Actuarial Analysis of Survival among Breast Cancer Patients in Lithuania
Abstract
1. Introduction
2. Some Notations and Mathematical Preliminaries
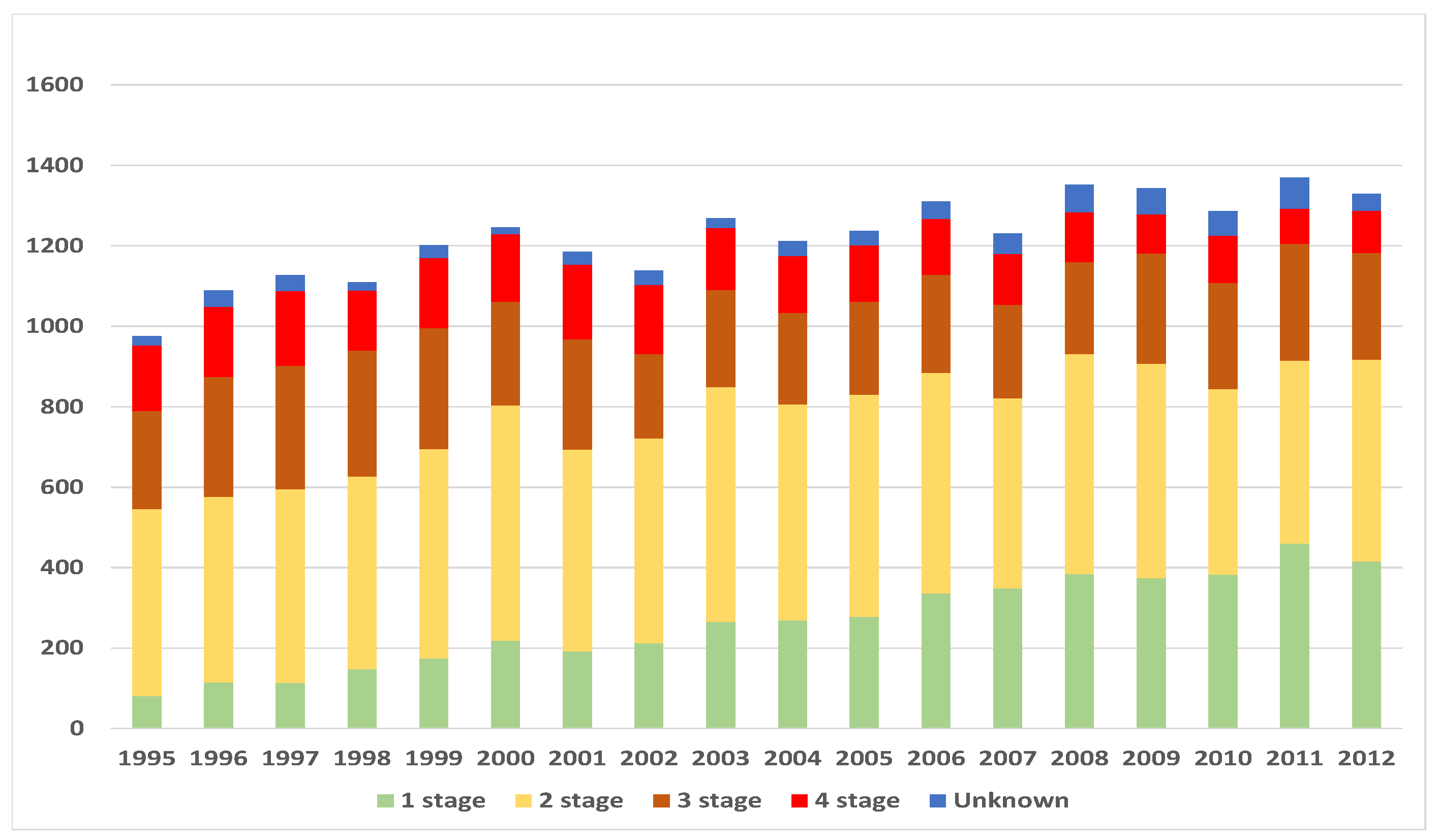
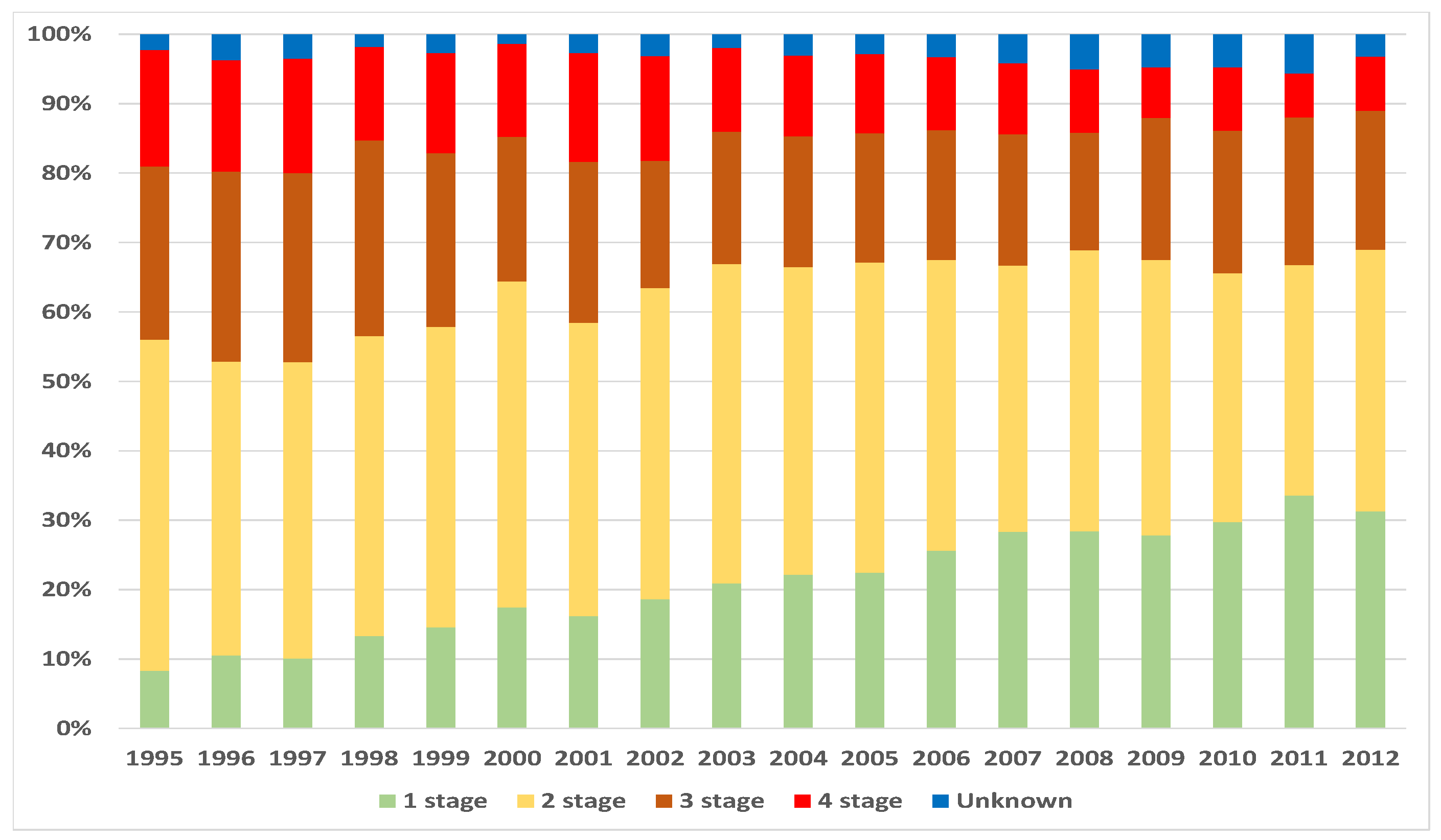
3. Data and Methodology
3.1. Exposure to Risk and Central Mortality Rate
3.2. Kaplan–Meier Method
- It is suitable for data sets with limited number of cases. Otherwise, using this method may become time consuming. For our data we used an Excel spreadsheet and built-in VBA programming language for this reason.
- It is very suitable for medical trials when time since onset of disease is more important than just the age of the patient, so it is difficult to apply standard actuarial procedures for construction of life tables.
- It is non-parametric, so no advance assumptions about analytic form of survival function are required, nor do parameters need to be estimated. Despite being non-parametric, it is still a statistical estimator, so standard error and confidence intervals may be calculated.
- The estimate for death probability is obtained. Interval h may be as short as one day, e.g., . Hence, the Kaplan–Meier method is suitable for estimation of death probabilities during quite a short period without making any assumptions about distribution of deaths within one year. Moreover, interval h may differ for different subintervals and is not determined a priori, but is based on data under investigation.
4. Main Results
4.1. Exposure to Risk and Central Mortality Rate
4.2. Kaplan–Meier Method
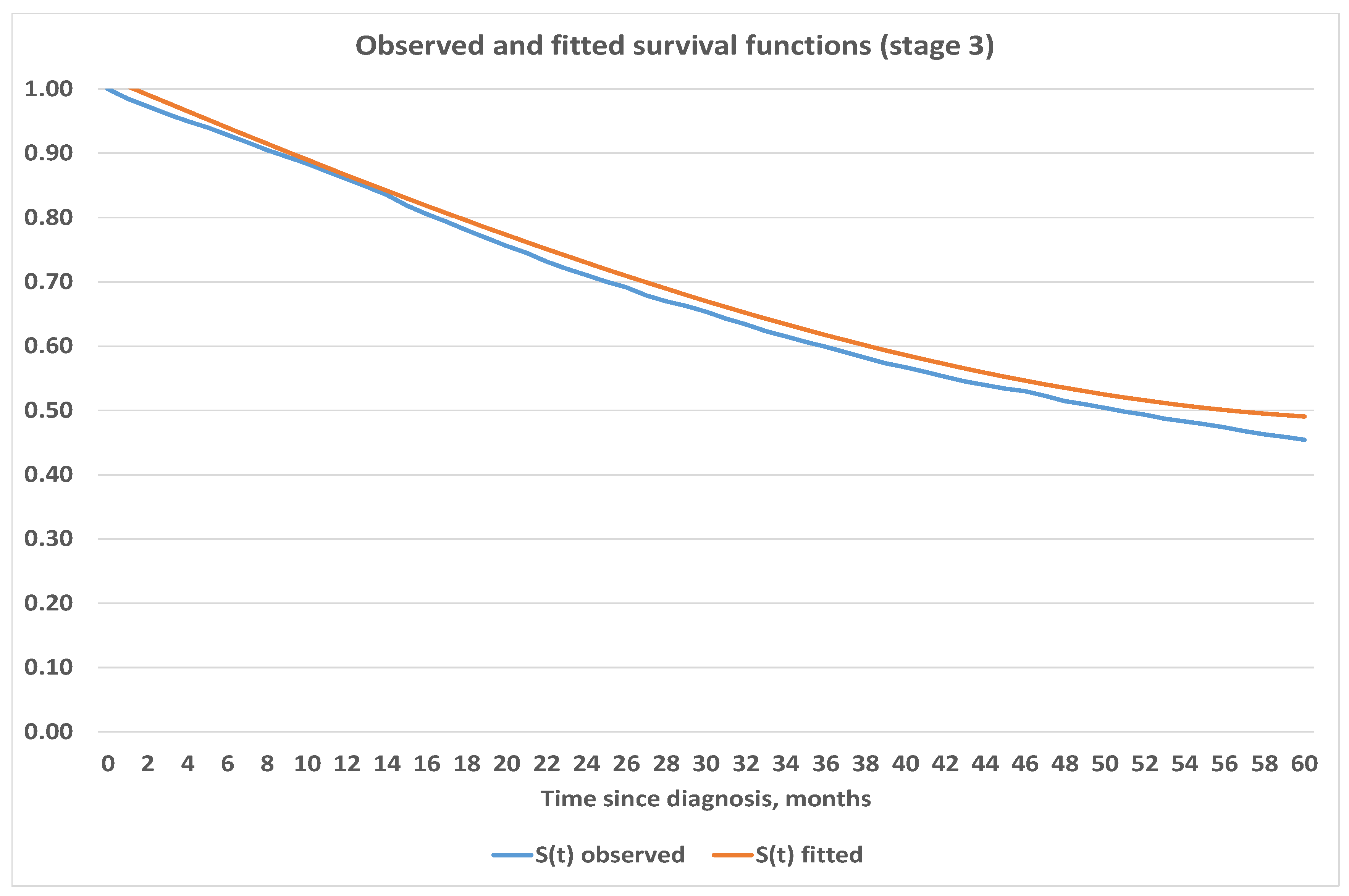
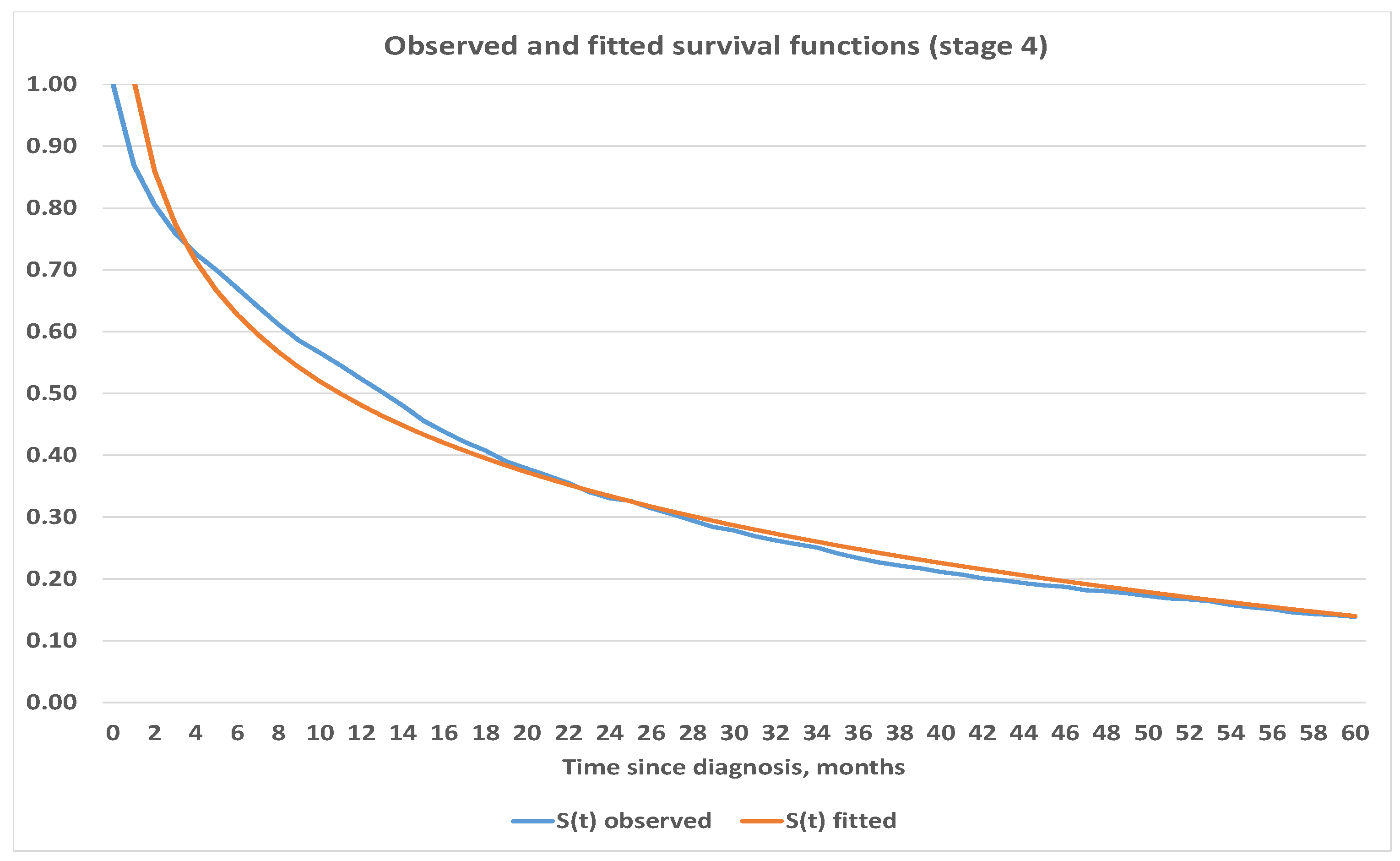
4.3. Construction of Analytic Survival Functions
5. Applications, Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer in Lithuania during 2012 (Lithuanian) [Vėžys Lietuvoje 2012 Metais]. Annual report of Lithuanian Cancer Registry. 2012. Available online: https://www.nvi.lt/uploads/pdf/Vezio%20registras/Vezys_lietuvoje_2012.pdf (accessed on 20 April 2020).
- Pyenson, B.S.; Sander, M.S.; Jiang, Y.; Kahn, H.; Mulshine, J.L. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff. 2012, 31, 770–779. [Google Scholar] [CrossRef]
- Dieguez, G.; Ferro, C.; Pyenson, B.S. A Multi-Year Look at the Cost Burden of Cancer Care. Milliman Research Report. 2017. Available online: https://www.milliman.com/en/insight/2017/a-multi-year-look-at-the-cost-burden-of-cancer-care (accessed on 20 April 2020).
- Fisher, S.; Gao, H.; Yasui, Y.; Dabbs, K.; Winget, M. Survival in stage I-III breast cancer patiens by surgical treatment in a publicly funded health care system. Ann. Oncol. 2015, 26, 1161–1169. [Google Scholar]
- Giordano, S.H.; Buzdar, A.U.; Smith, T.L.; Kau, S.W.; Yang, Y.; Hortobagyi, G.N. Is breast cancer survival improving? Cancer 2004, 100, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Iqbal, J.; Giannakeas, V.; Sopik, V.; Sun, P. Breast cancer mortality after a diagnosis of ductal carcinoma in silico. JAMA Oncol. 2015, 1, 888–896. [Google Scholar] [CrossRef]
- Narod, S.A.; Giannakeas, V.; Sopik, V. Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Res. Treat. 2018, 172, 659–669. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chan, C.H.; Lim, Y.B.; Yang, S.F.; Yeh, L.T.; Wang, Y.H.; Chou, M.C.; Yeh, C.B. Risk at breast cancer in women with mastitis: A retrospective population-based cohort study. Medicina 2020, 56, 372. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Narod, S.A. A generalizable relationship beetween mortality and time-to-death among breast cancer patients can be explained by tumour dormancy. Breast Cancer Res. Treat. 2019, 177, 691–703. [Google Scholar] [CrossRef]
- Seung, S.J.; Traore, A.N.; Pourmirza, B.; Fathers, K.E.; Coombes, M.; Jerzak, K.J. A population-based analysis of breast cancer incidence and survival by subtypes in Ontario women. Curr. Oncol. 2020, 27, 191–198. [Google Scholar] [CrossRef]
- Tyuryumina, E.V.; Neznanov, A.A.; Turumin, J.L. A mathematical model to predict Diagnostic periods for secondary distant metastasis in Patients ER/PR/HER2/Ki-67 subtypes of breast cancer. Cancers 2020, 12, 2344. [Google Scholar] [CrossRef]
- Smith, T.J. Breast cancer surveillance guidelines. J. Oncol. Pract. 2013, 9, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Soldato, D.; Rubagotti, A.; Zinoli, L.; Zanardi, E.; Barboro, P.; Messina, C.; Castro, E.; Olmos, D.; Boccardo, F. Epidemiological characteristics and survival in patients with de Novo Metastatic prostate cancer. Cancers 2020, 12, 2855. [Google Scholar] [CrossRef]
- Das, S.; Camphausen, K.; Shankavaram, V. Cancer-specific immune prognostic signature in solid tumor and its relation to immune checkpoint therapies. Cancers 2020, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Devos, G.; Berghen, C.; Van Eecke, H.; Vander Stichele, A.; Van Poppel, H.; Goffin, K.; Mai, C.; De Wever, L.; Albersen, M.; Everaerts, W.; et al. Oncological outcomes of metastasis-directed therapy in oligorecurrent prostate cancer patients following radical prostatectomy. Cancers 2020, 12, 2271. [Google Scholar] [CrossRef]
- Frank, F.; Hecht, M.; Loy, F.; Rutzner, S.; Fietkau, R.; Distel, L. Differences in and prognostic value of quality of live data in rectal cancer patients with and without distant metastases. Healtcare 2021, 9, 1. [Google Scholar]
- Garsia-Velasco, A.; Zacarias-Pons, L.; Texidor, H.; Valeros, M.; Liñan, L.; Carmona-Garsia, M.C.; Puigdemont, M.; Carbajal, W.; Guardeño, R.; Malats, N.; et al. Incidence and survival trends of pancreatic cancer in Girona: Impact of the change in patient care in the last 25 years. Int. J. Environ. Rec. Public. Health 2020, 17, 9538. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.F.; Owen, T.; Griffiths, R.J.; Brennan, K. Does the frequency of routine follow-up after curative treatement for head and neck cancer affect survival. Curr. Oncol. 2019, 26, 295–306. [Google Scholar] [CrossRef]
- Hendricks, A.; Amallraja, A.; Meißner, T.; Forster, P.; Rosenstiel, P.; Burmeister, G.; Schafmayer, C.; Franke, A.; Hinz, S.; Forster, M.; et al. Stage IV colorectal cancer patients with high risk mutation survived 16 months longer with individualized therapies. Cancers 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Lin, S.K.; Hsu, R.J.; Pang, S.T.; Chuang, C.K.; Chang, Y.H.; Liu, J.M. Spirit-quieting traditional chinese medicine may improve survival in prostate cancer patients with depression. J. Clin. Med. 2019, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; O’Brien, P.; Zhao, Y.; Hopman, W.M.; Lamond, N.; Ramjeesingh, R. Adjuvant treatment in older patients with rectal cancer: A population based review. Curr. Oncol. 2018, 25, 499–506. [Google Scholar] [CrossRef]
- Luo, S.D.; Chen, W.C.; Wu, C.N.; Yang, Y.H.; Li, S.H.; Fang, F.M.; Huang, T.L.; Wang, Y.M.; Chiu, T.J.; Wu, S.C. Low dose aspirin use significantly improves the survival of late-stage NPC: A propensity score-matched cohort study in Taiwan. Cancers 2020, 12, 1551. [Google Scholar] [CrossRef]
- Mariani, P.; Dureau, S.; Savignoni, A.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Piperno-Neumann, S.; Rodrigues, M.J.; Desjardins, L.; Cassoux, N.; Servois, V. Development of a prognostic nomogram for liver metastasis of uveal melanoma patients selected by liver MRI. Cancers 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Nemes, S.; Bülow, E.; Gustavsson, A. A brief overview of restricted mean survival time estimators and associated variances. Stats 2020, 3, 107–119. [Google Scholar] [CrossRef]
- Stahl, K.A.; Olecki, E.J.; Dixon, M.E.; Peng, J.S.; Torres, M.B.; Gusani, N.J.; Shen, C. Gastric cancer treatments and survival trends in the United States. Curr. Oncol. 2021, 28, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef]
- National Cancer Institute’s Cancer Registry. Available online: https://www.nvi.lt/cancerregistry/ (accessed on 5 September 2019).
- Pitacco, E. Health Insurance. Basic Actuarial Models; EAA Series; Springer: New York, NY, USA, 2014. [Google Scholar]
- Macdonald, A.S.; Richards, S.J.; Currie, I.D. Modelling Mortality with Actuarial Applications; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Steponavičiene, L.; Vincerževskienė, I.; Briedienė, R.; Urbonas, V. Vansevičiūtė-Petkevičiene, R.; Smailytė, G. Breast cancer screening program in Lithuania: Interval cancers and program sensitivity after 7 years of mammography screening. Cancer Control 2017, 26, 1–7. [Google Scholar]
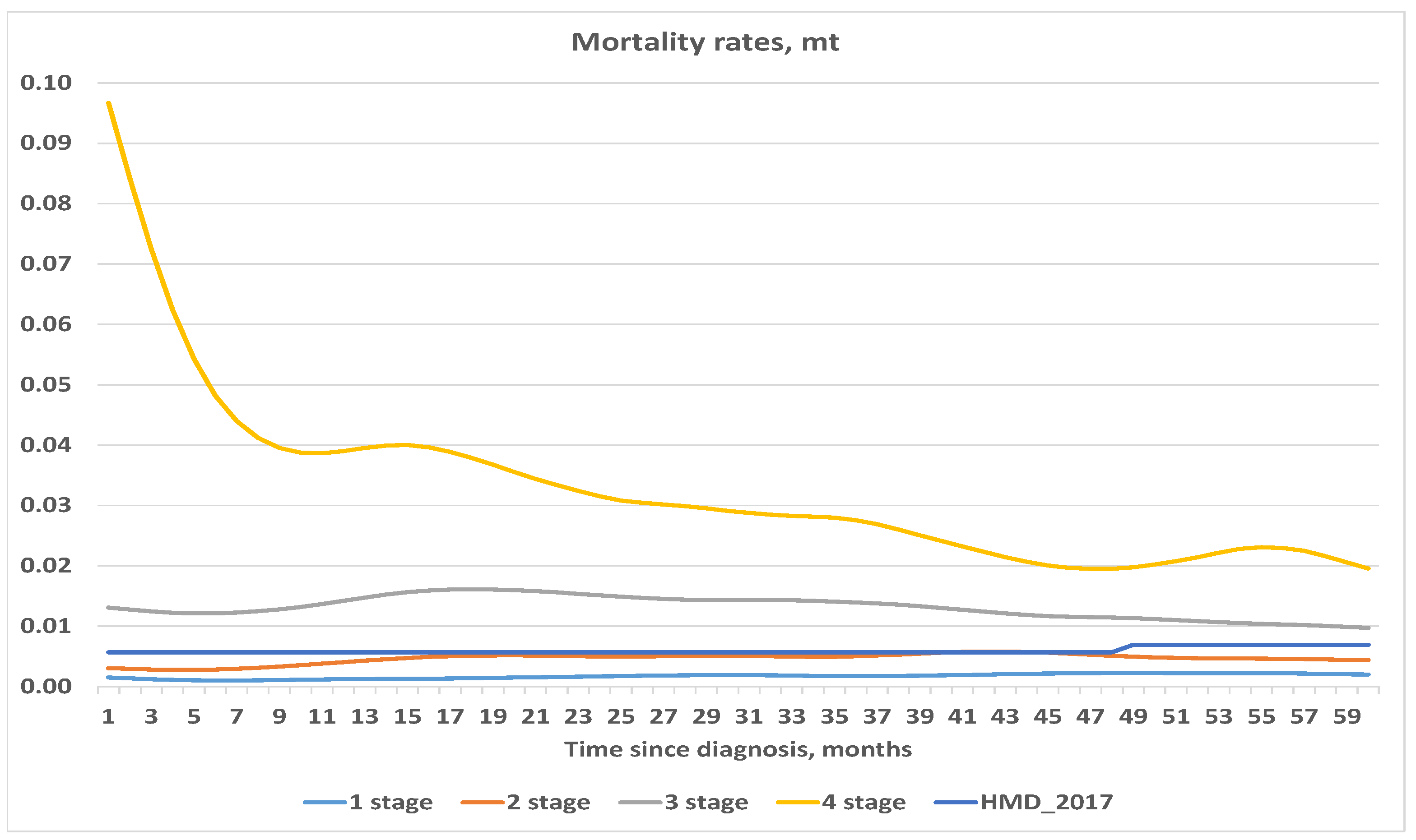
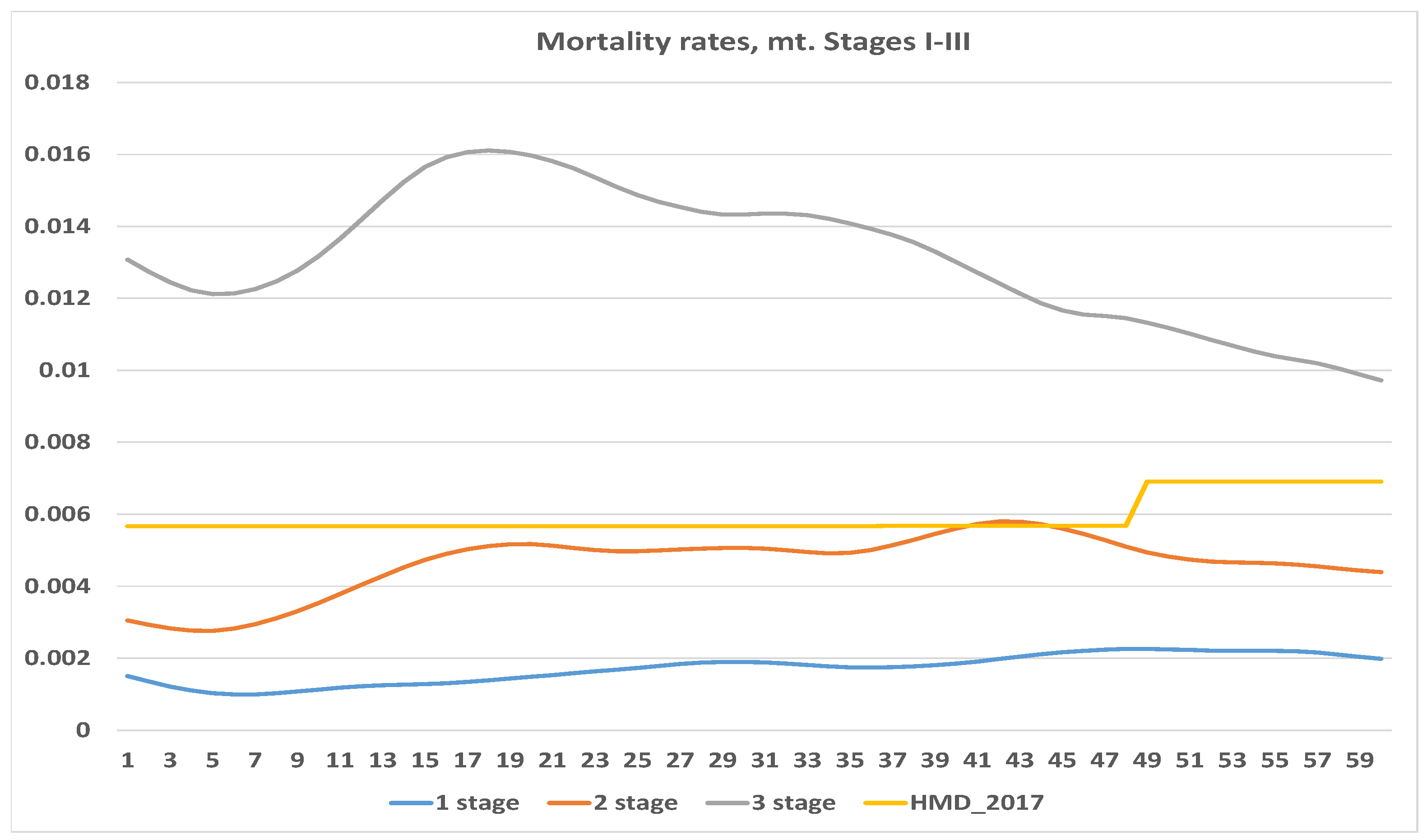

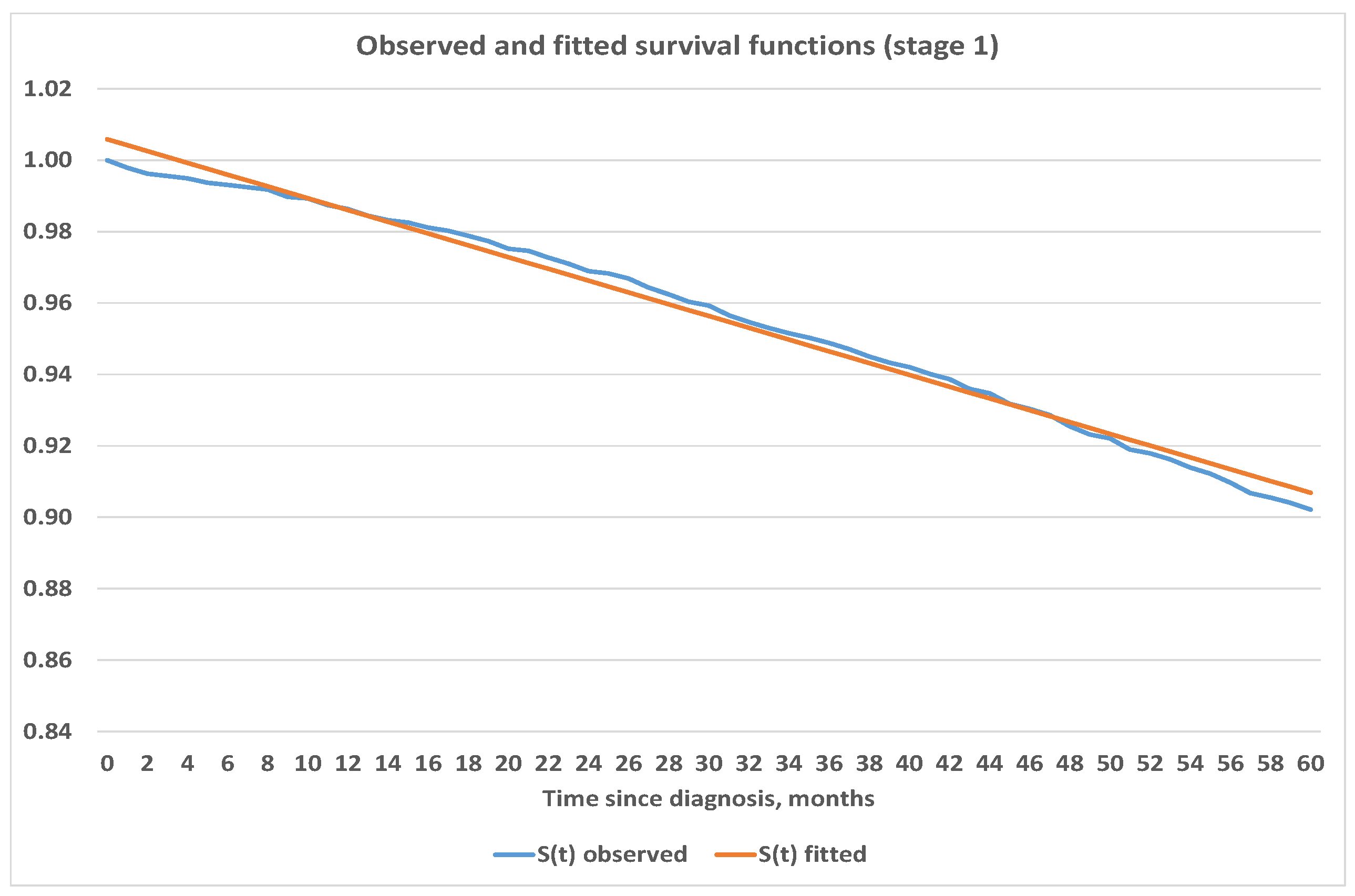
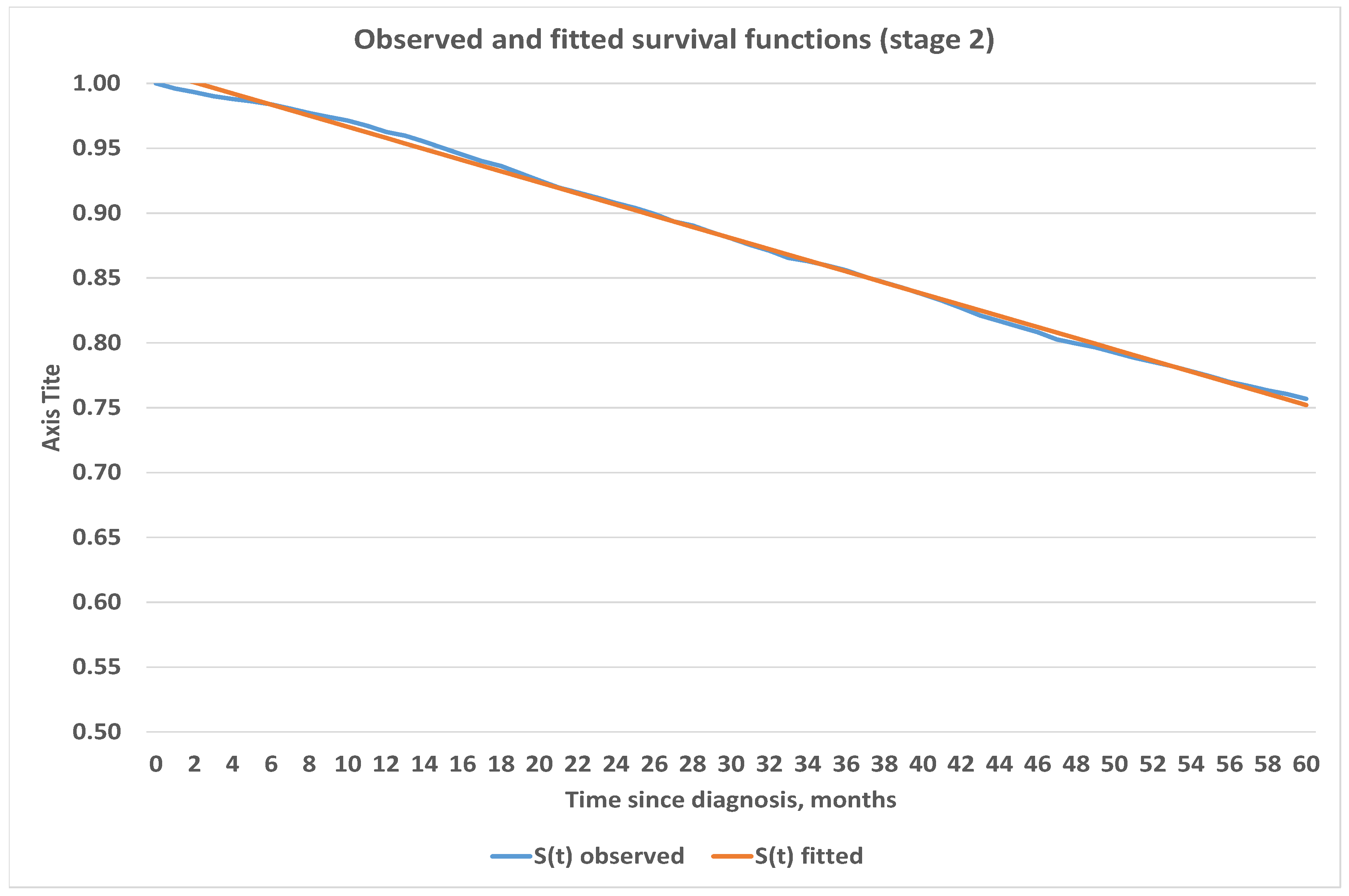
| Stage, i | Number of Cases, | Deaths, | Censored, * |
|---|---|---|---|
| 1 | 4767 | 1258 | 3509 |
| 2 | 9190 | 4631 | 4559 |
| 3 | 4707 | 3588 | 1119 |
| 4 | 2600 | 2480 | 120 |
| Unknown | 738 | - | - |
| Total | 22,002 | 11,957 | 9307 |
| Duration Since Diagnosis in Months | Stage at Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Standard Error | Standard Error | Standard Error | Standard Error | |||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 1 | 0.997902 | 0.000663 | 0.9961 | 0.000652 | 0.984916 | 0.001777 | 0.869615 | 0.006604 |
| 2 | 0.996224 | 0.000888 | 0.9931 | 0.000861 | 0.972806 | 0.002371 | 0.805769 | 0.007758 |
| 3 | 0.995595 | 0.000959 | 0.9901 | 0.001033 | 0.960909 | 0.002825 | 0.759615 | 0.00838 |
| 4 | 0.994965 | 0.001025 | 0.988 | 0.001134 | 0.950074 | 0.003174 | 0.725769 | 0.008749 |
| 5 | 0.993707 | 0.001145 | 0.9861 | 0.001222 | 0.940089 | 0.003459 | 0.699615 | 0.00899 |
| 6 | 0.993077 | 0.001201 | 0.9839 | 0.001313 | 0.928617 | 0.003753 | 0.670385 | 0.009219 |
| 7 | 0.992448 | 0.001254 | 0.9806 | 0.001438 | 0.916932 | 0.004023 | 0.640385 | 0.009411 |
| 8 | 0.991818 | 0.001305 | 0.9771 | 0.001559 | 0.904823 | 0.004277 | 0.611154 | 0.00956 |
| 9 | 0.98972 | 0.001461 | 0.9742 | 0.001653 | 0.8942 | 0.004483 | 0.585385 | 0.009662 |
| 10 | 0.989301 | 0.00149 | 0.9713 | 0.001742 | 0.88379 | 0.004671 | 0.565769 | 0.009721 |
| 11 | 0.987412 | 0.001615 | 0.9674 | 0.001854 | 0.87168 | 0.004875 | 0.545 | 0.009766 |
| 12 | 0.986363 | 0.00168 | 0.9627 | 0.001977 | 0.859783 | 0.005061 | 0.523462 | 0.009795 |
| 13 | 0.984475 | 0.001791 | 0.9597 | 0.002051 | 0.84746 | 0.005241 | 0.502308 | 0.009806 |
| 14 | 0.983216 | 0.001861 | 0.9551 | 0.002161 | 0.835135 | 0.005409 | 0.480385 | 0.009798 |
| 15 | 0.982586 | 0.001895 | 0.9499 | 0.002275 | 0.81856 | 0.005617 | 0.455769 | 0.009767 |
| 16 | 0.981117 | 0.001972 | 0.945 | 0.002377 | 0.805172 | 0.005773 | 0.438077 | 0.00973 |
| 17 | 0.980278 | 0.002014 | 0.9401 | 0.002474 | 0.793272 | 0.005903 | 0.421154 | 0.009683 |
| 18 | 0.978809 | 0.002086 | 0.9363 | 0.002547 | 0.780307 | 0.006035 | 0.407308 | 0.009636 |
| 19 | 0.97734 | 0.002156 | 0.9308 | 0.002648 | 0.767979 | 0.006153 | 0.39 | 0.009566 |
| 20 | 0.975241 | 0.002251 | 0.9251 | 0.002745 | 0.755863 | 0.006262 | 0.378462 | 0.009512 |
| 21 | 0.974612 | 0.002279 | 0.9197 | 0.002835 | 0.74481 | 0.006355 | 0.366923 | 0.009452 |
| 22 | 0.972723 | 0.00236 | 0.9159 | 0.002896 | 0.731419 | 0.006461 | 0.355 | 0.009384 |
| 23 | 0.971044 | 0.002429 | 0.912 | 0.002956 | 0.720365 | 0.006543 | 0.340769 | 0.009295 |
| 24 | 0.968945 | 0.002513 | 0.9077 | 0.003019 | 0.710375 | 0.006612 | 0.330385 | 0.009224 |
| 25 | 0.968316 | 0.002537 | 0.9039 | 0.003075 | 0.700172 | 0.006679 | 0.326154 | 0.009194 |
| 26 | 0.966846 | 0.002593 | 0.8992 | 0.00314 | 0.691457 | 0.006733 | 0.314231 | 0.009104 |
| 27 | 0.964328 | 0.002687 | 0.8936 | 0.003217 | 0.678704 | 0.006807 | 0.304615 | 0.009026 |
| 28 | 0.962438 | 0.002754 | 0.8904 | 0.003259 | 0.669776 | 0.006856 | 0.29422 | 0.008937 |
| 29 | 0.960339 | 0.002827 | 0.8852 | 0.003326 | 0.662549 | 0.006893 | 0.283823 | 0.008842 |
| 30 | 0.959289 | 0.002863 | 0.8806 | 0.003383 | 0.653409 | 0.006937 | 0.278431 | 0.008791 |
| 31 | 0.95656 | 0.002953 | 0.8755 | 0.003444 | 0.642567 | 0.006986 | 0.269189 | 0.008699 |
| 32 | 0.95467 | 0.003014 | 0.8709 | 0.003498 | 0.633637 | 0.007024 | 0.26225 | 0.008627 |
| 33 | 0.952991 | 0.003066 | 0.8655 | 0.003559 | 0.623218 | 0.007064 | 0.256457 | 0.008566 |
| 34 | 0.951521 | 0.003111 | 0.8629 | 0.003588 | 0.614925 | 0.007094 | 0.250663 | 0.008502 |
| 35 | 0.950261 | 0.00315 | 0.8596 | 0.003624 | 0.606633 | 0.007122 | 0.241007 | 0.008391 |
| 36 | 0.948791 | 0.003193 | 0.8558 | 0.003665 | 0.598978 | 0.007145 | 0.233669 | 0.008302 |
| 37 | 0.947111 | 0.003242 | 0.8508 | 0.003717 | 0.59026 | 0.00717 | 0.226717 | 0.008215 |
| 38 | 0.945011 | 0.003303 | 0.8465 | 0.00376 | 0.581755 | 0.007191 | 0.22131 | 0.008146 |
| 39 | 0.943331 | 0.00335 | 0.8422 | 0.003803 | 0.57325 | 0.007211 | 0.217061 | 0.008089 |
| 40 | 0.942071 | 0.003384 | 0.8374 | 0.00385 | 0.567083 | 0.007224 | 0.211268 | 0.008011 |
| 41 | 0.940181 | 0.003436 | 0.8325 | 0.003896 | 0.559854 | 0.007237 | 0.207016 | 0.007951 |
| 42 | 0.938711 | 0.003475 | 0.827 | 0.003946 | 0.552199 | 0.00725 | 0.200824 | 0.007863 |
| 43 | 0.935981 | 0.003546 | 0.8211 | 0.003999 | 0.54497 | 0.00726 | 0.197729 | 0.007817 |
| 44 | 0.934721 | 0.003579 | 0.8167 | 0.004036 | 0.539442 | 0.007267 | 0.193086 | 0.007748 |
| 45 | 0.931781 | 0.003653 | 0.8125 | 0.004072 | 0.533701 | 0.007273 | 0.189603 | 0.007694 |
| 46 | 0.930311 | 0.003689 | 0.8082 | 0.004107 | 0.529873 | 0.007277 | 0.187281 | 0.007658 |
| 47 | 0.928631 | 0.00373 | 0.8026 | 0.004153 | 0.522431 | 0.007282 | 0.181477 | 0.007566 |
| 48 | 0.92548 | 0.003805 | 0.7994 | 0.004178 | 0.514348 | 0.007287 | 0.179929 | 0.007541 |
| 49 | 0.923169 | 0.003859 | 0.7965 | 0.004201 | 0.509456 | 0.007289 | 0.176447 | 0.007484 |
| 50 | 0.922119 | 0.003883 | 0.7925 | 0.00423 | 0.503925 | 0.00729 | 0.172191 | 0.007413 |
| 51 | 0.918968 | 0.003954 | 0.7886 | 0.00426 | 0.497755 | 0.00729 | 0.168321 | 0.007346 |
| 52 | 0.917917 | 0.003977 | 0.7854 | 0.004284 | 0.493283 | 0.007289 | 0.166773 | 0.00732 |
| 53 | 0.916236 | 0.004014 | 0.7821 | 0.004307 | 0.487103 | 0.007288 | 0.163678 | 0.007265 |
| 54 | 0.913924 | 0.004064 | 0.7781 | 0.004336 | 0.48262 | 0.007286 | 0.157874 | 0.00716 |
| 55 | 0.912243 | 0.0041 | 0.7741 | 0.004363 | 0.478561 | 0.007284 | 0.154004 | 0.007089 |
| 56 | 0.909721 | 0.004153 | 0.7698 | 0.004392 | 0.473636 | 0.007281 | 0.150909 | 0.00703 |
| 57 | 0.906778 | 0.004213 | 0.7667 | 0.004413 | 0.467633 | 0.007277 | 0.145491 | 0.006925 |
| 58 | 0.905517 | 0.004238 | 0.7633 | 0.004435 | 0.462698 | 0.007272 | 0.14317 | 0.006879 |
| 59 | 0.904045 | 0.004268 | 0.7604 | 0.004454 | 0.458834 | 0.007268 | 0.141235 | 0.006841 |
| 60 | 0.902153 | 0.004305 | 0.7568 | 0.004476 | 0.454323 | 0.007263 | 0.138523 | 0.006786 |
| Stage at Diagnosis | Average Age at Diagnosis | Probability to Survive 5 Years (Population Average), % * | Probability to Survive 5 Years (Patients), % |
|---|---|---|---|
| 1 | 57.55 | 97.13 | 90.22 |
| 2 | 58.77 | 96.85 | 75.68 |
| 3 | 61.79 | 95.62 | 45.43 |
| 4 | 62.97 | 95.23 | 13.85 |
| Stage | Estimated Survival Function | Mean Squared Error |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skučaitė, A.; Puvačiauskienė, A.; Puišys, R.; Šiaulys, J. Actuarial Analysis of Survival among Breast Cancer Patients in Lithuania. Healthcare 2021, 9, 383. https://doi.org/10.3390/healthcare9040383
Skučaitė A, Puvačiauskienė A, Puišys R, Šiaulys J. Actuarial Analysis of Survival among Breast Cancer Patients in Lithuania. Healthcare. 2021; 9(4):383. https://doi.org/10.3390/healthcare9040383
Chicago/Turabian StyleSkučaitė, Aldona, Alma Puvačiauskienė, Rokas Puišys, and Jonas Šiaulys. 2021. "Actuarial Analysis of Survival among Breast Cancer Patients in Lithuania" Healthcare 9, no. 4: 383. https://doi.org/10.3390/healthcare9040383
APA StyleSkučaitė, A., Puvačiauskienė, A., Puišys, R., & Šiaulys, J. (2021). Actuarial Analysis of Survival among Breast Cancer Patients in Lithuania. Healthcare, 9(4), 383. https://doi.org/10.3390/healthcare9040383





