Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Data Source
2.3. Participants
2.4. Study Variables and Measurements
2.5. Fundus Examination and Grading of Diabetic Retinopathy
2.6. Statistical Analysis
2.7. Ethics Consideration
3. Results
3.1. Characteristics of Study Subjects
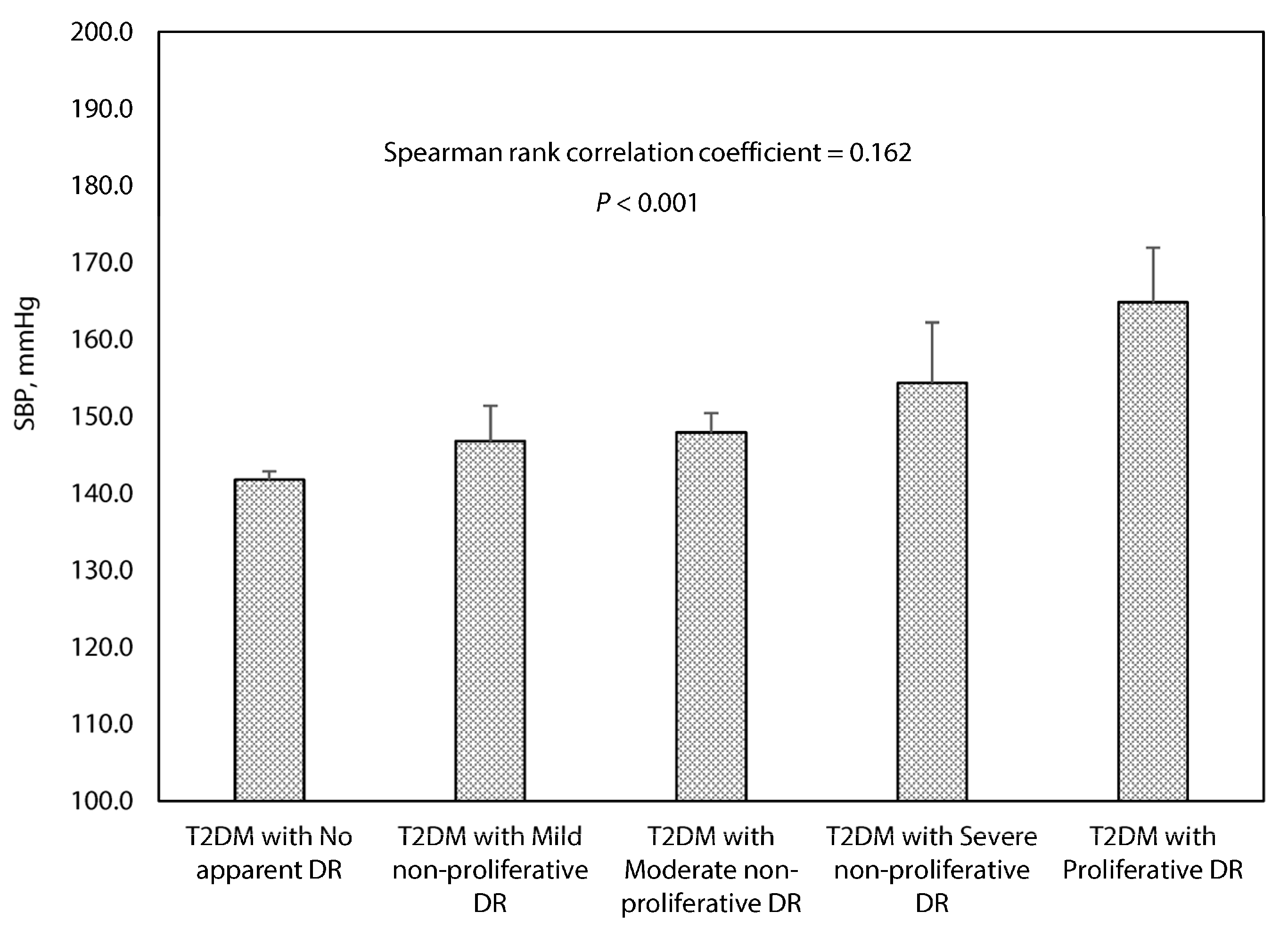
3.2. Association between Systolic Blood Pressure and the Grading of Diabetic Retinopathy
3.3. Risk Factors Associated with Diabetic Retinopathy
4. Discussion
4.1. Main Findings
4.2. Relationship with Other Studies
4.3. Implications for Research and Practice
4.4. Strengths and Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A. Vascular complications of diabetes. Circ Res. 2016, 118, 1771–1785. [Google Scholar] [CrossRef]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.J.; Scruggs, B.A.; Flaxel, C.J. Diabetic eye disease: A review of screening and management recommendations. Clin. Exp. Ophthalmol. 2021, 49, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- International Diabetes Federation and The Fred Hollows Foundation. Diabetes Eye Health: A Guide for Health Care Professionals; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Lanzetta, P.; Sarao, V.; Scanlon, P.H.; Barratt, J.; Porta, M.; Bandello, F.; Loewenstein, A. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020, 57, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Poncelas, A.; Miravet-Jiménez, S.; Casellas, A.; Barrot-De La Puente, J.F.; Franch-Nadal, J.; López-Simarro, F.; Mata-Cases, M.; Mundet-Tudurí, X. Prevalence of diabetic retinopathy in individuals with type 2 diabetes who had recorded diabetic retinopathy from retinal photographs in Catalonia (Spain). Br. J. Ophthalmol. 2015, 99, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- McGill, J.B. Improving microvascular outcomes in patients with diabetes through management of hypertension. Postgrad Med. 2009, 121, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; Handelsman, Y.; Grunberger, G.; Einhorn, D.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; American Association of Clinical Endocrinologists; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm-2020 Executive Summary. Endocr. Pract. 2020, 26, 107–139. [Google Scholar] [CrossRef]
- Valencia, W.M.; Florez, H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Wang, X.; Vedula, S.S.; Marrone, M.; Sleilati, G.; Hawkins, B.S.; Frank, R.N. Blood pressure control for diabetic retinopathy. Cochrane Database Syst. Rev. 2015, 1, CD006127. [Google Scholar] [PubMed]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Osataphan, S.; Chalermchai, T.; Ngaosuwan, K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: A retrospective cohort study. J. Diabetes 2016, 9, 267–274. [Google Scholar] [CrossRef]
- Weng, J.; Ji, L.; Jia, W.; Lu, J.; Zhou, Z.; Zou, D.; Zhu, D.; Chen, L.; Chen, L.; Guo, L.; et al. Standards of care for type 2 diabetes in China. Diabetes Metab. Res. Rev. 2016, 32, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Department of Primary Health, National Health Commission, PRC. 2017, No.13 Document. Standards for National Basic Public Health Services (3rd edition). Available online: http://www.nhc.gov.cn/jws/s3578/201703/d20c37e23e1f4c7db7b8e25f34473e1b.shtml (accessed on 12 April 2021).
- IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005. Available online: http://www.ipaq.ki.se/ (accessed on 12 April 2021).
- Writing Group of the 2018 Chinese Guidelines for the Management of Hypertension. 2018 Chinese guidelines for the management of hypertension. Chin. J. Cardiovasc. Med. 2019, 24, 24–56. [Google Scholar]
- Praidou, A.; Harris, M.; Niakas, D.; Labiris, G. Physical activity and its correlation to diabetic retinopathy. J. Diabetes Complicat. 2017, 31, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Rooney, D.; Lye, W.K.; Tan, G.; Lamoureux, E.L.; Ikram, M.K.; Cheng, C.Y.; Kumari, N.; Zheng, Y.F.; Mitchell, P.; Wang, J.J.; et al. Body mass index and retinopathy in Asian populations with diabetes mellitus. Acta Diabetol. 2015, 52, 73–80. [Google Scholar] [CrossRef]
- Zhang, X.; Saaddine, J.B.; Chou, C.-F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.K.; Klein, R. Prevalence of Diabetic Retinopathy in the United States, 2005–2008. JAMA 2010, 304, 649–656. [Google Scholar] [CrossRef]
- Raman, R.; Gupta, A.; Krishna, S.; Kulothungan, V.; Sharma, T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27). J. Diabetes Complicat. 2012, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sjølie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Chaturvedi, N. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008, 372, 1385–1393. [Google Scholar] [CrossRef]
- Adler, A.I.; Stratton, I.M.; Neil, H.A.; Yudkin, J.S.; Matthews, D.R.; Cull, C.A.; Holman, R.R. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ 2000, 321, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Morris, A.D.; Doney, A.S.; Leese, G.P.; Pearson, E.R.; Palmer, C.N. Glycemic exposure and blood pressure influencing progression and remission of diabetic retinopathy: A longitudinal cohort study in GoDARTS. Diabetes Care 2013, 36, 3979–3984. [Google Scholar] [CrossRef]
- Porta, M.; Taulaigo, A.V. The changing role of the endocrinologist in the care of patients with diabetic retinopathy. Endocrine 2014, 46, 199–208. [Google Scholar] [CrossRef]
- Foo, V.; Quah, J.; Cheung, G.; Tan, N.C.; Zar, K.L.M.; Chan, C.M.; Lamoureux, E.; Yin, W.T.; Tan, G.; Sabanayagam, C. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics. J. Diabetes 2016, 9, 200–207. [Google Scholar] [CrossRef]
- Liu, L.; Quang, N.D.; Banu, R.; Kumar, H.; Tham, Y.C.; Cheng, C.Y. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS ONE 2020, 15, e0229665. [Google Scholar] [CrossRef]
- Takao, T.; Matsuyama, Y.; Suka, M.; Yanagisawa, H.; Kikuchi, M.; Kawazu, S. Time-to-effect relationships between systolic blood pressure and the risks of nephropathy and retinopathy in patients with type 2 diabetes. J. Diabetes Complicat. 2014, 28, 674–678. [Google Scholar] [CrossRef]
- Williams, M.E. The goal of blood pressure control for prevention of early diabetic microvascular complications. Curr. Diab. Rep. 2011, 11, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.A.; Silva, K.C.; Rosales, M.A.; Lopes de Faria, J.B.; Lopes de Faria, J.M. The concomitance of hypertension and diabetes exacerbating retinopathy: The role of inflammation and oxidative stress. Curr. Clin. Pharmacol. 2013, 8, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.S.; Branch, W.T.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical Inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Berlowitz, D.R.; Ash, A.S.; Hickey, E.C.; Glickman, M.; Friedman, R.; Kader, B. Hypertension management in patients with diabetes: The need for more aggressive therapy. Diabetes Care 2003, 26, 355–359. [Google Scholar] [CrossRef][Green Version]
- Barton, A.B.; Okorodudu, D.E.; Bosworth, H.B.; Crowley, M.J. Clinical Inertia in a Randomized Trial of Telemedicine-Based Chronic Disease Management: Lessons Learned. Telemed. J. E Health 2018, 24, 742–748. [Google Scholar] [CrossRef]
- Shahab, L.; Mindell, J.; Poulter, N.R.; West, R. Hypertension and its identification among current, past and never smokers in an English population sample. Eur. J. Cardiovasc Prev. Rehabil. 2010, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.F.; Severo, M.D.; Ledur, P.S.; Becker, A.D.; Aguiar, F.M.; Massierer, D.; Freitas, V.C.; Schaan, B.D.; Gus, M. White coat effect and masked uncontrolled hypertension in treated hypertensive-diabetic patients: Prevalence and target organ damage. J. Diabetes. 2015, 7, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Head, G.A.; Shaw, J.E.; Dunstan, D.W.; Owen, N.; Magliano, D.J.; Chadban, S.; Zimmet, P. Hypertension, white-coat hypertension and masked hypertension in Australia: Findings from the Australian Diabetes, Obesity, and Lifestyle Study 3. J. Hypertens. 2019, 37, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Grubber, J.; Mruthyunjaya, P.; McCant, F.; Bosworth, H.B. Progression of diabetic retinopathy in the hypertension intervention nurse telemedicine study. JAMA Ophthalmol. 2013, 131, 957–958. [Google Scholar] [CrossRef]
- Schrier, R.W.; Estacio, R.O.; Mehler, P.S.; Hiatt, W.R. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: A summary of the ABCD trial. Nat. Clin. Pract. Nephrol. 2007, 3, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Vijan, S. Diabetes: Treating hypertension. BMJ Clin. Evid. 2014, 2014, 0608. [Google Scholar]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S125–S150. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Wang, H.H.X.; Cheung, C.S.K.; Tong, E.L.H.; Sek, A.C.H.; Cheung, N.T.; Yan, B.P.Y.; Yu, C.M.; Griffiths, S.M.; Coats, A.J.S. Factors associated with multimorbidity and its link with poor blood pressure control among 223,286 hypertensive patients. Int. J. Cardiol. 2014, 177, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.X. Taking a multidisciplinary team approach to better healthcare outcomes for society. Hong Kong Med. J. 2020, 26, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.X.; Wong, S.Y.S.; Wong, M.C.S.; Wei, X.L.; Wang, J.J.; Li, D.K.T.; Tang, J.L.; Gao, G.Y.; Griffiths, S.M. Patients’ experiences in different models of community health centers in southern China. Ann. Fam. Med. 2013, 11, 517–526. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 2510) | Hypertensives with T2DM (n = 1495) | Normotensives with T2DM (n = 1015) | p-Value | |
|---|---|---|---|---|
| Age, years | 64.84 (7.96) | 66.36 (7.65) | 62.61 (7.89) | <0.001 |
| Age (groups) | ||||
| <60 years | 616 (24.5%) | 274 (18.3%) | 342 (33.7%) | <0.001 |
| 60–69 years | 1230 (49.0%) | 728 (48.7%) | 502 (49.5%) | |
| ≥70 years | 664 (26.5%) | 493 (33.0%) | 171 (16.8%) | |
| Sex | ||||
| Women | 1445 (57.6%) | 888 (59.4%) | 557 (54.9%) | 0.024 |
| Men | 1065 (42.4%) | 607 (40.6%) | 458 (45.1%) | |
| Education level | ||||
| ≥Senior secondary | 1614 (64.3%) | 925 (61.9%) | 682 (67.2%) | 0.066 |
| Others | 896 (35.7%) | 570 (38.1%) | 333 (32.8%) | |
| Current smoking | ||||
| Yes | 286 (11.4%) | 138 (9.2%) | 148 (14.6%) | <0.001 |
| No | 2224 (88.6%) | 1357 (90.8%) | 867 (85.4%) | |
| Regular drinking | ||||
| Yes | 189 (7.5%) | 100 (6.7%) | 89 (8.8%) | 0.053 |
| No | 2321 (92.5%) | 1395 (93.3%) | 926 (91.2%) | |
| Levels of physical activity | ||||
| Low-to-moderate | 1353 (53.9%) | 852 (57.0%) | 501 (49.4%) | <0.001 |
| High | 1157 (46.1%) | 643 (43.0%) | 514 (50.6%) |
| Total (n = 2510) | Hypertensives with T2DM (n = 1495) | Normotensives with T2DM (n = 1015) | p-Value | |
|---|---|---|---|---|
| Duration of diabetes, years | 8.56 (7.10) | 9.00 (7.22) | 7.92 (6.87) | <0.001 |
| Duration of diabetes (groups) | ||||
| <4 years | 768 (30.6%) | 433 (29.0%) | 335 (33.0%) | 0.004 |
| 4–11 years | 1023 (40.8%) | 598 (40.0%) | 425 (41.9%) | |
| ≥12 years | 719 (28.6%) | 464 (31.0%) | 255 (25.1%) | |
| HbA1c, % | 7.02 (1.43) | 6.97 (1.38) | 7.10 (1.51) | 0.023 |
| HbA1c (groups) | ||||
| <6.5% | 1054 (42.0%) | 629 (42.1%) | 425 (41.9%) | 0.920 |
| ≥6.5% | 1456 (58.0%) | 866 (57.9%) | 590 (58.1%) | |
| Systolic BP, mmHg | 134.82 (18.74) | 143.39 (17.86) | 122.14 (11.40) | <0.001 |
| Diastolic BP, mmHg | 70.45 (10.47) | 72.89 (10.97) | 66.80 (8.44) | <0.001 |
| Presence of DR | ||||
| No | 1960 (78.1%) | 1155 (77.3%) | 805 (79.3%) | 0.222 |
| Yes | 550 (21.9%) | 340 (22.7%) | 210 (20.7%) | |
| Grading of DR 1 | ||||
| Mild NPDR | 119 (22.3%) | 61 (18.4%) | 58 (28.6%) | 0.081 |
| Moderate NPDR | 342 (64.0%) | 222 (67.1%) | 120 (59.1%) | |
| Severe NPDR | 47 (8.8%) | 30 (9.1%) | 17 (8.4%) | |
| Proliferative DR | 26 (4.9%) | 18 (5.4%) | 8 (3.9%) | |
| Variables | Crude Model | Adjusted Model | ||
|---|---|---|---|---|
| cOR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Duration, years | 1.077 (1.058–1.096) | <0.001 | 1.061 (1.042–1.081) | <0.001 |
| Male sex | 1.688 (1.294–2.202) | <0.001 | 1.733 (1.284–2.338) | <0.001 |
| Regular drinking | 1.605 (1.016–2.535) | 0.042 | 1.146 (0.691–1.901) | 0.596 |
| High physical activity | 0.717 (0.547–0.942) | 0.017 | 0.642 (0.478–0.861) | 0.003 |
| BMI | 0.956 (0.917–0.997) | 0.035 | 0.950 (0.908–0.994) | 0.028 |
| Systolic BP | 1.025 (1.017–1.033) | <0.001 | 1.020 (1.012–1.029) | <0.001 |
| HbA1c | 1.631 (1.488–1.787) | <0.001 | 1.527 (1.387–1.680) | <0.001 |
| Age, years | 0.989 (0.972–1.006) | 0.207 | - | - |
| High education level | 0.840 (0.635–1.110) | 0.219 | - | - |
| Current smoking | 1.130 (0.730–1.748) | 0.583 | - | - |
| Diastolic BP | 1.004 (0.992–1.016) | 0.517 | - | - |
| Triglyceride | 0.998 (0.926–1.075) | 0.955 | - | - |
| Total cholesterol | 0.967 (0.853–1.097) | 0.605 | - | - |
| LDL cholesterol | 0.962 (0.836–1.107) | 0.589 | - | - |
| HDL cholesterol | 0.982 (0.700–1.377) | 0.914 | - | - |
| Variables | Crude Model | Adjusted Model | ||
|---|---|---|---|---|
| cOR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Duration, years | 1.066 (1.043–1.091) | <0.001 | 1.057 (1.032–1.082) | <0.001 |
| Current smoking | 1.572 (1.015–2.435) | 0.043 | 1.358 (0.857–2.150) | 0.192 |
| Systolic BP | 1.024 (1.008–1.040) | 0.004 | 1.019 (1.003–1.037) | 0.018 |
| HbA1c | 1.465 (1.329–1.614) | <0.001 | 1.408 (1.275–1.555) | <0.001 |
| Male sex | 1.241 (0.883–1.742) | 0.213 | - | - |
| Age, years | 0.983 (0.962–1.004) | 0.120 | - | - |
| High education level | 0.819 (0.582–1.153) | 0.252 | - | - |
| Regular drinking | 0.843 (0.447–1.589) | 0.597 | - | - |
| High physical activity | 0.777 (0.553–1.092) | 0.146 | - | - |
| BMI | 0.964 (0.913–1.018) | 0.188 | - | - |
| Diastolic BP | 0.990 (0.971–1.010) | 0.344 | - | - |
| Triglyceride | 0.963 (0.858–1.082) | 0.530 | - | - |
| Total cholesterol | 0.924 (0.785–1.088) | 0.344 | - | - |
| LDL cholesterol | 0.991 (0.827–1.187) | 0.922 | - | - |
| HDL cholesterol | 0.728 (0.469–1.130) | 0.157 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-T.; Wang, Y.; Hu, X.-J.; Chen, J.-H.; Li, Y.-Y.; Zhong, Q.-Y.; Cheng, H.; Mohammed, B.H.; Liang, X.-L.; Hernandez, J.; et al. Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications. Healthcare 2021, 9, 580. https://doi.org/10.3390/healthcare9050580
Li Y-T, Wang Y, Hu X-J, Chen J-H, Li Y-Y, Zhong Q-Y, Cheng H, Mohammed BH, Liang X-L, Hernandez J, et al. Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications. Healthcare. 2021; 9(5):580. https://doi.org/10.3390/healthcare9050580
Chicago/Turabian StyleLi, Yu-Ting, Yi Wang, Xiu-Jing Hu, Jia-Heng Chen, Yun-Yi Li, Qi-Ya Zhong, Hui Cheng, Bedru H. Mohammed, Xiao-Ling Liang, Jose Hernandez, and et al. 2021. "Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications" Healthcare 9, no. 5: 580. https://doi.org/10.3390/healthcare9050580
APA StyleLi, Y.-T., Wang, Y., Hu, X.-J., Chen, J.-H., Li, Y.-Y., Zhong, Q.-Y., Cheng, H., Mohammed, B. H., Liang, X.-L., Hernandez, J., Huang, W.-Y., & Wang, H. H. X. (2021). Association between Systolic Blood Pressure and Diabetic Retinopathy in Both Hypertensive and Normotensive Patients with Type 2 Diabetes: Risk Factors and Healthcare Implications. Healthcare, 9(5), 580. https://doi.org/10.3390/healthcare9050580







