A Meta-Analysis on Prophylactic Donor Heart Tricuspid Annuloplasty in Orthotopic Heart Transplantation: High Hopes from a Small Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Search and Eligibility
2.2. Intraoperative Timing and Outcomes
2.3. Data Collection and Synthesis
2.4. Studies Quality Assessment
3. Results
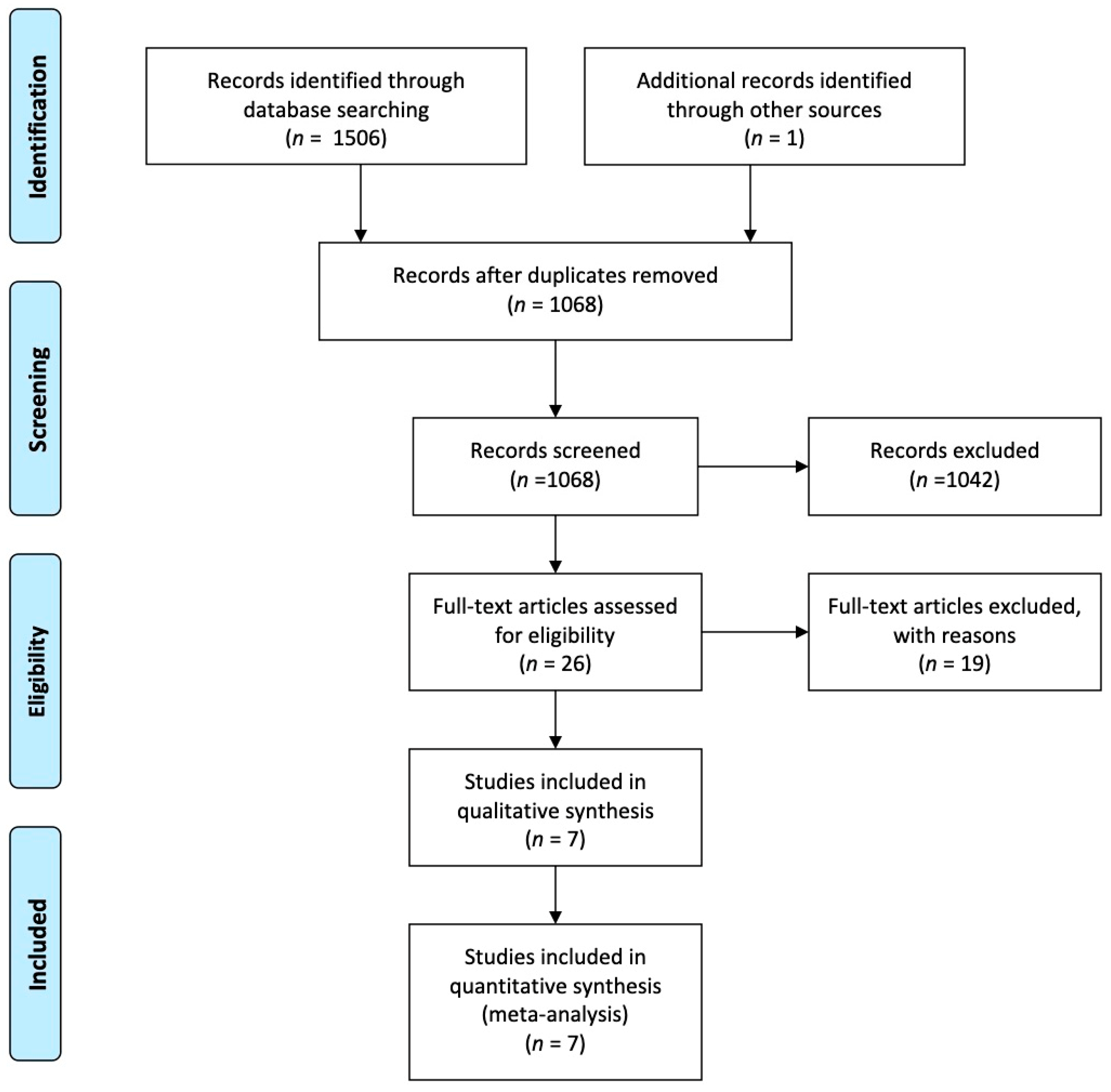
3.1. Literature Search and Study Selection
3.2. Study Characteristics and Risk of Bias
3.3. Patient and Periprocedural Characteristics
3.4. Intraoperative Times
3.5. Outcomes
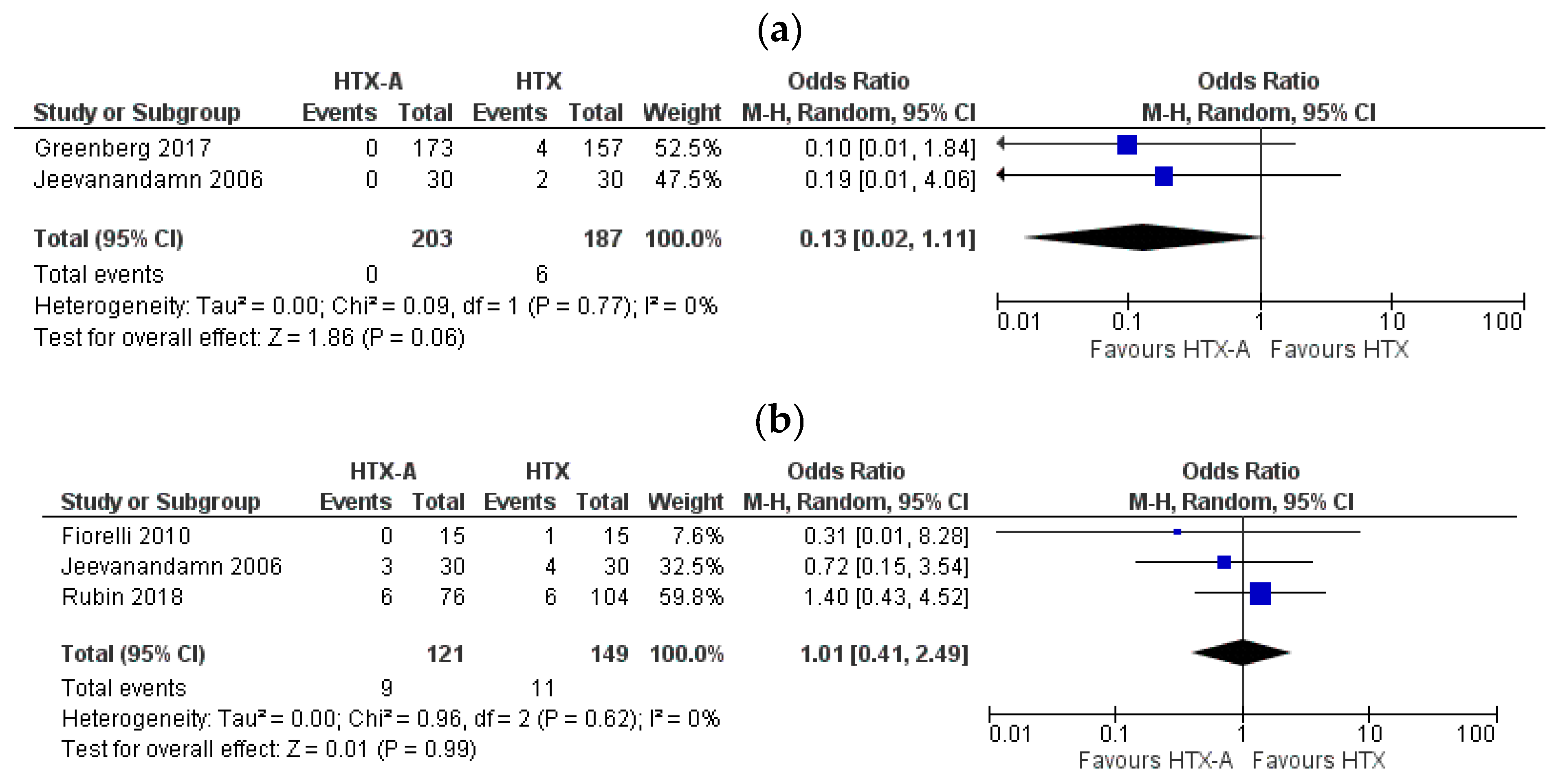
3.5.1. Tricuspid Regurgitation
3.5.2. Periprocedural Complications
3.5.3. Reoperation and Survival
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, R.C.C.; Abrahams, Z.; Hanna, M.; Pangrace, J.; Gonzalez-Stawinski, G.; Starling, R.; Taylor, D. Tricuspid Regurgitation After Cardiac Transplantation: An Old Problem Revisited. J. Heart Lung Transplant. 2008, 27, 247–252. [Google Scholar] [CrossRef]
- Fiorelli, A.I.; Oliveira, J.L.; Santos, R.H.; Coelho, G.B.; Oliveira, A.S.; Lourenço-Filho, D.D.; Lapenna, G.; Dias, R.R.; Bacal, F.; Bocchi, E.A.; et al. Can tricuspid annuloplasty of the donor heart reduce valve insufficiency following cardiac transplantation with bicaval anastomosis? Heart Surg. Forum 2010, 13, E168–E171. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Shernan, S.K.; Leacche, M.; Rawn, J.D.; Paul, S.; Mihaljevic, T.; Jarcho, J.A.; Stevenson, L.W.; Fang, J.C.; Lewis, E.F.; et al. Severity of intraoperative tricuspid regurgitation predicts poor late survival following cardiac transplantation. Ann. Thorac. Surg. 2004, 78, 1635–1642. [Google Scholar] [CrossRef]
- Bainbridge, A.D.; Cave, M.; Roberts, M.; Casula, R.; Mist, B.A.; Parameshwar, J.; Wallwork, J.; Large, S.R. A prospective randomized trial of complete atrioventricular transplantation versus ventricular transplantation with atrioplasty. J. Heart Lung Transpl. 1999, 18, 407–413. [Google Scholar] [CrossRef]
- Aziz, T.M.; Burgess, M.I.; Rahman, A.N.; Campbell, C.S.; Deiraniya, A.K.; Yonan, N.A. Risk factors for tricuspid valve regurgitation after orthotopic heart transplantation. Ann. Thorac. Surg. 1999, 68, 1247–1251. [Google Scholar] [CrossRef]
- De Simone, R.; Lange, R.; Sack, F.U.; Mehmanesh, H.; Hagl, S. Atrioventricular valve insufficiency and atrial geometry in orthotopic heart transplantation. Cardiologia 1994, 39, 325–334. [Google Scholar] [CrossRef]
- Leyh, R.G.; Jahnke, A.W.; Kraatz, E.G.; Sievers, H.H. Cardiovascular dynamics and dimensions after bicaval and standard cardiac transplantation. Ann. Thorac. Surg. 1995, 59, 1495–1500. [Google Scholar] [CrossRef]
- Deleuze, P.; Benvenuti, C.; Mazzucotelli, J.P.; Perdrix, C.; Le Besnerais, P.; Mourtada, A.; Hillion, M.L.; Patrat, J.F.; Loisance, D.Y. Orthotopic cardiac transplantation with caval anastomoses: A comparative randomised study with the standard procedure in 81 cases. Arch. Des Mal. Du Coeur Et Des Vaiss. 1996, 89, 43–48. [Google Scholar]
- Algarni, K.D.; Arafat, A.A.; Pragliola, C.; Alhebaishi, Y.S.; AlFayez, L.A.; AlOtaibi, K.; Bakhsh, A.M.; Amro, A.A.; Adam, A.I. Tricuspid Valve Regurgitation After Heart Transplantation: A Single-Center 10-year Experience. J. Saudi Heart Assoc. 2020, 32, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Giannetti, N.; Kato, T.; Kornbluth, M.; Oyer, P.; Valantine, H.A.; Robbins, R.C.; Hunt, S.A. Severe tricuspid regurgitation after heart transplantation. J. Heart Lung Transplant. 2001, 20, 709–717. [Google Scholar] [CrossRef]
- Farag, M.; Arif, R.; Raake, P.; Kreusser, M.; Karck, M.; Ruhparwar, A.; Schmack, B. Cardiac surgery in the heart transplant recipient: Outcome analysis and long-term results. Clin. Transplant. 2019, 33, e13709. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, L.; Haddad, H.; Davies, R.A.; Veinot, J.P. Tricuspid valve chordal tissue in endomyocardial biopsy specimens of patients with significant tricuspid regurgitation. J. Heart Lung Transplant. 2005, 24, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Iacovoni, A.; Agnese, V.; Falletta, C.; Coronnello, C.; Pasta, S.; Novo, G.; di Gesaro, G.; Senni, M.; Maalouf, J.; et al. Usefulness of regional right ventricular and right atrial strain for prediction of early and late right ventricular failure following a left ventricular assist device implant: A machine learning approach. Int. J. Artif. Organs 2020, 43, 297–314. [Google Scholar] [CrossRef]
- Scardulla, F.; Agnese, V.; Romano, G.; Di Gesaro, G.; Sciacca, S.; Bellavia, D.; Clemenza, F.; Pilato, M.; Pasta, S. Modeling Right Ventricle Failure After Continuous Flow Left Ventricular Assist Device: A Biventricular Finite-Element and Lumped-Parameter Analysis. Cardiovasc. Eng. Technol. 2018, 9, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Kitamura, M.; Noji, S.; Aomi, S.; Endo, M.; Koyanagi, H. Long-term results after De Vega’s tricuspid annuloplasty. J. Cardiovasc. Surg. 2002, 43, 773–777. [Google Scholar]
- Kuwaki, K.; Morishita, K.; Tsukamoto, M.; Abe, T. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur. J. Cardiothorac. Surg. 2001, 20, 577–582. [Google Scholar] [CrossRef]
- Lee, C.H.; Wei, J. Successful continuous-flow left ventricular assist device implantation with adjuvant tricuspid valve repair for advanced heart failure. Cardiovasc. J. Afr. 2016, 27, e14–e16. [Google Scholar] [CrossRef][Green Version]
- Bishawi, M.; Zanotti, G.; Shaw, L.; MacKenzie, M.; Castleberry, A.; Bartels, K.; Schroder, J.; Velazquez, E.; Swaminathan, M.; Rogers, J.; et al. Tricuspid Valve Regurgitation Immediately After Heart Transplant and Long-Term Outcomes. Ann. Thorac. Surg. 2019, 107, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.E.; Muehlebach, G.F.; Jones, P.; Gorton, M.E.; Stuart, R.S.; Borkon, A.M. Tricuspid annuloplasty significantly reduces early tricuspid regurgitation after biatrial heart transplantation. J. Heart Lung Transplant. 2004, 23, 1160–1162. [Google Scholar] [CrossRef]
- Fiorelli, A.I.; Stolf, N.A.; Abreu Filho, C.A.; Santos, R.H.; Buco, F.H.; Fiorelli, L.R.; Issa, V.; Bacal, F.; Bocchi, E.A. Prophylactic donor tricuspid annuloplasty in orthotopic bicaval heart transplantation. Transplant. Proc. 2007, 39, 2527–2530. [Google Scholar] [CrossRef]
- Greenberg, J.; Teman, N.R.; Haft, J.W.; Romano, M.A.; Pagani, F.D.; Aaronson, K.D.; Wu, A.H. Association of Donor Tricuspid Valve Repair With Outcomes After Cardiac Transplantation. Ann. Thorac. Surg. 2018, 105, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, V.; Russell, H.; Mather, P.; Furukawa, S.; Anderson, A.; Grzywacz, F.; Raman, J. A one-year comparison of prophylactic donor tricuspid annuloplasty in heart transplantation. Ann. Thorac. Surg. 2004, 78, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, V.; Russell, H.; Mather, P.; Furukawa, S.; Anderson, A.; Raman, J. Donor Tricuspid Annuloplasty During Orthotopic Heart Transplantation: Long-Term Results of a Prospective Controlled Study. Ann. Thorac. Surg. 2006, 82, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.A.; Sanchez, J.; Bayne, J.; Avula, U.M.R.; Takayama, H.; Takeda, K.; Naka, Y.; Garan, H.; Farr, M.A.; Wan, E.Y. Conduction Abnormalities Associated with Tricuspid Annuloplasty in Cardiac Transplantation. ASAIO J. 2019, 65, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Filsoufi, F.; Salzberg, S.P.; Anderson, C.A.; Couper, G.S.; Cohn, L.H.; Adams, D.H. Optimal surgical management of severe tricuspid regurgitation in cardiac transplant patients. J. Heart Lung Transplant. 2006, 25, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Cladellas, M.; Abadal, M.L.; Pons-Lladó, G.; Ballester, M.; Carreras, F.; Obrador, D.; Garcia-Moll, M.; Padró, J.M.; Aris, A.; Caralps, J.M. Early transient multivalvular regurgitation detected by pulsed Doppler in cardiac transplantation. Am. J. Cardiol. 1986, 58, 1122–1124. [Google Scholar] [CrossRef]
- Yankah, A.C.; Musci, M.; Weng, Y.; Loebe, M.; Zurbruegg, H.R.; Siniawski, H.; Mueller, J.; Hetzer, R. Tricuspid valve dysfunction and surgery after orthotopic cardiac transplantation. Eur. J. Cardiothorac. Surg. 2000, 17, 343–348. [Google Scholar] [CrossRef]
- Braverman, A.C.; Coplen, S.E.; Mudge, G.H.; Lee, R.T. Ruptured chordae tendineae of the tricuspid valve as a complication of endomyocardial biopsy in heart transplant patients. Am. J. Cardiol. 1990, 66, 111–113. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, J.J.; Kim, J.B.; Kim, D.H.; Song, J.M.; Yun, T.J.; Choo, S.J.; Kang, D.H.; Chung, C.H.; Song, J.K.; et al. Fate of atrioventricular valve function of the transplanted heart. Circ. J. 2014, 78, 1654–1660. [Google Scholar] [CrossRef]
- Kalra, N.; Copeland, J.G.; Sorrell, V.L. Tricuspid regurgitation after orthotopic heart transplantation. Echocardiography 2010, 27, 1–4. [Google Scholar] [CrossRef]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J. Heart Lung Transplant. 2005, 24, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, F.; Matos, V.; Gonçalves, L.; Antunes, M.; Providência, L.A. Complications of endomyocardial biopsy in heart transplant patients: A retrospective study of 2117 consecutive procedures. Transplant. Proc. 2011, 43, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Mügge, A.; Daniel, W.G.; Herrmann, G.; Simon, R.; Lichtlen, P.R. Quantification of tricuspid regurgitation by Doppler color flow mapping after cardiac transplantation. Am. J. Cardiol. 1990, 66, 884–887. [Google Scholar] [CrossRef]
- Fiorelli, A.I.; Coelho, G.H.B.; Oliveira, J.L., Jr.; Aiello, V.D.; Benvenuti, L.A.; Santos, A.; Chi, A.; Tallans, A.; Igushi, M.L.; Bacal, F.; et al. Endomyocardial Biopsy as Risk Factor in the Development of Tricuspid Insufficiency After Heart Transplantation. Transplant. Proc. 2009, 41, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, H.; Müller, J.; Cohnert, T.; Hummel, M.; Spiegelsberger, S.; Siniawski, H.K.; Lieback, E.; Hetzer, R. Clinical heart transplantation without routine endomyocardial biopsy. J. Heart Lung Transplant. 1992, 11, 1093–1102. [Google Scholar]
- Camargo, P.R.; Mazzieri, R.; Snitcowsky, R.; Higuchi, M.L.; Meneghetti, J.C.; Soares Júnior, J.; Fiorelli, A.; Ebaid, M.; Pileggi, F. Correlation between gallium-67 imaging and endomyocardial biopsy in children with severe dilated cardiomyopathy. Int. J. Cardiol. 1990, 28, 293–297. [Google Scholar] [CrossRef]
- Fiorelli, A.I.; Coelho, G.H.; Aiello, V.D.; Benvenuti, L.A.; Palazzo, J.F.; Santos Júnior, V.P.; Canizares, B.; Dias, R.R.; Stolf, N.A. Tricuspid valve injury after heart transplantation due to endomyocardial biopsy: An analysis of 3550 biopsies. Transplant. Proc. 2012, 44, 2479–2482. [Google Scholar] [CrossRef]
- Bollano, E.; Karason, K.; Lidén, H.; Dellgren, G. How should we manage early tricuspid valve regurgitation after heart transplantation? Int. J. Cardiol. 2016, 214, 191–193. [Google Scholar] [CrossRef]
- Wei, J.; Chang, C.Y.; Lee, F.Y.; Lai, W.Y. De Vega’s semicircular annuloplasty for tricuspid valve regurgitation. Ann. Thorac. Surg. 1993, 55, 482–485. [Google Scholar] [CrossRef]
- Kanter, K.R.; Doelling, N.R.; Fyfe, D.A.; Sharma, S.; Tam, V.K.H. De Vega tricuspid annuloplasty for tricuspid regurgitation in children. Ann. Thorac. Surg. 2001, 72, 1344–1348. [Google Scholar] [CrossRef]
- Cantillon, D.J.; Tarakji, K.G.; Hu, T.; Hsu, A.; Smedira, N.G.; Starling, R.C.; Wilkoff, B.L.; Saliba, W.I. Long-term outcomes and clinical predictors for pacemaker-requiring bradyarrhythmias after cardiac transplantation: Analysis of the UNOS/OPTN cardiac transplant database. Heart Rhythm 2010, 7, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, H.R.; Bates, M. Pacemaker Use Following Heart Transplantation. Ochsner J. 2017, 17, 20–24. [Google Scholar] [PubMed]
- Hamon, D.; Taleski, J.; Vaseghi, M.; Shivkumar, K.; Boyle, N.G. Arrhythmias in the Heart Transplant Patient. Arrhythmia Electrophysiol. Rev. 2014, 3, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Kobashigawa, J.; Margarian, A.; Sen, L. Cause of atrioventricular block in patients after heart transplantation. Transplantation 2003, 76, 137–142. [Google Scholar] [CrossRef] [PubMed]



| Author | Year | Country | No. of Centers | Type of Study | Time Period | Type of Surgery | Patient Group | No. of Patients Per Group | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Jeevanandam | 2004 | USA | 1 | RCT | April 1997–March 1998 | Bicaval orthotopic heart transplantation with DeVega TVA | HTX | 30 | 1 year |
| HTX-A | 30 | ||||||||
| Jeevanandam | 2006 | USA | 1 | RCT | April 1997–December 2003 | Bicaval orthotopic heart transplantation with DeVega TVA | HTX | 30 | 5.7 to 6.7 years |
| HTX-A | 30 | ||||||||
| Rubin, G | 2018 | USA | 1 | Retrospective observational | 2013–2017 | Orthotopic heart transplantation with DeVega TVA | HTX | 104 | 32 months |
| HTX-A | 76 | ||||||||
| Greenberg J | 2017 | USA | 18 | Retrospective observational- Propensity score-matched | January 2002–December 2016 | Bicaval orthotopic heart transplantation with DeVega TVA | HTX | 117 | 7.9 ± 4.3 years |
| HTX-A | 130 | 5.2 ± 2.9 years | |||||||
| Fiorelli | 2007 | Brazil | 1 | Prospective Observational- nonrandomized | March 1985–December 2005 | Bicaval orthotopic heart transplantation with DeVega TVA | HTX | 10 | 14.6 ± 4.3 months |
| HTX-A | 10 | ||||||||
| Fiorelli | 2010 | Brazil | 1 | Prospective Observational- nonrandomized | 2002–2010 | Bicaval orthotopic heart transplantation with DeVega TVA | HTX | 15 | 26.9 ± 5.4 months |
| HTX-A | 15 | ||||||||
| Brown | 2004 | USA | 1 | Retrospective Observational | November 1999–July 2001 | Biatrial cardiac transplantation with a Cabrol modification with either a DeVega (n = 10) or Ring (n = 15) TVA | HTX | 25 | 6 months |
| HTX-A | 25 |
| Parameters | No. of Studies | No. of HTX Patients | No. of HTX-A Patients | HTX Mean ± SD or (%) | HTX-A Mean ± SD or (%) | p-Value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 5 | 331 | 319 | 51.48 ± 10.20 | 51.92± 11.32 | 0.6 |
| Male | 5 | 331 | 319 | 72.2% | 73.3% | 0.8 |
| Preoperative data | ||||||
| Ischemic etiology of the end-stage heart failure | 4 | 132 | 148 | 88.63% | 67.57% | 0.0001 |
| Inotropic medication | 2 | 172 | 188 | 30.23% | 37.76% | 0.2 |
| Preoperative renal function | ||||||
| Creatinine | 2 | 187 | 203 | 1.26 ± 0.93 | 1.22 ± 0.46 | 0.6 |
| BUN | 2 | 187 | 203 | 23.73 ± 11.90 | 23.56 ± 11.82 | 0.9 |
| Hemodynamic parameters | ||||||
| Pulmonary capillary wedge pressure | 2 | 187 | 203 | 17.16 ± 8.55 | 19.71± 9.14 | 0.005 |
| Pulmonary vascular resistance woods units | 5 | 298 | 300 | 2.29 ± 1.00 | 2.17 ± 1.02 | 0.15 |
| Mechanical circulatory support | 4 | 306 | 304 | 55.88% | 52.63% | 0.5 |
| Intraoperative times | ||||||
| CPB duration | 3 | 144 | 116 | 173.33± 27.75 | 154.15 ± 25.89 | <0.0001 |
| Ischemic time | 5 | 326 | 314 | 181.75 ± 40.83 | 165.32 ± 41.72 | <0.0001 |
| Aortic cross-clamp | 4 | 169 | 141 | 88.02 ± 20.50 | 86.89± 13.86 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacusca, A.E.; Tarus, A.; Burlacu, A.; Enache, M.; Tinica, G. A Meta-Analysis on Prophylactic Donor Heart Tricuspid Annuloplasty in Orthotopic Heart Transplantation: High Hopes from a Small Intervention. Healthcare 2021, 9, 306. https://doi.org/10.3390/healthcare9030306
Bacusca AE, Tarus A, Burlacu A, Enache M, Tinica G. A Meta-Analysis on Prophylactic Donor Heart Tricuspid Annuloplasty in Orthotopic Heart Transplantation: High Hopes from a Small Intervention. Healthcare. 2021; 9(3):306. https://doi.org/10.3390/healthcare9030306
Chicago/Turabian StyleBacusca, Alberto Emanuel, Andrei Tarus, Alexandru Burlacu, Mihail Enache, and Grigore Tinica. 2021. "A Meta-Analysis on Prophylactic Donor Heart Tricuspid Annuloplasty in Orthotopic Heart Transplantation: High Hopes from a Small Intervention" Healthcare 9, no. 3: 306. https://doi.org/10.3390/healthcare9030306
APA StyleBacusca, A. E., Tarus, A., Burlacu, A., Enache, M., & Tinica, G. (2021). A Meta-Analysis on Prophylactic Donor Heart Tricuspid Annuloplasty in Orthotopic Heart Transplantation: High Hopes from a Small Intervention. Healthcare, 9(3), 306. https://doi.org/10.3390/healthcare9030306







