Effects of a Home-Based Pulmonary Rehabilitation Program in Patients with Chronic Obstructive Pulmonary Disease in GOLD B Group: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
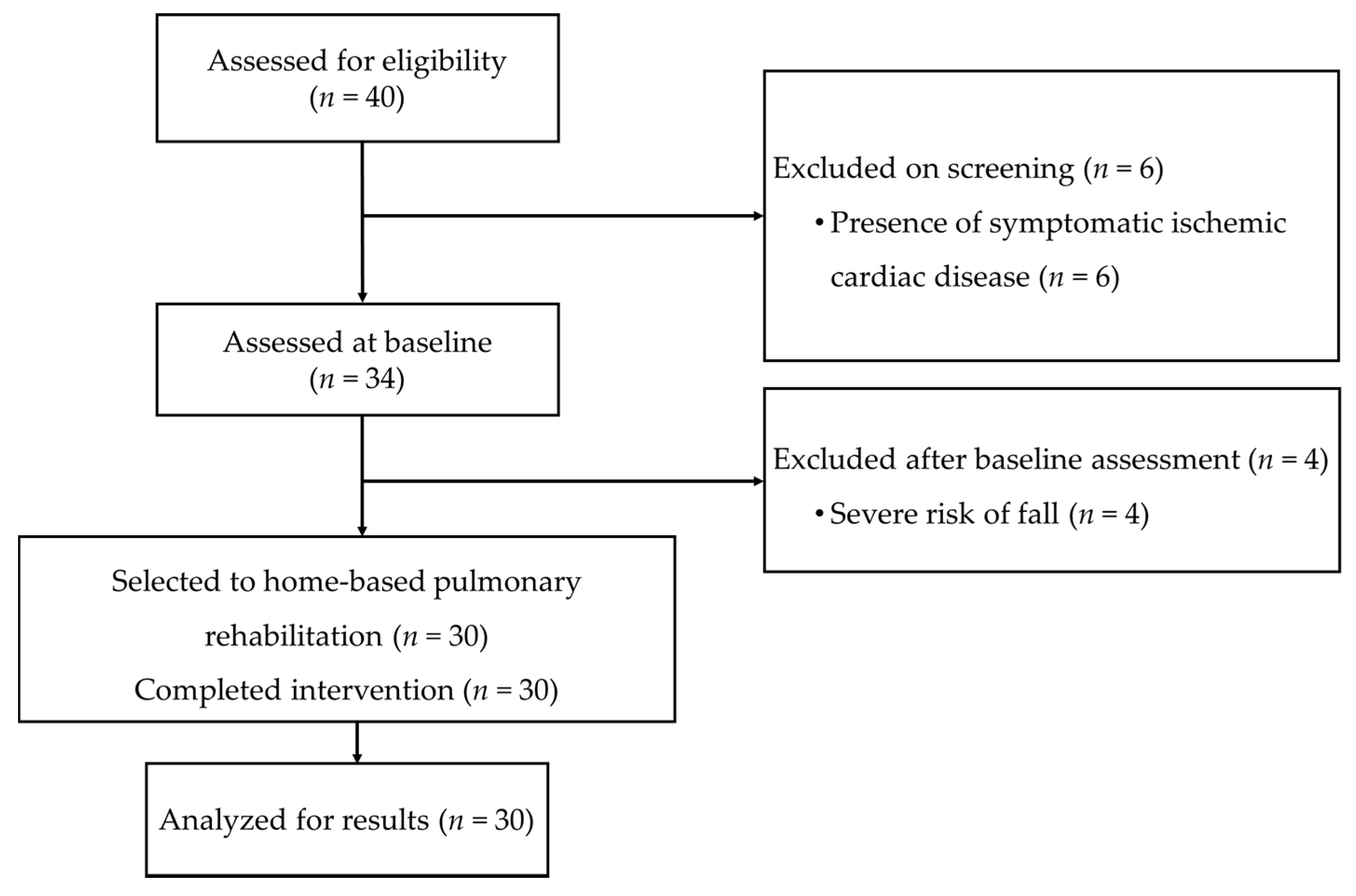
2.1. Study Design, Referrals, and Study Criteria
2.2. Sample Size
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Impact of the Home-Based Pulmonary Rehabilitation Program
3.2. Correlations between Changes in Outcomes after Home-Based Pulmonary Rehabilitation Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2, CD003793. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Mahal, A.; Hill, C.J.; Lee, A.L.; Burge, A.T.; Cox, N.S.; Moore, R.; Nicolson, C.; O’Halloran, P.; Lahham, A.; et al. Home-based rehabilitation for COPD using minimal resources: A randomised, controlled equivalence trial. Thorax 2017, 72, 57–65. [Google Scholar] [CrossRef]
- Maltais, F.; Bourbeau, J.; Shapiro, S.; Lacasse, Y.; Perrault, H.; Baltzan, M.; Hernandez, P.; Rouleau, M.; Julien, M.; Parenteau, S.; et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2008, 149, 869–878. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Wuytack, F.; Devane, D.; Stovold, E.; McDonnell, M.; Casey, M.; McDonnell, T.J.; Gillespie, P.; Raymakers, A.; Lacasse, Y.; McCarthy, B. Comparison of outpatient and home-based exercise training programmes for COPD: A systematic review and meta-analysis. Respirology 2018, 23, 272–283. [Google Scholar] [CrossRef]
- Neves, L.F.; Reis, M.H.; Gonçalves, T.R. Home or community-based pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Cad. Saude Publica 2016, 32. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Malaguti, C.; Hoffman, M.; Lahham, A.; Burge, A.T.; Dowman, L.; May, A.K.; Bondarenko, J.; Graco, M.; Tikellis, G.; et al. Home-based or remote exercise testing in chronic respiratory disease, during the COVID-19 pandemic and beyond: A rapid review. Chron Respir. Dis. 2020, 17, 1479973120952418. [Google Scholar] [CrossRef]
- Garvey, C.; Boylan, A.M.; Miller, D.L.; Holland, A.E.; Singh, S.J.; Spruit, M.A.; Wilson, K.C.; Thomson, C.C. Field walking tests in chronic respiratory disease. Ann. Am. Thorac. Soc. 2015, 12, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; de Bisschop, C.; Beaumont, M.; Ouksel, H.; Jean, V.; Dessables, F.; Chambellan, A. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int. J. Chronic Obs. Pulm. Dis. 2016, 11, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef]
- Ozalevli, S.; Ozden, A.; Itil, O.; Akkoclu, A. Comparison of the Sit-to-Stand Test with 6min walk test in patients with chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 286–293. [Google Scholar] [CrossRef]
- Caneiras, C.; Mayoralas-Alise, S.; Esteves, C.M.; Sampaio Silva, J.; Vilarinho, R.; Pinto, I.; Cantante, R.; Carvalho, J.; Sedeño, M.F.; Bourbeau, J.; et al. The First Nationwide Home-Based Pulmonary Rehabilitation Program for Chronic Obstructive Pulmonary Disease (COPD) Patients in Portugal: The Possible Combination of Patient’s Empowerment and Add Value to the System. In Proceedings of the 27th Congress of the European Association of Hospital Managers, Estoril, Lisbon, 26–28 September 2018; p. 14. [Google Scholar]
- Direção Geral da Saúde de Portugal. Orientação Técnica–Programas de Reabilitação Respiratória nos Cuidados de Saúde Primários. Orientação nº 014/2019 de 07/08/2019. Available online: https://www.dgs.pt/directrizes-da-dgs/orientacoes-e-circulares-informativas/orientacao-n-0142019-de-07082019.aspx (accessed on 1 March 2021).
- Bourbeau, J.; Julien, M.; Maltais, F.; Rouleau, M.; Beaupré, A.; Bégin, R.; Renzi, P.; Nault, D.; Borycki, E.; Schwartzman, K.; et al. Reduction of Hospital Utilization in Patients With Chronic Obstructive Pulmonary Disease: A Disease-Specific Self-management Intervention. Arch. Intern. Med. 2003, 163, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Clini, E.M. Measures of dyspnea in pulmonary rehabilitation. Multidiscip. Respir. Med. 2010, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Dodd, J.W.; Hogg, L.; Nolan, J.; Jefford, H.; Grant, A.; Lord, V.M.; Falzon, C.; Garrod, R.; Lee, C.; Polkey, M.I.; et al. The COPD assessment test (CAT): Response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011, 66, 425–429. [Google Scholar] [CrossRef]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–235; quiz 235–237. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Garrod, R.; Bestall, J.C.; Paul, E.A.; Wedzicha, J.A.; Jones, P.W. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: The London Chest Activity of Daily Living scale (LCADL). Respir. Med. 2000, 94, 589–596. [Google Scholar] [CrossRef]
- de Torres, J.P.; Pinto-Plata, V.; Ingenito, E.; Bagley, P.; Gray, A.; Berger, R.; Celli, B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002, 121, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.S.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Clark, A.L.; Dickson, M.J.; Haselden, B.M.; Polkey, M.I.; Man, W.D. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir. Med. 2014, 2, 195–203. [Google Scholar] [CrossRef]
- Puhan, M.A.; Frey, M.; Büchi, S.; Schünemann, H.J. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual. Life Outcomes 2008, 6, 46. [Google Scholar] [CrossRef]
- Bisca, G.W.; Proença, M.; Salomão, A.; Hernandes, N.A.; Pitta, F. Minimal detectable change of the London chest activity of daily living scale in patients with COPD. J. Cardiopulm. Rehabil. Prev. 2014, 34, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.Y.; Chien, C.W.; Hsueh, I.P.; Sheu, C.F.; Wang, C.H.; Hsieh, C.L. Developing a short form of the Berg Balance Scale for people with stroke. Phys. Ther. 2006, 86, 195–204. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Academic Press, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Correlation and Regression. Available online: https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression (accessed on 10 February 2021).
- World Health Assembly, 55. Diet, Physical Activity and Health: Report by the Secretariat. 2002. Available online: https://apps.who.int/iris/handle/10665/78471 (accessed on 10 February 2021).
- Main, E.; Denehy, L. Cardiorespiratory Physiotherapy: Adults and Paediatrics (E-Book); Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Spencer, L.M.; McKeough, Z.J. Maintaining the benefits following pulmonary rehabilitation: Achievable or not? Respirology 2019, 24, 909–915. [Google Scholar] [CrossRef]
- Mendes de Oliveira, J.C.; Studart Leitão Filho, F.S.; Malosa Sampaio, L.M.; Negrinho de Oliveira, A.C.; Hirata, R.P.; Costa, D.; Donner, C.F.; de Oliveira, L.V.F. Outpatient vs. home-based pulmonary rehabilitation in COPD: A randomized controlled trial. Multidiscip. Respir. Med. 2010, 5, 401. [Google Scholar] [CrossRef]
- Boxall, A.-M.; Barclay, L.; Sayers, A.; Caplan, G.A. Managing Chronic Obstructive Pulmonary Disease in the Community: A Randomized Controlled Trial of Home-Based Pulmonary Rehabilitation for Elderly Housebound Patients. J. Cardiopulm. Rehabil. Prev. 2005, 25, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Lahham, A.; McDonald, C.F.; Moore, R.; Cox, N.S.; Rawlings, S.; Nichols, A.; Liacos, A.; Holland, A.E. The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: A randomised controlled trial. Clin. Respir. J. 2020, 14, 335–344. [Google Scholar] [CrossRef]
- de Sousa Pinto, J.M.; Martín-Nogueras, A.M.; Calvo-Arenillas, J.I.; Ramos-González, J. Clinical Benefits of Home-Based Pulmonary Rehabilitation in Patients With Chronic Obstructive Pulmonary Disease. J. Cardiopulm. Rehabil. Prev. 2014, 34, 355–359. [Google Scholar] [CrossRef]
- Fernández, A.M.; Pascual, J.; Ferrando, C.; Arnal, A.; Vergara, I.; Sevila, V. Home-Based Pulmonary Rehabilitation in Very Severe COPD: IS IT SAFE AND USEFUL? J. Cardiopulm. Rehabil. Prev. 2009, 29, 325–331. [Google Scholar] [CrossRef]
- Güell, M.R.; de Lucas, P.; Gáldiz, J.B.; Montemayor, T.; Rodríguez González-Moro, J.M.; Gorostiza, A.; Ortega, F.; Bellón, J.M.; Guyatt, G. Home vs. hospital-based pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: A Spanish multicenter trial. Arch. Bronconeumol. 2008, 44, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, C.; Jin, Y.S.; Oh, Y.M.; Lee, S.D.; Yang, Y.J.; Park, Y.B. Effects of home-based pulmonary rehabilitation with a metronome-guided walking pace in chronic obstructive pulmonary disease. J. Korean Med. Sci. 2013, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Kawagoshi, A.; Kiyokawa, N.; Sugawara, K.; Takahashi, H.; Sakata, S.; Satake, M.; Shioya, T. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir. Med. 2015, 109, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Wijkstra, P.J.; van der Mark, T.W.; Kraan, J.; van Altena, R.; Koëter, G.H.; Postma, D.S. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). Eur. Respir. J. 1996, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Elaal, E.A.; Mehany, M.; Tolba, K. Home-based pulmonary rehabilitation program: Effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann. Thorac. Med. 2010, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Akinci, A.C.; Olgun, N. The Effectiveness of Nurse-Led, Home-Based Pulmonary Rehabilitation in Patients with COPD in Turkey. Rehabil. Nurs. 2011, 36, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Candemir, I.; Ergun, P.; Kaymaz, D.; Demir, N.; McCurdy, S.A. Comparison of unsupervised home-based pulmonary rehabilitation versus supervised hospital outpatient pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2019, 13, 1195–1203. [Google Scholar] [CrossRef]
- do Nascimento, E.S.; Sampaio, L.M.; Peixoto-Souza, F.S.; Dias, F.D.; Gomes, E.L.; Greiffo, F.R.; Ligeiro de Oliveira, A.P.; Stirbulov, R.; Vieira, R.P.; Costa, D. Home-based pulmonary rehabilitation improves clinical features and systemic inflammation in chronic obstructive pulmonary disease patients. Int. J. Chronic Obs. Pulm. Dis. 2015, 10, 645–653. [Google Scholar] [CrossRef]
- Horton, E.J.; Mitchell, K.E.; Johnson-Warrington, V.; Apps, L.D.; Sewell, L.; Morgan, M.; Taylor, R.S.; Singh, S.J. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: A randomised non-inferiority trial. Thorax 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Vilarinho, R.; Caneiras, C.; Montes, A.M. Measurement properties of step tests for exercise capacity in COPD: A systematic review. Clin. Rehabil. 2020, 35, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Meys, R.; Stoffels, A.A.F.; Houben-Wilke, S.; Janssen, D.J.A.; Burtin, C.; van Hees, H.W.H.; Franssen, F.M.E.; van den Borst, B.; Wouters, E.F.M.; Spruit, M.A.; et al. Association between patient-reported outcomes and exercise test outcomes in patients with COPD before and after pulmonary rehabilitation. Health Qual. Life Outcomes 2020, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.L.; Nyberg, A.; Maltais, F.; Saey, D. Functional Tests in Chronic Obstructive Pulmonary Disease, Part 1: Clinical Relevance and Links to the International Classification of Functioning, Disability, and Health. Ann. Am. Thorac. Soc. 2017, 14, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Coke, T.; Alday, R.; Biala, K.; Luna, S.; Martines, P. The new role of physical therapy in home care. Home Healthc. Nurse 2005, 23, 594–599. [Google Scholar] [CrossRef]
- Resqueti, V.R.; Gorostiza, A.; Gáldiz, J.B.; De Santa María, E.L.; Clarà, P.C.; Güell Rous, R. Beneficios de un programa de rehabilitación respiratoria domiciliaria en pacientes con EPOC grave. Arch. Bronconeumol. 2007, 43, 599–604. [Google Scholar] [CrossRef]
- Grosbois, J.M.; Heluain-Robiquet, J.; Machuron, F.; Terce, G.; Chenivesse, C.; Wallaert, B.; Le Rouzic, O. Influence Of Socioeconomic Deprivation On Short- And Long-Term Outcomes Of Home-Based Pulmonary Rehabilitation In Patients With Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obs. Pulm. Dis. 2019, 14, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Lahham, A.; McDonald, C.F.; Mahal, A.; Lee, A.L.; Hill, C.J.; Burge, A.T.; Cox, N.S.; Moore, R.; Nicolson, C.; O’Halloran, P.; et al. Home-based pulmonary rehabilitation for people with COPD: A qualitative study reporting the patient perspective. Chronic Respir. Dis. 2018, 15, 123–130. [Google Scholar] [CrossRef]
| Characteristics | n = 30 |
|---|---|
| Age, years | 71.6 ± 9.4 |
| Sex, male/female (%) | 12/18 (40.0/60.0) |
| BMI, kg/m2 | 26.4 ± 5.3 |
| GOLD stages, n (%) | |
| I | 2 (6.6) |
| II | 11 (36.7) |
| III | 16 (53.4) |
| IV | 1 (3.3) |
| FEV1, %predicted | 52.8 ± 18.3 |
| FEV1/FVC (%) | 50.6 ± 11.9 |
| Noninvasive ventilation, n (%) | 9 (30.0) |
| Long-term oxygen therapy, n (%) | 9 (30.0) |
| Comorbid illness, n (%) | |
| Coronary artery disease | 5 (17) |
| Arrhythmia | 6 (20) |
| Hypertension | 11 (37) |
| Diabetes | 6 (20) |
| Musculoskeletal | 11 (37) |
| Medication, n (%) | |
| SABA | 5 (17) |
| LABA | 4 (13) |
| SAMA | 4 (13) |
| LAMA | 9 (30) |
| LABA + LAMA | 11 (37) |
| LABA + ICS | 11 (37) |
| LABA + LAMA + ICS | 2 (7) |
| ICS | 11 (37) |
| Xanthines | 11 (37) |
| Heart rate at rest (bpm) | 76.7 ± 12.1 |
| Systolic BP at rest (mmHg) | 133.6 ± 15.7 |
| Diastolic BP at rest (mmHg) | 73.3 ± 11.3 |
| SpO2 at rest (%) | 94.1 ± 2.8 |
| Variables (n = 30) | Pre-HBPR | Post-HBPR | p Value | ES |
|---|---|---|---|---|
| 1MSTS (repetitions) | 17.2 ± 4.4 | 21.2 ± 5.3 | <0.001 | 1.137 |
| Dyspnea (mBorg) | 1.0 [0.0; 3.0] | 0.0 [0.0; 0.3] | 0.002 | −0.569 |
| Fatigue (mBorg) | 0.0 [0.0; 1.3] | 0.0 [0.0; 0.0] | 0.048 | −0.361 |
| mMRC (score) | 2.0 [1.0; 2.5] | 1.0 [1.0; 2.0] | 0.010 | −0.468 |
| CAT (score) | 16.3 ± 4.9 | 9.9 ± 5.2 | <0.001 | −1.020 |
| HADS (score) | 14.4 ± 5.8 | 9.6 ± 5.8 | 0.001 | −0.734 |
| HADS A (score) | 7.8 ± 4.2 | 5.1 ± 3.4 | <0.001 | −0.766 |
| HADS D (score) | 6.6 ± 2.8 | 4.6 ± 3.1 | 0.009 | −0.531 |
| LCADL (score) | 21.0 ± 7.4 | 15.8± 3.3 | <0.001 | −0.743 |
| Outcomes | ∆1MSTS | ∆mMRC | ∆CAT | ∆HADS | ∆LCADL |
|---|---|---|---|---|---|
| ∆1MSTS | --- | NS | −0.48 * | NS | NS |
| ∆mMRC | --- | --- | 0.51 ** | 0.62 ** | 0.55 ** |
| ∆CAT | --- | --- | --- | 0.66 *** | NS |
| ∆HADS | --- | --- | --- | --- | NS |
| ∆LCADL | --- | --- | --- | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarinho, R.; Serra, L.; Coxo, R.; Carvalho, J.; Esteves, C.; Montes, A.M.; Caneiras, C. Effects of a Home-Based Pulmonary Rehabilitation Program in Patients with Chronic Obstructive Pulmonary Disease in GOLD B Group: A Pilot Study. Healthcare 2021, 9, 538. https://doi.org/10.3390/healthcare9050538
Vilarinho R, Serra L, Coxo R, Carvalho J, Esteves C, Montes AM, Caneiras C. Effects of a Home-Based Pulmonary Rehabilitation Program in Patients with Chronic Obstructive Pulmonary Disease in GOLD B Group: A Pilot Study. Healthcare. 2021; 9(5):538. https://doi.org/10.3390/healthcare9050538
Chicago/Turabian StyleVilarinho, Rui, Lúcia Serra, Ricardo Coxo, João Carvalho, Cátia Esteves, António Mesquita Montes, and Cátia Caneiras. 2021. "Effects of a Home-Based Pulmonary Rehabilitation Program in Patients with Chronic Obstructive Pulmonary Disease in GOLD B Group: A Pilot Study" Healthcare 9, no. 5: 538. https://doi.org/10.3390/healthcare9050538
APA StyleVilarinho, R., Serra, L., Coxo, R., Carvalho, J., Esteves, C., Montes, A. M., & Caneiras, C. (2021). Effects of a Home-Based Pulmonary Rehabilitation Program in Patients with Chronic Obstructive Pulmonary Disease in GOLD B Group: A Pilot Study. Healthcare, 9(5), 538. https://doi.org/10.3390/healthcare9050538






