaluation of the Effectiveness of Ibuprofen and Manual Therapy in Young Women with Dysmenorrhea—A Pilot Study †
Abstract
1. Introduction
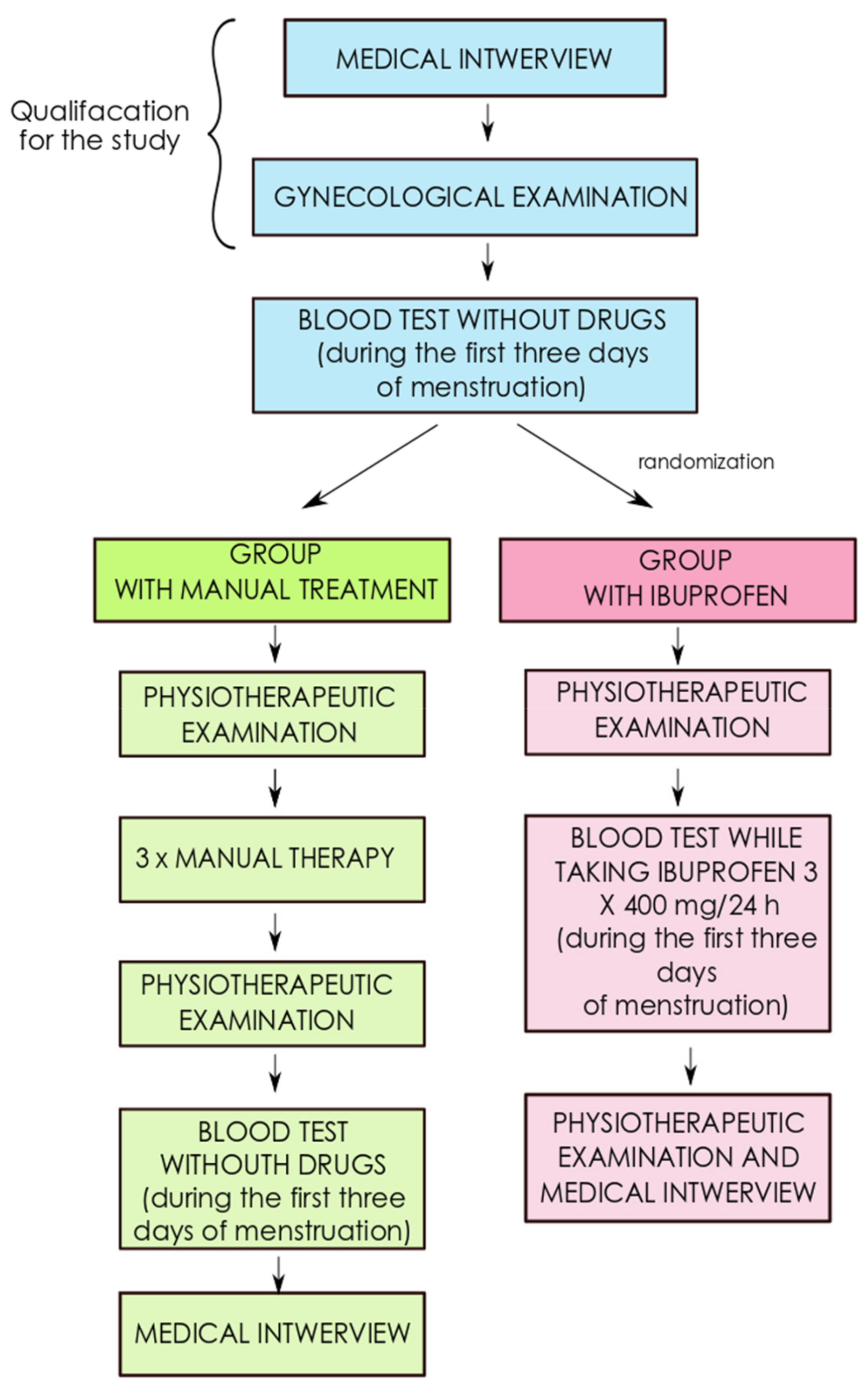
2. Materials and Methods
2.1. Testing the Concentration of Sex Hormones
2.2. Physiotherapeutic Examination
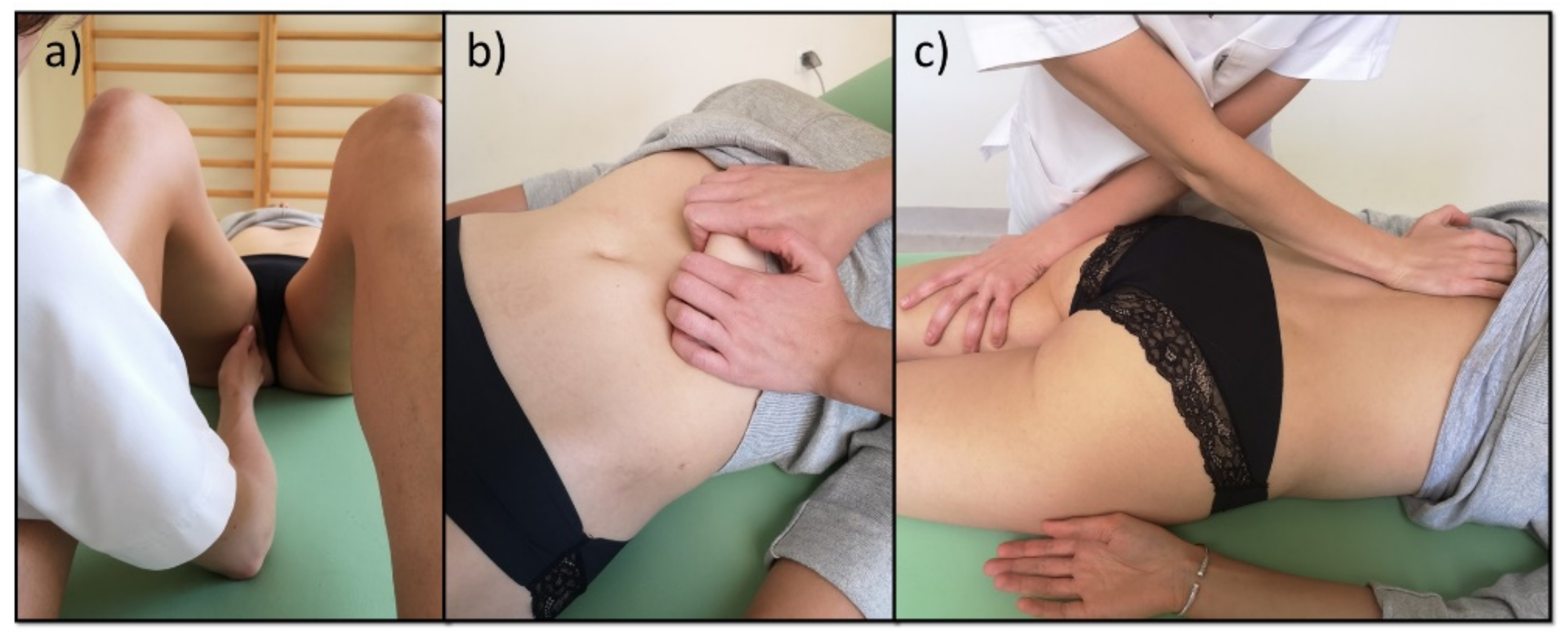
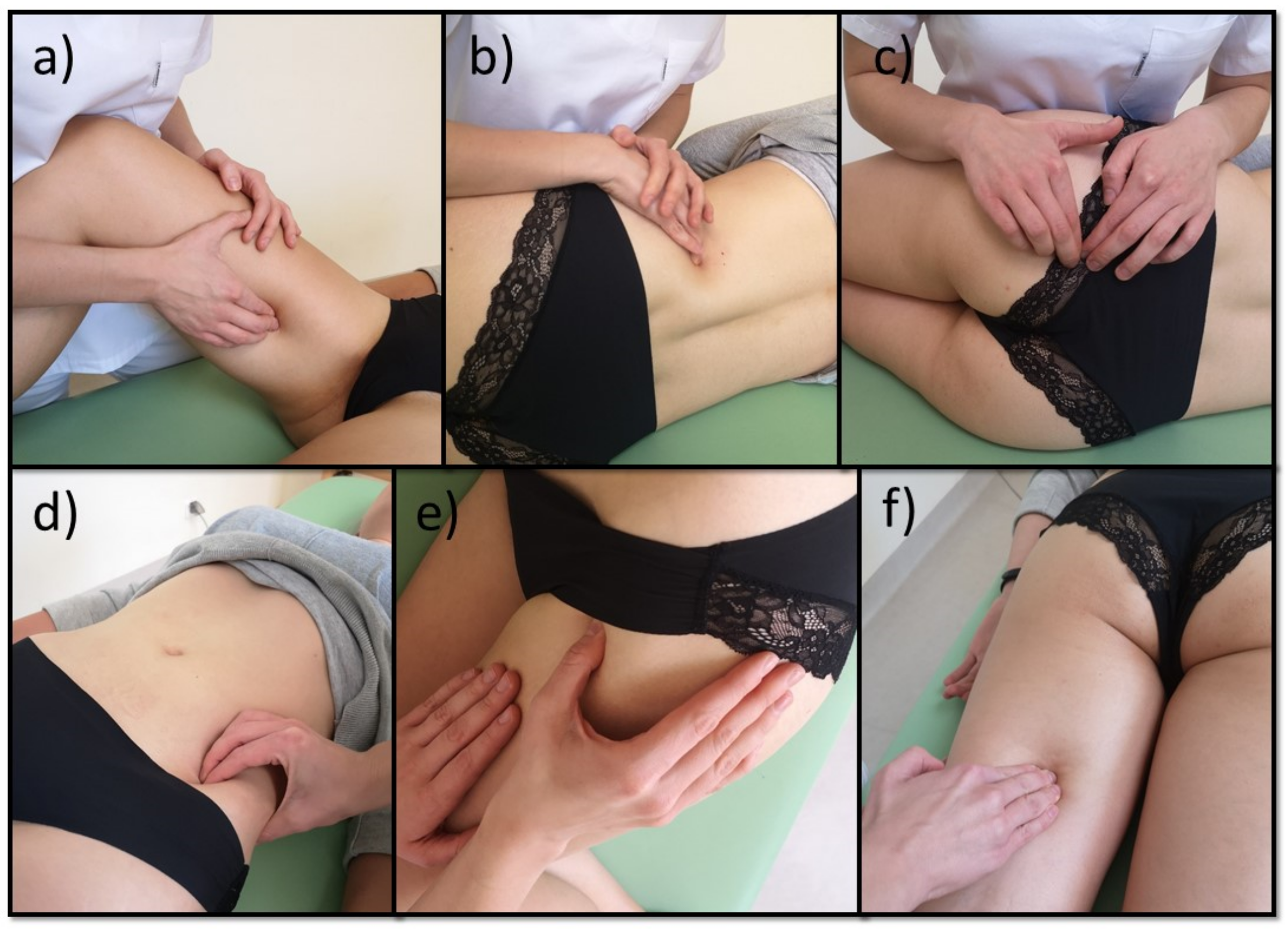
2.3. Manual Therapy and Pharmacotherapy
3. Results
3.1. The Characteristics of Patients with Dysmenorrhea from the Study Subgroup (A) and Control Subgroup (B)
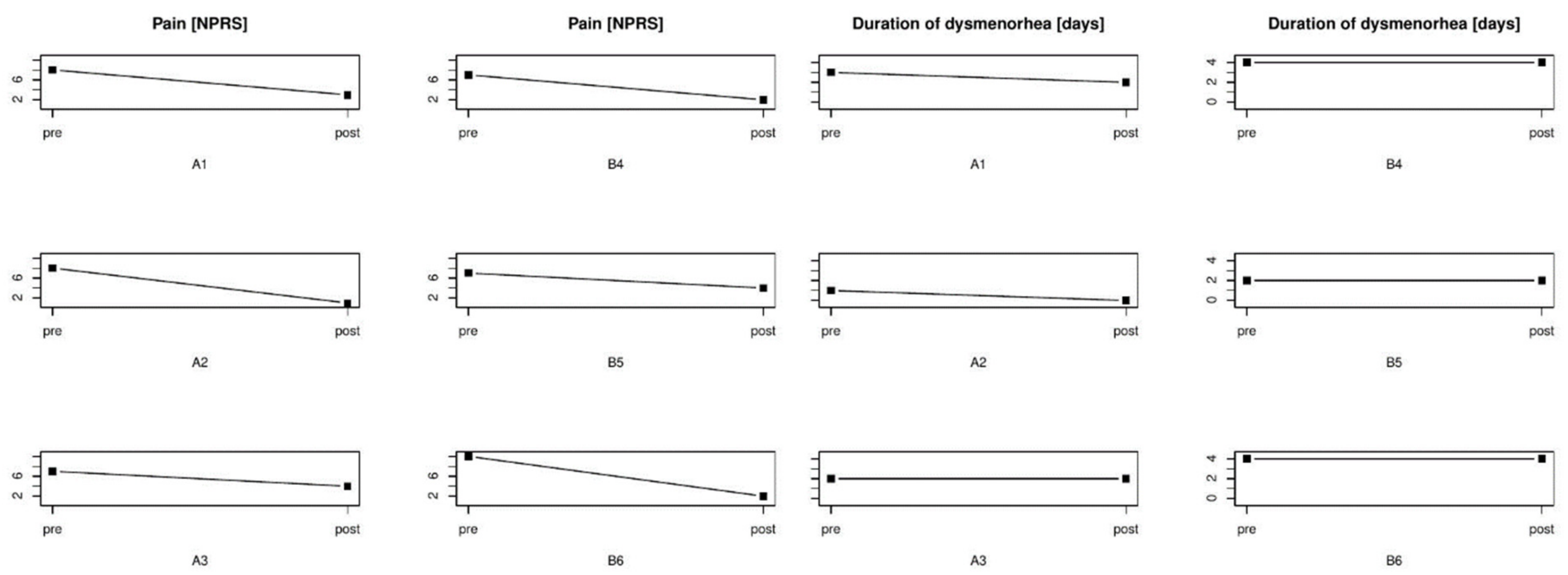
3.2. Patients with Dysmenorrhea Subjected to Manual Therapy
3.2.1. Patient No. 1
3.2.2. Patient No. 2
3.2.3. Patient No. 3
3.3. Patients with Dysmenorrhea Treated with Ibuprofen
3.3.1. Patient No. 4
3.3.2. Patient No. 5
3.3.3. Patient No. 6
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, G.; Grygoruk, C.; Sieczyński, P.; Grusza, M.; Bołkun, I.; Pietrewicz, P. Etiopathogenesis of Dysmenorrhea. Dev. Period Med. 2013, 17, 85–89. [Google Scholar]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef] [PubMed]
- Dogru, H.Y.; Ozsoy, A.Z.; Karakus, N.; Delibas, I.B.; Isguder, C.K.; Yigit, S. Association of Genetic Polymorphisms in TNF and MIF Gene with the Risk of Primary Dysmenorrhea. Biochem. Genet. 2016, 54, 457–466. [Google Scholar] [CrossRef]
- Hailemeskel, S.; Demissie, A.; Assefa, N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: Evidence from female university students in Ethiopia. Int. J. Womens Health 2016, 8, 489–496. [Google Scholar]
- Martinez, E.; Zafra, M.D.O.; Fernandez, M.L. Lifestyle and prevalence of dysmenorrhea among Spanish female university students. PLoS ONE 2018, 13, e0201894. [Google Scholar]
- Barcikowska, Z.; Wójcik-Bilkiewicz, K.; Sobierajska-Rek, A.; Grzybowska, M.E.; Wąż, P.; Zorena, K. Dysmenorrhea and Associated Factors among Polish Women: A Cross-Sectional Study. Pain Res. Manag. 2020. [Google Scholar] [CrossRef]
- Szmidt, M.K.; Granda, D.; Sicinska, E.; Kaluza, J. Primary dysmenorrhea in relation to oxidative stress and antioxidant status: A systematic review of case-control studies. Antioxidants 2020, 9, 994. [Google Scholar] [CrossRef]
- Messinis, I.E.; Messini, C.I.; Dafopoulos, K. Novel aspects of the endocrinology of the menstrual cycle. Reprod. Biomed. Online 2014, 28, 714–722. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D. Progesterone: A pivotal hormone at menstruation. Ann. N. Y. Acad. Sci. 2011, 1221, 88–97. [Google Scholar] [CrossRef]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.; Farquhar, C.; Proctor, M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 7, 1–188. [Google Scholar] [CrossRef]
- Zahradnik, H.P.; Hanjalic-Beck, A.; Groth, K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: A review. Contraception 2010, 81, 185–196. [Google Scholar] [CrossRef]
- Wang, S.F.; Lee, J.P.; Hwa, H.L. Effect of transcutaneous electrical nerve stimulation on primary dysmenorrhea. Neuromodulation 2009, 12, 302–309. [Google Scholar] [CrossRef]
- Trybulec, B.; Wyżycka, E. The use of selected techniques of manual therapy in conservative treatment of primary dysmenorrhea. Med. Rev. 2015, 14, 162–172. [Google Scholar] [CrossRef]
- Parra-Fernández, M.L.; Onieva-Zafra, M.D.; Abreu-Sánchez, A.; Ramos-Pichardo, J.D.; Iglesias-López, M.T.; Fernández-Martínez, E. Management of primary dysmenorrhea among university students in the south of spain and family influence. Int. J. Environ. Res. Public Health 2020, 17, 5570. [Google Scholar] [CrossRef]
- Armour, M.; Smith, C.A.; Steel, K.A.; Macmillan, F. The effectiveness of self-care and lifestyle interventions in primary dysmenorrhea: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Toprak Celenay, S.; Kavalci, B.; Karakus, A.; Alkan, A. Effects of kinesio tape application on pain, anxiety, and menstrual complaints in women with primary dysmenorrhea: A randomized sham-controlled trial. Complement Ther. Clin. Pract. 2020, 39, 101148. [Google Scholar] [CrossRef]
- Çelik, A.S.; Apay, S.E. Effect of progressive relaxation exercises on primary dysmenorrhea in Turkish students: A randomized prospective controlled trial. Complement Ther. Clin. Pract. 2021, 42, 101280. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, T.T.; Qi, X.F.; Yao, M.; Cui, X.J.; Wang, Y.J.; Liang, Q.Q. Manual therapy for hip osteoarthritis: A systematic review and meta-analysis. Pain Phys. 2015, 18, E1005–E1020. [Google Scholar]
- Carnes, D.; Mars, T.S.; Mullinger, B.; Froud, R.; Underwood, M. Adverse events and manual therapy: A systematic review. Man. Ther. 2010, 15, 355–363. [Google Scholar] [CrossRef]
- Demirtürk, F.; Yilar Erkek, Z.; Alparslan, Ö.; Demirtürk, F.; Demir, O.; Inanir, A. Comparison of Reflexology and Connective Tissue Manipulation in Participants with Primary Dysmenorrhea. J. Altern. Complement. Med. 2016, 22, 38–44. [Google Scholar] [CrossRef]
- Ernst, E. A systematic review of systematic reviews of spinal manipulation. J. R. Soc. Med. 2006, 99, 192–196. [Google Scholar] [CrossRef]
- Ruffini, N.; D’Alessandro, G.; Cardinali, L.; Frondaroli, F.; Cerritelli, F. Osteopathic manipulative treatment in gynecology and obstetrics: A systematic review. Complement Ther. Med. 2016, 26, 72–78. [Google Scholar] [CrossRef]
- Wolny, T.; Saulicz, E.; Linek, P.; Shacklock, M.; Myśliwiec, A. Efficacy of Manual Therapy Including Neurodynamic Techniques for the Treatment of Carpal Tunnel Syndrome: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2017, 40, 263–272. [Google Scholar] [CrossRef]
- Proctor, M.L.; Hing, W.; Johnson, T.C.; Murphy, P.A. Spinal manipulation for primary and secondary dysmenorrhoea (Cochrane review) [with consumer summary]. Cochrane Database Syst. Rev. 2006, 3, CD002119. [Google Scholar]
- Holtzman, D.A.; Petrocco-Napuli, K.L.; Burke, J.R. Prospective Case Series on the Effects of Lumbosacral Manipulation on Dysmenorrhea. J. Manip. Physiol Ther. 2008, 31, 237–246. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P. The Challenge of Pain; Penguin: London, UK, 1996. [Google Scholar]
- Tano-Rast, H. Praxisbuch Myofasziale Triggerpunkte: Diagnostik—Therapie—Wirkungen; Elsevier, Urban & Fischer: New York, NY, USA, 2014. [Google Scholar]
- Howland, R.H. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Shoja, M.M.; Sharma, A.; Mirzayan, N.; Groat, C.; Watanabe, K.; Loukas, M.; Shane Tubbs, R. Neuroanatomy of the female abdominopelvic region: A review with application to pelvic pain syndromes. Clin. Anat. 2013, 26, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 20, 5781–5790. [Google Scholar] [CrossRef]
- Shermon, S.; Iii, O.M.S.; Docherty, J.; Iii, O.M.S.; Yao, S.; Capobianco, J. An Osteopathic Approach to Diagnosing and Treating Perimenstrual Disorders. Osteopath Fam. Phys. 2019, 11, 32–40. [Google Scholar]
- Corner, J. Pain, Clinical Manual for Nursing Practice (Book). J. Adv. Nurs. 1989, 14, 988. [Google Scholar]
- Simons, D.G.; Travell, J.G.; Simons, L.S. Myofascial Pain and Dysfunction: The Trigger Point Manual; William & Wilkins: Philadelphia, PA, USA, 1999; Volume 1. [Google Scholar]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual; The Lower Extremities. Clin. J. Pain. 1992, 8, 178. [Google Scholar]
- Peeler, J.; Anderson, J.E. Reliability of the Thomas test for assessing range of motion about the hip. Phys. Ther. Sport. 2007, 8, 14–21. [Google Scholar] [CrossRef]
- Chaitow, L. Sequential assessment and MET treatment of main postural muscles. In Muscle Energy Techniques; Elsevier Urban & Partner: New York, NY, USA, 2011; pp. 131–198. [Google Scholar]
- Kuszewski, M.; Saulicz, E.; Gnat, R.; Knapik, H.; Saulicz, M.K. Effectivenes of hamstring muscles stretching assessed by means of “lacking—angle” test. Ann. Univ. Mariae Curie-Skłodowska Lublin Pol Vol. LX Suppl. XVI, 272. 2005, 10, 212–215. [Google Scholar]
- Schwerla, F.; Wirthwein, P.; Rütz, M.; Resch, K.-L. Osteopathic treatment in patients with primary dysmenorrhoea: A randomised controlled trial. Int. J. Osteopath Med. 2014, 17, 222–231. [Google Scholar] [CrossRef]
- Zecchillo, D.; Acquati, A.; Aquino, A.; Pisa, V.; Uberti, S.; Ratti, S. Osteopathic Manipulative Treatment of Primary Dysmenorrhea and Related Factors: A Randomized Controlled Trial. Int. J. Med. Res. Health Sci. 2017, 6, 165–174. [Google Scholar]
- Cronin, C.G.; Lohan, D.G.; Meehan, C.P.; Delappe, E.; McLoughlin, R.; O’Sullivan, G.J.; McCarthy, P. Anatomy, pathology, imaging and intervention of the iliopsoas muscle revisited. Emerg. Radiol. 2008, 15, 295–310. [Google Scholar] [CrossRef]
- Boyajian-O’Neill, L.A.; McClain, R.L.; Coleman, M.K.; Thomas, P.P. Diagnosis and management of piriformis syndrome: An osteopathic approach. J. Am. Osteopath Assoc. 2008, 108, 657–664. [Google Scholar] [CrossRef]
- Rossetti, S.R. Functional anatomy of pelvic floor. Arch. Ital. Urol Androl. 2016, 88, 28–37. [Google Scholar] [CrossRef]
- Barassi, G.; Bellomo, R.G.; Porreca, A.; Di Felice, P.A.; Prosperi, L.; Saggini, R. Somato-Visceral Effects in the Treatment of Dysmenorrhea: Neuromuscular Manual Therapy and Standard Pharmacological Treatment. J. Altern. Complement. Med. 2017, 24, 291–299. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Sirivichayakul, S.; Matsumoto, A.K.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Barbosa, D.S.; Moreira, E.G.; Maes, M. Menstruation distress is strongly associated with hormone-immune-metabolic biomarkers. J. Psychosom. Res. 2021, 142, 110355. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, J.; Xue, Y.; Wang, Q.; Li, S.; Zhou, Y.; Zhang, W. Comparison between manual acupuncture and electroacupuncture for hot flashes and sex hormone of perimenopausal syndrome. Zhongguo Zhen Jiu 2017, 37, 247–252. [Google Scholar]
- Hernandez-Reif, M.; Martinez, A.; Field, T.; Quintero, O.; Hart, S.; Burman, I. Premenstrual symptoms are relieved by massage therapy. J. Psychosom. Obstet. Gynaecol. 2000, 21, 9–15. [Google Scholar] [CrossRef]



| Subgroup | Age (years) | BMI (kg/m2) | Duration of Menstrual Cycle (days) | Duration of Menstruation (days) |
|---|---|---|---|---|
| A (n = 3) manual therapy | 22 | 21 | 27 | 5 |
| B (n = 3) ibuprofen | 21 | 23 | 30 | 6 |
| The data presented are the mean of each group | ||||
| Patient | Duration of Menstrual Cycle (days) | Duration of Menstruation (days) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| 1A | 28 | 30 | 6 | 6 |
| 2A | 25 | 25 | 5 | 5 |
| 3A | 28 | 33 | 5 | 5 |
| 4B | 34 | 34 | 7 | 7 |
| 5B | 29 | 29 | 6 | 6 |
| 6B | 28 | 26 | 6 | 6 |
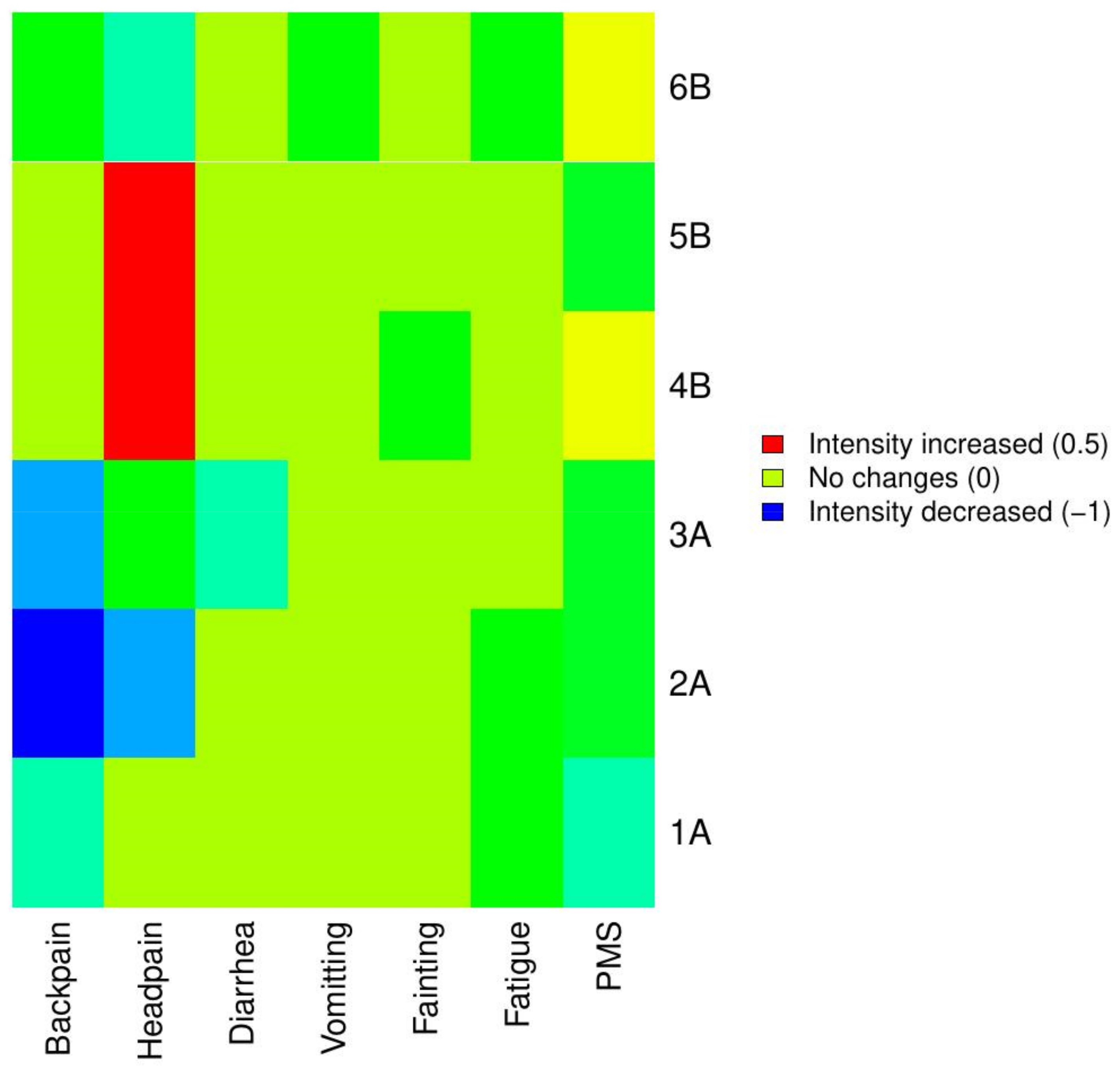
| Patient | Backpain | Headpain | Diarrhea | Vomiting | Fainting | Fatigue | PMS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1A | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 8 | 3 |
| 2A | 3 | 2 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 10 | 3 |
| 3A | 3 | 0 | 1 | 0 | 3 | 1 | 1 | 1 | 0 | 0 | 2 | 2 | 3 | 0 |
| 4B | 0 | 0 | 0 | 2 | 4 | 4 | 0 | 0 | 1 | 0 | 3 | 3 | 6 | 7 |
| 5B | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 3 | 0 |
| 6B | 3 | 2 | 2 | 0 | 3 | 3 | 1 | 0 | 0 | 0 | 3 | 2 | 7 | 8 |
| Patient | Progesterone (ng/mL) | Estradiol 17-Beta (pg/mL) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| 1A | 0.54 | 0.31 | 53 | 60 |
| 2A | 0.42 | 0.38 | 26 | 36 |
| 3A | 0.26 | 0.22 | 5 | 13 |
| 4B | 0.58 | 0.33 | 13 | <5 |
| 5B | 0.21 | 0.17 | 50 | 21 |
| 6B | 0.14 | 0.31 | 18 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Wąż, P.; Zorena, K. An Evaluation of the Effectiveness of Ibuprofen and Manual Therapy in Young Women with Dysmenorrhea—A Pilot Study. Healthcare 2021, 9, 617. https://doi.org/10.3390/healthcare9060617
Barcikowska Z, Rajkowska-Labon E, Grzybowska ME, Hansdorfer-Korzon R, Wąż P, Zorena K. An Evaluation of the Effectiveness of Ibuprofen and Manual Therapy in Young Women with Dysmenorrhea—A Pilot Study. Healthcare. 2021; 9(6):617. https://doi.org/10.3390/healthcare9060617
Chicago/Turabian StyleBarcikowska, Zofia, Elżbieta Rajkowska-Labon, Magdalena Emilia Grzybowska, Rita Hansdorfer-Korzon, Piotr Wąż, and Katarzyna Zorena. 2021. "An Evaluation of the Effectiveness of Ibuprofen and Manual Therapy in Young Women with Dysmenorrhea—A Pilot Study" Healthcare 9, no. 6: 617. https://doi.org/10.3390/healthcare9060617
APA StyleBarcikowska, Z., Rajkowska-Labon, E., Grzybowska, M. E., Hansdorfer-Korzon, R., Wąż, P., & Zorena, K. (2021). An Evaluation of the Effectiveness of Ibuprofen and Manual Therapy in Young Women with Dysmenorrhea—A Pilot Study. Healthcare, 9(6), 617. https://doi.org/10.3390/healthcare9060617







