Mucinous Cystic Neoplasm of Pancreas in a Pregnant Woman Presenting with Severe Anemia and Gastric Bleeding: Case Report and Review of the Literature
Abstract
1. Introduction
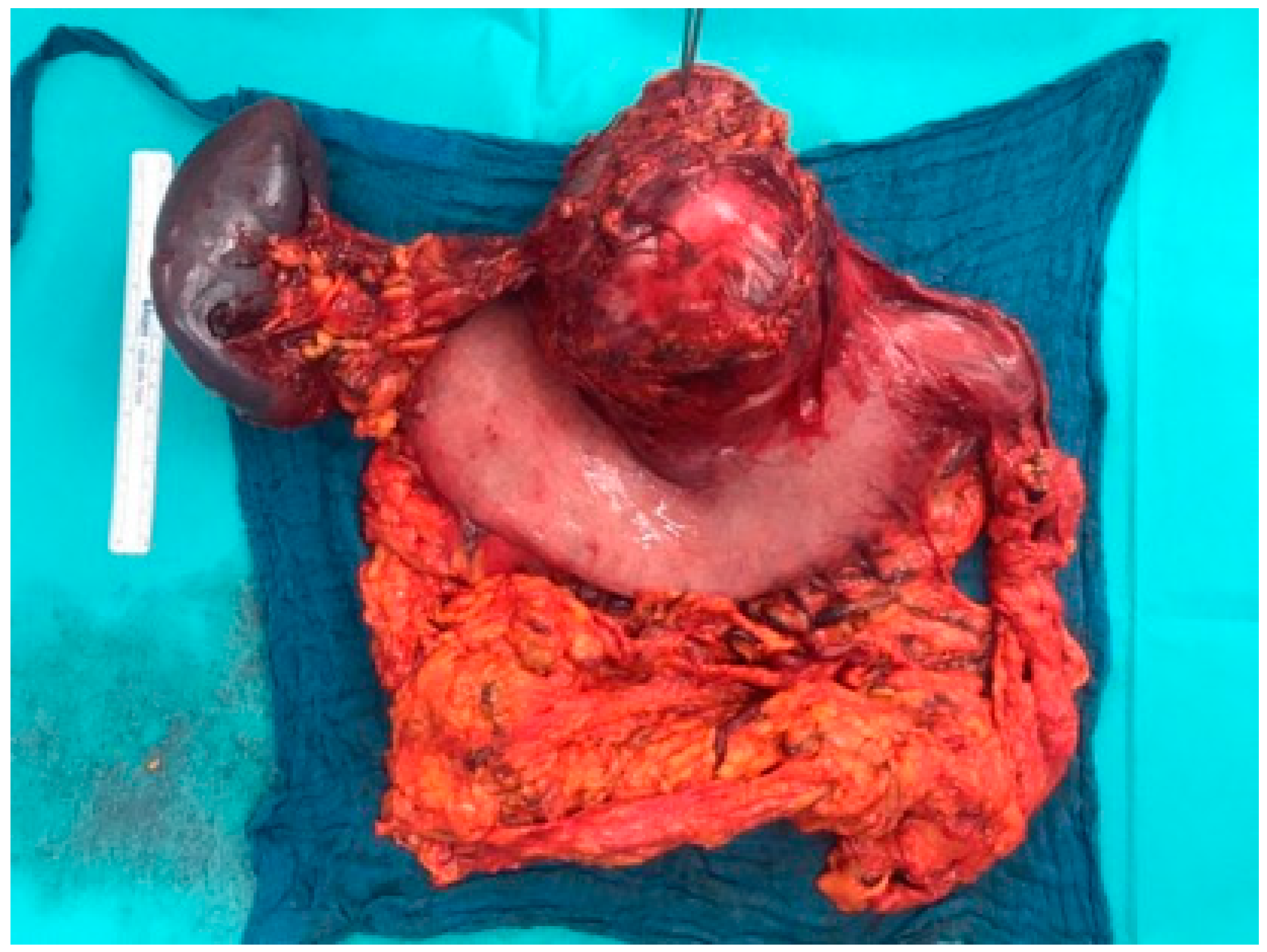
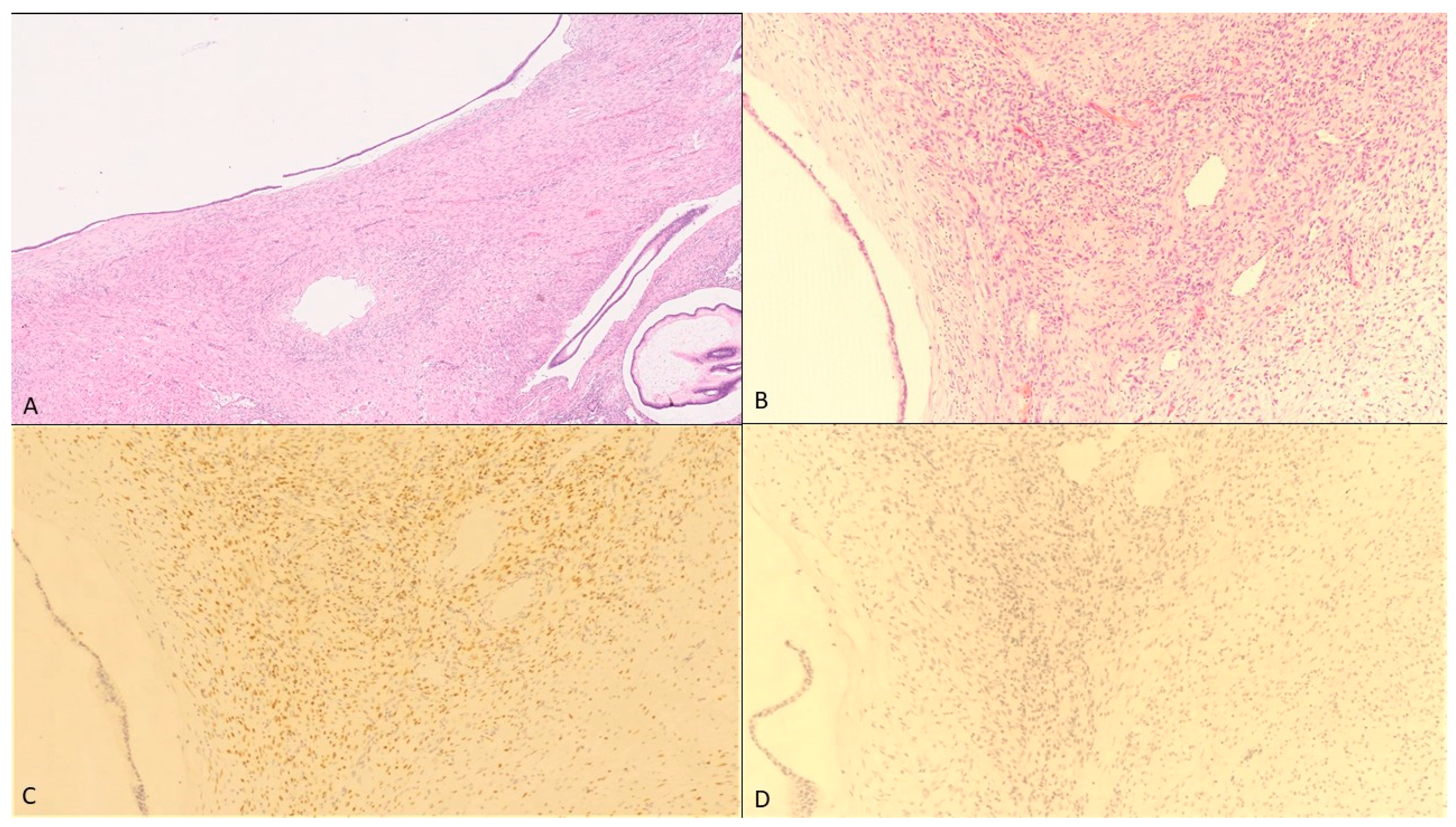
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Author/Citation | Age at Diagnosis | Gestational Age at Diagnosis (Weeks) | Maximum Diameter of Tumor (cm) | Location in Pancreas | Timing of Operation | Complications | Surgical Procedure | Histological Diagnosis | ER/PR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Smithers [31] | 33 | 7 | 10 | Body/tail | AD (abortion in week 9) | Tumor rupture | DP | adenocarcinoma | NA/NA |
| 2 | Baiocchu [32] | 29 | 40 | 10 | Tail | AD (NA) | No | DP | adenocarcinoma | NA/NA |
| 3 | Olsen [33] | 25 | 5 | 5 | Tail | DPr (week 18) | NR | DP | Adenoma | NA/NA |
| 4 | Ganepola [34] | 37 | 4 | 12 | Tail | DPr (week 23) | NR | DP | Adenoma | +/+ |
| 5 | Kato [21] | 33 | 15 | 22 | Body/tail | DPr (week 23) | IUGR | DP | Adenoma | +/+ |
| 6 | Lopez-Tomassetti Fernández [23] | 26 | 20 | 15 | Tail | DPr (week 26) | Episodic epigastric pain | DP | Adenoma | NA/NA |
| 7 | Kitagawa [35] | 25 | AD | 15 | Body | AD (11 months) | No | DP | Adenoma | −/+ |
| 8 | Herring [36] | 34 | 3 | 20 | Body/tail | DPr (week 17) | NR | DP | adenocarcinoma | +/+ |
| 9 | Ozden [37] | 32 | 36 | 15 | tail | AD (emergency Caesarean: week 34) | Tumor rupture | DP | adenocarcinoma | −/− |
| 10 | Berindoague [38] | 31 | AD | 12 | Body/tail | AD (2 months) | NR | DP | adenocarcinoma | −/− |
| 11 | Ishikawa [39] | 33 | 17 | 18 | Body/tail | AD (3 months) | NR | DP | Adenoma | −/− |
| 12 | Ikuta [40] | 30 | 10 | 18 | Tail | AD (abortion: week 10) | Missed abortion | DP | Moderate dysplasia | +/+ |
| 13 | Hakamada [41] | 38 | 2 years before pregnancy | 14 | Tail | DPr (second trimester) | nausea, hematemesis, tarry stool | DP, partial stomach resection | Borderline | NA/+ |
| 14 | Wiseman [42] | 32 | 11 | 15 | Tail | DPr (week 15) | Intractable nausea | DP | Low grade dysplasia | +/+ |
| 15 | Brown [16] | 38 | 8 | 10 | Body/tail | DPr (week 8) | Gastrointestinal bleeding | DP | Severe dysplasia | NA/NA |
| 16 | Shirakawa [43] | 34 | 26 | 19 | Body/tail | AD (3 months) | No | DP | Adenoma | +/+ |
| 17 | Shirakawa [43] | 36 | AD | 16 | Body/tail | AD (NA) | No | DP | adenocarcinoma | −/− |
| 18 | Asciutti [44] | 31 | 23 | 8 | Tail | AD (1 month) | Pancreatitis | DP | Adenoma | NA/NA |
| 19 | Coral [45] | 26 | 3 | 32 | Body/tail | AD (1 month) | No | DP | Adenoma | −/+ |
| 20 | Naganuma [22] | 32 | 33 | 11 | Head | AD (emergency Caesarean: week 34) | Tumor rupture | PD | adenocarcinoma | −/+ |
| 21 | Martins-Filho [46] | 20 | 20 | 15 | Body/tail | DPr (week 20) | No | DP | Adenoma | NA/NA |
| 22 | Boyd [47] | 21 | 7 months before pregnancy | 17 | Body/tail | DPr (week 20) | Abdominal distension and fullness | DP | Moderate dysplasia | NA/NA |
| 23 | Iusco [48] | 28 | AD | 16 | Body/tail | AD (NA) | No | DP | Associated adenocarcinoma | +/+ |
| 24 | Tsuda [49] | 28 | Some years before | 15 | Body/tail | DPr (week 18) | No | DP | Severe dysplasia | +/+ |
| 25 | Tica [8] | 27 | 29 | 15 | Body/tail | AD (2 months) | NR | DP | Adenoma | −/− |
| 26 | Urabe [50] | 34 | 16 | 16.5 | Body | AD (1 month) | No | DP | Adenoma | NA/NA |
| 27 | Urabe [50] | 40 | 33 | 12 | Tail | AD (emergency Caesarean: week 33) | Rupture | DP | Adenoma | +/+ |
| 28 | Takashima [51] | 28 | 12 | 13 | Head | AD (1 month) | No | Enucleation | High grade dysplasia | +/+ |
| 29 | Kleeff [52] | 41 | Three years before pregnancy | 7 | Body/tail | AD (3 months) | No | DP | Moderate dysplasia | +/+ |
| 30 | Kosumi [26] | 33 | 4 | 7.6 | Body/tail | AD (2 weeks) | No | DP | NA | +/+ |
| 31 | Veits [53] | 28 | 11 | 4.7 | Tail | DPr | Pancreatitis | DP | Low grade dysplasia | NA/NA |
| 32 | Revoredo [12] | 38 | 17 | 20 | Body/tail | DPr (week 29) | Rupture | DP | Moderate dysplasia | −/+ |
| 33 | Revoredo [12] | 30 | 18 | 13.6 | Body/tail | DPr (week 20) | no | DP | adenocarcinoma | +/+ |
| 34 | Carvalho [13] | 32 | 31 | 24 | Tail | AD (1 month) | no | DP | Low grade dysplasia | NA/NA |
| 35 | Present case | 33 | 34 | 12.5 | Body/tail | AD (emergency Caesarean: week 34) | Gastrointestinal bleeding | DP + 4/5-gastrectomy | Low grade dysplasia | −/+ |
References
- World Health Organization. Prevalence of Anaemia in Pregnant Women (%). 2021. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-pregnant-women- (accessed on 15 December 2020).
- Sifakis, S.; Pharmakides, G. Anemia in pregnancy. Ann. N. Y. Acad. Sci. 2000, 900, 125–136. [Google Scholar] [CrossRef]
- Horowitz, K.M.; Ingardia, C.J.; Borgida, A.F. Anemia in pregnancy. Clin. Lab. Med. 2013, 33, 281–291. [Google Scholar] [CrossRef]
- Albright, C.M.; Wenstrom, K.D. Malignancies in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 33, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Eibye, S.; Kjær, S.K.; Mellemkjær, L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013, 122, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.V.; Al-Sehli, H.; Parrish, J.; D’Souza, R. Breast cancer in pregnancy: A retrospective cohort study. Gynecol. Obstet. Invest. 2019, 84, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Berveiller, P.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Tica, A.A.; Tica, O.S.; Saftoiu, A.; Camen, D.; Tica, V.I. Large pancreatic mucinous cystic neoplasm during pregnancy: What should be done? Gynecol. Obstet. Invest. 2013, 75, 132–138. [Google Scholar] [CrossRef]
- Nilsson, L.N.; Keane, M.G.; Shamali, A.; Bocos, J.M.; van Zanten, M.M.; Antila, A.; Gil, C.V.; Del Chiaro, M.; Laukkarinen, J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology 2016, 16, 1028–1036. [Google Scholar] [CrossRef]
- Löhr, J.M.; Hackert, T. Zystische pankreasneoplasie—Eine interdisziplinäre herausforderung. Gastroenterology 2018, 13, 444–449. [Google Scholar] [CrossRef]
- Testini, M.; Gurrado, A.; Lissidini, G.; Venezia, P.; Greco, L.; Piccinni, G. Management of mucinous cystic neoplasms of the pancreas. World J. Gastroenterol. 2010, 16, 5682–5692. [Google Scholar] [CrossRef] [PubMed]
- Revoredo, F.; de Vinatea, J.; Reaño, G.; Villanueva, L.; Kometter, F.; Arenas, J.; Polanco, P.M. Mucinous cystic neoplasms of the pancreas associated with pregnancy: Two case reports. Medicine 2020, 99, e21471. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Ferreira, T.; Frutuoso, L.; Matos, L.; Castro, T.; Rodrigues, D.; Oliveira, V.; Gonçalves, G.; Nora, M.; Scigliano, H. Large mucinous cystic neoplasm of the pancreas during pregnancy: A case report. J. Surg. Case Rep. 2020, 2020, rjaa085. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.K.; Tan, Y.M.; Chung, Y.F.; Chow, P.K.; Cheow, P.C.; Wong, W.K.; Ooi, L.L. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: Clinicopathological features of 344 patients. World J. Surg. 2006, 30, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.; Becker, R.C.; Przygodzki, R.M.; Adair, C.F.; Heffess, C.S. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: A clinicopathologic study of 130 cases. Am. J. Surg. Pathol. 1999, 23, 1–16. [Google Scholar] [CrossRef]
- Li, W.H.; Cheuk, E.C.; Kowk, P.C.; Cheung, M.T. Gastrointestinal bleeding in a pregnant woman: Mucinous cystic neoplasm of pancreas mimicking gastrointestinal stromal tumor of stomach. J. Hepatobiliary Pancreat. Surg. 2009, 16, 681–683. [Google Scholar] [CrossRef]
- Tanaka, M.; Chari, S.; Adsay, V.; Castillo, F.D.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef]
- Wenig, B.M.; Albores-Saavedra, J.; Buetow, P.C.; Heffess, C.S. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: A report of three cases. Am. J. Surg. Pathol. 1997, 21, 70–80. [Google Scholar] [CrossRef]
- WHO. Classification of Tumors Editorial Board. Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 319–321.
- Esposito, I.; Schlitter, A.M.; Sipos, B.; Klöppel, G. Classification and malignant potential of pancreatic cystic tumors. Pathologe 2015, 36, 94–99. [Google Scholar] [CrossRef]
- Kato, M.; Kubota, K.; Kita, J.; Shimoda, M.; Rokkaku, K.; Inaba, N.; Fukasawa, I.; Honma, K. Huge mucinous cystadenoma of the pancreas developing during pregnancy: A case report. Pancreas 2005, 30, 186–188. [Google Scholar] [CrossRef]
- Naganuma, S.; Honda, K.; Noriki, S.; Kimura, S.; Murakami, M.; Koneri, K.; Katayama, K.; Yamaguchi, A.; Itoh, H. Ruptured mucinous cystic neoplasm with an associated invasive carcinoma of pancreatic head in a pregnant woman: Report of a case and review of literature. Pathol. Int. 2011, 61, 28–33. [Google Scholar] [CrossRef]
- Fernández, E.M.; Malagón, A.M.; Gonzalez, I.A.; Montes, J.R.; Luis, H.D.; Hermoso, F.G.; Pallares, Á.C. Mucinous cystic neoplasm of the pancreas during pregnancy: The importance of proper management. J. Hepatobiliary Pancreat. Surg. 2005, 12, 494–497. [Google Scholar] [CrossRef]
- Babiker, H.; Hoilat, G.; Recio-Boiles, A. Mucinous Cystic Pancreatic Neoplasms; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Yamao, K.; Yanagisawa, A.; Takahashi, K.; Kimura, W.; Doi, R.; Fukushima, N.; Ohike, N.; Shimizu, M.; Hatori, T.; Nobukawa, B.; et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: A multi-institutional study of the Japan pancreas society. Pancreas 2011, 40, 67–71. [Google Scholar] [CrossRef]
- Kosumi, K.; Takamori, H.; Hashimoto, D.; Tanaka, H.; Abe, S.; Nakahara, O.; Horino, K.; Baba, H. Mucinous cystic neoplasm of the pancreas activated during pregnancy. Surg. Case Rep. 2015, 1, 13. [Google Scholar] [CrossRef]
- Zamboni, G.; Capelli, P.; Pesci, A.; Brighenti, A. Imaging of the Pancreas: Cystic and Rare Tumors; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Yeh, M.M.; Tang, L.H.; Wang, S.; Robert, M.E.; Zheng, W.; Jain, D. Inhibin expression in ovarian-type stroma in mucinous cystic neoplasms of the pancreas. Appl. Immunohistochem. Mol. Morphol. AIMM 2004, 12, 148–152. [Google Scholar] [CrossRef]
- Robles-Diaz, G.; Duarte-Rojo, A. Pancreas: A sex steroid-dependent tissue. ISR Med. Assoc. J. 2001, 3, 364–368. [Google Scholar]
- Verbeke, C.S.; Hauge, T. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Smithers, B.M.; Welch, C.; Goodall, P. Cystadenocarcinoma of the pancreas presenting in pregnancy. Br. J. Surg. 1986, 73, 591. [Google Scholar] [CrossRef]
- Baiocchi, C.; Landonio, G.; Majno, M.; Scanzi, F.; Ghislandi, E. Pancreatic cystadenocarcinoma and pregnancy: A case report. Tumori 1990, 76, 294–295. [Google Scholar] [CrossRef]
- Olsen, M.; Greer, M.; Feintuch, T. Pancreatic mucinous cystadenoma during pregnancy. Am. J. Gynecol. Heal 1993, 4, 27–30. [Google Scholar]
- Ganepola, G.A.; Gritsman, A.Y.; Asimakopulos, N.; Yiengpruksawan, A. Are pancreatic tumors hormone dependent? A case report of unusual, rapidly growing pancreatic tumor during pregnancy, its possible relationship to female sex hormones, and review of the literature. Am. Surg. 1999, 65, 105–111. [Google Scholar] [PubMed]
- Kitagawa, H.; Okabayashi, T.; Nishimori, I.; Kobayashi, M.; Sugimoto, T.; Akimori, T.; Kohsaki, T.; Miyaji, E.; Onishi, S.; Araki, K. Rapid growth of mucinous cystic adenoma of the pancreas following pregnancy. Int. J. Gastrointest. Cancer 2006, 37, 45–48. [Google Scholar] [CrossRef]
- Herring, A.A.; Graubard, M.B.; Gan, S.I.; Schwaitzberg, S.D. Mucinous cystadenocarcinoma of the pancreas during pregnancy. Pancreas 2007, 34, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Ozden, S.; Haliloglu, B.; Ilter, E.; Akin, F.T.; Kebudi, A.; Peker, O. An extremely rare cause of acute abdomen in pregnancy: Ruptured pancreatic mucinous cystadenocarcinoma. Pancreas 2007, 34, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Berindoague, R.; Targarona, E.; Savelli, A.; Pernas, J.; Lloreta, J. Mucinous cystadenocarcinoma of the pancreas diagnosed in postpartum. Langenbeck Arch. Surg. 2007, 392, 493–496. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hirashita, T.; Kinoshita, H.; Kitano, M.; Matsuo, S.; Matsumata, T.; Kitano, S. Large mucinous cystadenoma of the pancreas during pregnancy: Report of a case. Surg. Today 2007, 37, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, S.; Aihara, T.; Yasui, C.; Iida, H.; Yanagi, H.; Mitsunobu, M.; Kakuno, A.; Yamanaka, N. Large mucinous cystic neoplasm of the pancreas associated with pregnancy. World J. Gastroenterol. 2008, 14, 7252–7255. [Google Scholar] [CrossRef] [PubMed]
- Hakamada, K.; Miura, T.; Kimura, A.; Nara, M.; Toyoki, Y.; Narumi, S.; Sasak, M. Anaplastic carcinoma associated with a mucinous cystic neoplasm of the pancreas during pregnancy: Report of a case and a review of the literature. World J. Gastroenterol. 2008, 14, 132–135. [Google Scholar] [CrossRef]
- Wiseman, J.E.S.; Yamamoto, M.; Nguyen, T.D.; Bonadio, J.; Imagawa, D.K. Cystic pancreatic neoplasm in pregnancy: A case report and review of the literature. Arch. Surg. 2008, 143, 84–86. [Google Scholar] [CrossRef]
- Shirakawa, S.; Matsumoto, I.; Nakayama, S.; Mukubo, H.; Toyama, H.; Shinzeki, M.; Fukumoto, T.; Ajiki, T.; Ku, Y. Mucinous cystic neoplasm of the pancreas associated with pregnancy: Report of two cases. Nihon Shokakibyo Gakkai Zasshi 2010, 107, 1828–1834. [Google Scholar] [PubMed]
- Asciutti, S.; Kanninen, T.T.; Clerici, G.; Nardi, E.; Castellani, D.; DI Renzo, G.C.; Clerici, C. Acute pancreatitis with a mucinous cystoadenoma of the pancreas in pregnancy. Anticancer Res. 2010, 30, 1025–1028. [Google Scholar]
- Coral, R.P.; Coral, R.V.; De Bacco, F.W.; Rinaldi, N.; Zettler, C.G. Cistoadenoma mucinoso de pâncreas durante a gravidez. Rev. AMRIGS 2010, 54, 328–330. [Google Scholar]
- Martins Filho, E.D.; Lima, T.; Jaques, R.P.M.; Guerra, G.V.; Adeodato, L.; Kreimer, F. Mucinous cystic neoplasm of the pancreas during pregnancy: Case report. Rev. Bras. Saúde Matern. Infant. 2011, 11, 187–190. [Google Scholar] [CrossRef]
- Boyd, C.A.; Benarroch-Gampel, J.; Kilic, G.; Kruse, E.J.; Weber, S.M.; Riall, T.S. Pancreatic neoplasms in pregnancy: Diagnosis, complications, and management. J. Gastrointest. Surg. 2012, 16, 1064–1071. [Google Scholar] [CrossRef]
- Iusco, D.R.; Navarra, G.; Bonomi, S.; Grassi, A.; Vicari, S.; Virzì, S. Pancreatic large mucinous cystoadenoma with invasive ductal carcinoma in pregnancy. Case report. G. Chir. 2012, 33, 163–167. [Google Scholar] [PubMed]
- Tsuda, H.; Kotani, T.; Sumigama, S.; Mano, Y.; Shimoyama, Y.; Kikkawa, F. Mucinous cystic neoplasm of the pancreas with severe dysplasia during pregnancy: Case report and review of the literature. Taiwan J. Obstet. Gynecol. 2012, 51, 635–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urabe, K.; Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Sasaki, H.; Ohge, H.; Sueda, T. Clinicopathological features of 11 resected cases of mucinous cystic neoplasms of the pancreas. Suizo 2014, 29, 721–728. [Google Scholar] [CrossRef]
- Takashima, S.; Wato, M.; Inaba, T.; Mizukawa, S.; Izumikawa, K.; Ishikawa, S.; Miyoshi, M.; Kawai, K. A case of pancreatic mucinous cystic neoplasm that enlarged during pregnancy and was resected after childbirth. Nihon Shokakibyo Gakkai Zasshi 2014, 111, 1789–1797. [Google Scholar]
- Kleeff, J.; Holzapfel, K.; Steiger, K.; Esposito, I. Progression of a cystic pancreatic lesion during pregnancy. JOP 2015, 16, 394–396. [Google Scholar]
- Veits, L.; Deuerling, J.; Henneking, K.; Falkeis, C.; Sterlacci, W.; Vieth, M. Ungewöhnlicher fall einer muzinösen zystischen neoplasie des pankreasschwanzes bei einer schwangeren frau TT—Unusual case of pancreatic mucinous cystic neoplasia in a pregnant woman. Endosk Heute 2015, 28, 192–194. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farahmandi, S.; Elessawy, M.; Bauerschlag, D.O.; Pecks, U.; Abdullazade, S.; Beckmann, J.H.; Heilmann, T.; Rumpf, A.-L.; Maass, N.; Jansen, P.; et al. Mucinous Cystic Neoplasm of Pancreas in a Pregnant Woman Presenting with Severe Anemia and Gastric Bleeding: Case Report and Review of the Literature. Healthcare 2021, 9, 540. https://doi.org/10.3390/healthcare9050540
Farahmandi S, Elessawy M, Bauerschlag DO, Pecks U, Abdullazade S, Beckmann JH, Heilmann T, Rumpf A-L, Maass N, Jansen P, et al. Mucinous Cystic Neoplasm of Pancreas in a Pregnant Woman Presenting with Severe Anemia and Gastric Bleeding: Case Report and Review of the Literature. Healthcare. 2021; 9(5):540. https://doi.org/10.3390/healthcare9050540
Chicago/Turabian StyleFarahmandi, Susan, Mohamed Elessawy, Dirk O. Bauerschlag, Ulrich Pecks, Samir Abdullazade, Jan Henrik Beckmann, Thorsten Heilmann, Anna-Lena Rumpf, Nicolai Maass, Peer Jansen, and et al. 2021. "Mucinous Cystic Neoplasm of Pancreas in a Pregnant Woman Presenting with Severe Anemia and Gastric Bleeding: Case Report and Review of the Literature" Healthcare 9, no. 5: 540. https://doi.org/10.3390/healthcare9050540
APA StyleFarahmandi, S., Elessawy, M., Bauerschlag, D. O., Pecks, U., Abdullazade, S., Beckmann, J. H., Heilmann, T., Rumpf, A.-L., Maass, N., Jansen, P., & Winkler, V. (2021). Mucinous Cystic Neoplasm of Pancreas in a Pregnant Woman Presenting with Severe Anemia and Gastric Bleeding: Case Report and Review of the Literature. Healthcare, 9(5), 540. https://doi.org/10.3390/healthcare9050540






