Abstract
Surgical procedures are key drivers of pain development and opioid utilization globally. Various organizations have generated guidance on postoperative pain management, enhanced recovery strategies, multimodal analgesic and anesthetic techniques, and postoperative opioid prescribing. Still, comprehensive integration of these recommendations into standard practice at the institutional level remains elusive, and persistent postoperative pain and opioid use pose significant societal burdens. The multitude of guidance publications, many different healthcare providers involved in executing them, evolution of surgical technique, and complexities of perioperative care transitions all represent challenges to process improvement. This review seeks to summarize and integrate key recommendations into a “roadmap” for institutional adoption of perioperative analgesic and opioid optimization strategies. We present a brief review of applicable statistics and definitions as impetus for prioritizing both analgesia and opioid exposure in surgical quality improvement. We then review recommended modalities at each phase of perioperative care. We showcase the value of interprofessional collaboration in implementing and sustaining perioperative performance measures related to pain management and analgesic exposure, including those from the patient perspective. Surgery centers across the globe should adopt an integrated, collaborative approach to the twin goals of optimal pain management and opioid stewardship across the care continuum.
1. Introduction
Surgery is an indispensable part of healthcare, and over 300 million surgical procedures are performed around the world annually [1]. Despite tremendous benefits to survival and quality of life, surgical procedures frequently result in acute pain, among other risks. Suboptimal postoperative pain management is associated with worsened humanistic and economic outcomes, including the development of chronic pain and opioid dependence [2]. In the U.S., opioid analgesics have been the cornerstone of postoperative pain management, driven by earlier efforts to improve treatment of pain and societal expectations for surgical recovery [3,4,5]. The significant risks and costs associated with opioid overuse are now better understood: opioid-related adverse events frequently potentiate complications in postoperative populations and postsurgical opioid prescribing patterns have contributed to the modern U.S. opioid epidemic [6,7,8,9,10,11]. Postoperative opioid prescribing in the U.S. remains alarmingly high and in stark contrast to that of non-U.S. countries, underscoring the need for more widespread adoption of multimodal analgesia and enhanced recovery strategies by American centers [4,12,13,14].
Perioperative pain management and opioid stewardship are therefore comparable in necessity and interrelated in execution. To this end, many organizations have offered guidance on components of their application. This has included general postoperative pain management [15,16,17], perioperative management of patients on preoperative opioids [18], surgery-specific guidelines [19,20,21,22,23,24], medication-specific recommendations [25,26], conceptual frameworks for opioid stewardship [27,28,29], collaborative postoperative opioid prescribing guidelines [30,31,32], statements on perioperative opioid use [33,34], legal opioid prescribing limits [35], and various quality measures for healthcare institutions [36,37,38]. Despite the multitude of recommendations available, a large proportion of surgical patients report inadequately treated pain and high rates of adverse events, alongside many institutions exhibiting overreliance on opioids and underutilization of multimodal strategies [2,39,40]. This narrative review enhances awareness and adoption of perioperative pain management and opioid stewardship strategies by integrating available guidance into a single “roadmap” for interprofessional stakeholders across the surgical care continuum.
2. Statistics and Definitions
2.1. The Burdens of Perioperative Opioid Overuse and of Uncontrolled Postoperative Pain
Approximately one out of every ten opioid-exposed postoperative patients will experience at least one opioid-related adverse event (ORAE), conferring significant morbidity and economic burden [7,41]. Many postoperative complications may be appropriately classified as ORAEs, including nausea and vomiting, ileus, urinary retention, delirium, and respiratory depression, underscoring the interrelatedness of perioperative opioid use and surgical outcomes [6,41]. Despite their toxicities, opioids appear to be overprescribed for postoperative pain [42,43,44,45,46,47]. Available data suggest 42–71% of prescribed opioid pills go unused after surgery, with 73% of postoperative orthopedic patients reporting unused opioid pills at one month post-procedure [42,46]. This reservoir of unused prescription opioids in community settings has been identified as a potential contributor to the U.S. opioid epidemic. Over 80% of modern heroin users report nonmedical prescription opioid use prior to heroin initiation, and two-thirds of prescription opioids used for nonmedical purposes are obtained from a friend or relative [11,48,49].
Despite an apparent overreliance on opioids by prescribers, less than half of postoperative patients endorse adequate pain relief, with 75–88% reporting a pain severity of moderate, severe, or extreme [2,15]. Short-term morbidities related to uncontrolled acute postoperative pain span nearly every organ system, including increased risks for thrombotic events, pneumonia, ileus, oliguria, and impaired wound healing. Furthermore, inadequate acute pain control negatively impacts long-term functional recovery, mental health, and quality of life. Collectively, the economic burden of uncontrolled acute postoperative pain is vast, driven by significantly longer surgery center stays and higher rates of unplanned admissions and readmissions to emergency departments and hospitals [2].
An additional risk of poorly managed acute postoperative pain is the development of persistent postoperative pain, frequently defined as new and enduring pain of the operative or related area without other evident causes lasting more than 2 months after surgery. While prevalence of such “chronic” postsurgical pain (CPSP) varies by surgery type and generally decreases with time, it may occur in 10–60% of patients after common procedures [2,50,51,52,53]. The physical and mental consequences of persistent postoperative pain are frequently complicated by the development of persistent opioid use, which is also variably defined but largely refers to ongoing opioid use for postoperative pain in the timeframe of 90 days to 1 year after surgery [2,34]. The incidence of persistent postoperative opioid use appears highest after spine surgery and not uncommon (i.e., 5–30%) after arthroplasty and thoracic procedures. Patients on opioids prior to surgery demonstrate a 10-fold increase in the development of persistent postoperative opioid use. Still, previously opioid-naïve patients are converted to persistent opioid users by the surgical process at an alarming 6–10% rate [10,34]. Considering that 1 in 4 chronic opioid users may develop an opioid use disorder, the mitigation of persistent postoperative pain and opioid use should be a priority to healthcare providers and systems [10,54].
2.2. Opioid Stewardship, Multimodal Analgesia, and Equianalgesic Opioid Dosing
“Perioperative opioid stewardship” may be defined as the judicious use of opioids to treat surgical pain and optimize postoperative patient outcomes. The paradigm is not simply “opioid avoidance,” and requires balancing the risks of both over- and under-utilization of these high-risk agents. To this end, postoperative opioid minimization should be pursued only in the greater context of optimizing acute pain management, reducing adverse events, and preventing persistent postoperative pain through comprehensive multimodal analgesia [19,33,55,56,57,58,59,60,61]. Multimodal analgesia, or the use of multiple modalities of differing mechanisms of action, is key to decreasing surgical recovery times and complications, and so is also a fundamental component of the enhanced recovery paradigm promoted by the international Enhanced Recovery After Surgery (ERAS®) Society [19,24,62,63,64,65]. Dedicated resources and care coordination are often required for institutions to align analgesic use with best practices, so Opioid Stewardship Programs (OSPs) are taking hold, modeled after antimicrobial stewardship practices [29,38,66,67,68].
Quantifying opioid exposure for patient care, process improvement, or research purposes requires the use of a standardized assessment. Opioid doses can be normalized to their equianalgesic oral morphine amounts, i.e., Oral Morphine Equivalent (OME), oral Morphine Milligram Equivalent (MME), or oral Morphine Equivalent Dose (MED) [69,70,71]. Current evidence-based recommendations for equianalgesic dosing of opioids commonly encountered in perioperative settings are summarized in Table 1 [71]. Guidelines on the use of opioids for chronic pain are also available and provide slightly different conversions for MME doses, citing earlier literature [54,72]. All opioid conversions for patient care purposes should include careful consideration of the limitations of these factors, including extremely wide ranges for ratios found in clinical trials, clinical inter-patient variability, incomplete cross-tolerance between opioids, and other patient-specific factors (e.g., renal impairment or genetic variants in metabolism, see Section 3.5). The newly calculated opioid dose should therefore be reduced by 25–50% when changing between opioids or routes of administration, as discussed in detail elsewhere [71].

Table 1.
Current Recommendations for Equianalgesic Dosing of Opioids Commonly Encountered in Perioperative Settings.
3. Pain Management and Opioid Stewardship across the Perioperative Continuum of Care
Perioperative care consists of a complex orchestra of medical professionals, physical locations, processes, and temporal phases. This continuum begins prior to the day of surgery (DOS), continues across inpatient or ambulatory stay, and extends through recovery and follow-up phases of care. A maximally effective institutional strategy for perioperative pain management and opioid stewardship includes all phases and providers across this continuum. Though there is no definitive evidence-based regimen, effective multimodal analgesia requires institutional culture and protocols for pre-admission optimization, consistent use of regional anesthesia, routine scheduled administration of nonopioid analgesics and nonpharmacologic therapies, and reservation of systemic opioids to an “as needed” basis at doses tailored to expected pain and preexisting tolerance [15,18,33]. Figure 1 summarizes the recommended strategies at each phase of care, which will be discussed in greater detail.

Figure 1.
Perioperative Pain Management and Opioid Stewardship Interventions across the Continuum of Care. Legend: DOS = day of surgery, IV = intravenous, MAT = medication-assisted treatment (i.e., for substance use disorders), O-NET+ = opioid-naïve, -exposed or -tolerant, plus modifiers classification system, ORAE = opioid-related adverse event, PCA = patient-controlled (intravenous) analgesia, PDMP = prescription drug monitoring program.
3.1. Pre-Admission Phase
The pre-admission phase of care occurs prior to the day of surgery (DOS) and represents the ideal opportunity for patient optimization. Safe and effective interventions exist during the pre-admission phase to improve pain control and decrease opioid requirements in the subsequent perioperative period. Recommended pre-admission interventions include evaluation of patient pain and pain history, education to patients and caregivers, assessment of patient risk for perioperative opioid-related adverse events (ORAEs) and implementation of mitigation strategies, optimization of preoperative opioid and multimodal therapies, and advance planning for perioperative management of chronic therapies for chronic pain and medication-assisted therapy for substance use disorders.
3.1.1. Patient Pain History, Evaluation and Education
Perioperative pain management planning should be pursued through a shared decision-making approach and necessitates an accurate pre-admission history and evaluation. Pain assessment should include classification of pain type(s) (e.g., neuropathic, visceral, somatic, or spastic), duration, impact on physical function and quality of life, and current therapies. Other key patient evaluation components include past medical and psychiatric comorbidities, concomitant medications, medication allergies and intolerances, assessment of chronic pain and/or substance use histories, and previous experiences with surgery and analgesic therapies [15]. Barriers to the safe use of regional anesthetic and analgesic strategies can be identified and considered, such as certain anatomic abnormalities, prior medication reactions, a history of bleeding disorders, or need for anticoagulant use [73]. Likewise, chronic medications that synergize postoperative risks for ORAEs and complications can be managed expectantly, such as benzodiazepines (e.g., respiratory depression, delirium). While such medications may not be avoided feasibly due to the risk of withdrawal syndromes, consideration could be given to preoperative tapering and/or increased education and monitoring for adverse effects in the perioperative period [15,74].
Psychosocial comorbidities and behaviors that could negatively affect the patient’s perioperative pain management and general recovery include anxiety, depression, frailty, and maladaptive coping strategies such as pain catastrophizing [15,18,52,75,76,77,78]. Additionally, patients with chronic pain and/or history of a substance use disorder frequently experience anxiety regarding their perioperative pain management and/or risk of relapse [18]. While high-quality data is currently lacking to support specific pre-admission strategies for decreasing postoperative adverse events associated with mental health comorbidities, pilot studies and expert opinion support the integration of psychosocial optimization into the “prehabilitation” paradigm for surgical readiness [18,52,75,79]. Cognitive function, language barriers, health literacy, and other social determinants of health also significantly influence postoperative pain management and recovery [51,80,81,82]. Validated health literacy assessments have been applied to surgical populations [83,84,85,86,87]. Prospective identification of these challenges, including the application of standardized cognitive and psychosocial assessments, can allow for appropriate preoperative referral, patient optimization, and future study of risk mitigation strategies [15,18,52,75,78,80,88]. To this end, various predictive tools for postoperative pain are being explored [88,89,90,91].
Patient-centered education and expectation management during the pre-admission phase of care are effective strategies for improving postoperative pain control, limiting postoperative opioid use, decreasing complications and readmissions, and increasing postoperative function and quality of life [15,18,92,93,94,95,96,97,98]. Insufficient evidence exists to support specific educational strategies or components, but current guidelines recommend an individualized discussion about expected severity and duration of postoperative pain to generate realistic goals about pain management, a description of how pain will be assessed, and an overview of available analgesic options, including the judicious use of opioids and their associated risks, multimodal therapies in the form of nonopioid medications, local anesthetic or regional (central and peripheral) techniques, and nonpharmacologic modalities [15]. Patients with chronic pain or substance use disorders should especially be introduced to the concepts of multimodal analgesia and educated on the risks of perioperative opioids, beginning at the pre-admission phase of care [18]. Education should be provided in an effective manner considering the patient’s age, health literacy, language, and cognitive ability [15,99]. The patient’s prior experiences, preferences, and expectations should then be integrated into a collaborative, documented, goal-based plan [15].
Provider education, resources, and time constraints in pre-admission clinics currently limit the widespread uptake of these best practices into routine care. The pre-admission phase therefore represents an important target for process improvement related to perioperative pain management and opioid stewardship [94]. To support such efforts, some organizations have made patient education materials publicly available [100,101,102,103].
3.1.2. Pre-Admission Opioid Use Assessment, Risk Stratification for Perioperative ORAEs, and Optimization
Recent guidelines have provided an updated tool recommended for preoperative opioid assessment, termed the Opioid-Naïve, -Exposed, or -Tolerant plus Modifiers (O-NET+) classification system (Table 2) [18]. Patients are deemed opioid-naïve if they have had no opioid exposure in the 90 days prior to surgery, opioid-exposed if they have taken any amount less than 60 milligram (mg) oral morphine equivalents per day (MED) in the same time period, or opioid-tolerant if they have taken 60 MED or more in the seven days before surgery. Risk modifiers are then utilized to stratify the patient’s risk for perioperative ORAEs, such as uncontrolled psychiatric disorders, any substance use disorder history, maladaptive behavioral tendencies that could impact pain management, and the surgical risk for persistent pain. These categories can then be used to guide perioperative risk mitigation strategies and optimization goals. Patients at every risk level benefit from preoperative education and expectation management in addition to multimodal analgesia throughout the perioperative care continuum. Additionally, patients at moderate risk for perioperative ORAEs should be referred for optimization of psychobehavioral comorbidities, and high risk patients should also be referred to a pain management specialist prior to surgery (Table 2). While not all identified risk factors may be modifiable in time for surgery, the O-NET+ classification system affords the ability to identify higher risk patients proactively to inform perioperative planning and support future practice research [18].

Table 2.
O-NET+ Classification System and Recommended Optimization for Patients on Preoperative Opioids.
Patients using opioids prior to surgery should also receive a customized evaluation of their current analgesic regimen for optimization opportunities, which may include maximizing pre-admission multimodal therapies and/or tapering of opioid therapies. Conversely, certain pain medications may need to be interrupted for surgery (e.g., aspirin or other anti-inflammatory agents), in which case clinicians should provide clear rationale and education on safe resumption after surgery. Patients on long-term opioid therapies prior to surgery experience increased rates of postoperative complications in addition to higher rates of persistent postsurgical pain and prolonged opioid use, so preoperative opioid minimization has emerged as a potentially modifiable risk factor. To this end, current consensus statements and expert opinion suggest titrating preoperative opioid therapies to the lowest effective dose, depending on the patient’s underlying condition [18,104,105,106]. Patients currently taking more than 60 mg MED may be evaluated for a goal of tapering to less than this threshold by one week prior to surgery as a possible mechanism for reducing risk of perioperative ORAEs, since this should theoretically reduce postoperative opioid requirements. One study found similar postoperative outcomes between opioid-naïve patients and chronic opioid users who successfully reduced their preoperative opioid dose by at least 50% before surgery, and both of these cohorts experienced significantly improved outcomes compared to chronic opioid users who were unable to wean to this threshold [107]. Some experts have proposed delaying elective surgery in chronic pain patients for a structured 12-week prehabilitation program focused on opioid reduction (general goal of ~10% per week) and increasing psychological reserve ahead of painful procedures [108]. The ultimate goals of preoperative opioid minimization include improving postoperative pain control, limiting perioperative opioid exposure and associated ORAEs, and avoiding persistent dose escalations of chronic opioid therapies [18].
High-quality data does not exist at this time to support strong recommendations regarding preoperative opioid reduction strategies, so a patient-specific, collaborative approach informed by appropriate expertise is vital. General guidance exists for opioid tapering in patients on chronic opioid therapy, but application to the preoperative setting is not discussed [109,110]. Opioid tapering must always be accompanied by patient education and respectful support from the healthcare team [104,109]. Transitional pain services or other perioperative pain management specialist consultation is recommended for opioid-tolerant or otherwise high-risk patients by current guidelines and is supported by implementation reports [15,18,111,112,113,114]. Current institutional expertise and resources limit availability of such services at many centers, representing an important area for future investment by health-systems and institutions.
3.1.3. Planning for Perioperative Management of Chronic Long-Acting Opioids and/or Medication Assisted Treatment (MAT)
Patients with chronic pain and/or substance use disorders pose significant challenges to perioperative pain management and opioid stewardship. These complex surgical populations are expected to continue growing, necessitating increased clinical knowledge and creativity from perioperative providers [115]. It is imperative that surgery centers create mechanisms for identifying these high-risk patients prior to surgery to allow for preoperative optimization and coordination of perioperative care. Pre-admission expert consultation is recommended, as is coordination with the patient’s chronic therapy prescriber, to allow for optimal perioperative care and safe transitions throughout the recovery period [15,18].
Perioperative management of chronic long-acting opioid receptor therapies, including those used as medication-assisted treatment (MAT) for substance use disorders, should be planned during the pre-admission phase of care. These high-risk medications include long-acting pure mu-opioid receptor agonists (e.g., OxyContin®), methadone, a multitude of buprenorphine products, and the pure opioid antagonist naltrexone (Table 3). A thorough pre-admission medication reconciliation is imperative, including the assessment of available prescription drug monitoring program (PDMP) data, since the use of these products span many formulations and therapeutic indications that may not be evident upon history and physical alone. For example, buccal, transdermal, and implanted formulations of buprenorphine are increasingly used for chronic pain indications. Additionally, naltrexone is used off-label for self-mutilation behavior, and is also available in a combination oral product labeled for weight management (Contrave®). Table 3 summarizes current general recommendations for perioperative management of chronic opioid receptor therapies.

Table 3.
Recommendations for Perioperative Management of Long-Acting Opioids and Medication Assisted Therapy (MAT).
Chronic pain and opioid tolerance are frequently complicated by opioid-induced hyperalgesia, physical dependence, psychological comorbidities, and/or substance use disorders, making postoperative pain more difficult to manage in this population [104,116,117,118]. These factors contribute to current expert recommendations to continue chronic long-acting opioid agonists throughout the perioperative period, including methadone and buprenorphine [18,115,116,119,120,121,122]. Methadone and buprenorphine can be prescribed for either chronic pain treatment or as medication-assisted treatment for opioid use disorder (OUD) in the outpatient setting.
Conventional belief has been to discontinue buprenorphine therapy prior to surgery to allow for unencumbered mu-opioid receptors and more effective perioperative analgesia. Current data and clinical experience have challenged this practice, and experts cite multiple reasons for supporting perioperative continuation over interruption. Firstly, buprenorphine is now better understood as an efficacious analgesic, and likely one without ceiling dose effect for analgesia. Little data exists to support better pain control with buprenorphine cessation. Ceiling effects are observed for respiratory depression and sedation, however, likely conferring a safer risk profile than pure mu-opioid agonists [104,122,129,130,131,132]. Buprenorphine has also demonstrated protective effects against opioid-induced hyperalgesia, likely improving postoperative pain responsiveness to therapy [121]. This notion is supported by retrospective evidence that chronic buprenorphine users exhibit lower postoperative opioid requirements when buprenorphine is given on day of surgery versus when it is not [133]. These unique qualities suggest buprenorphine continuation is beneficial to pain control and opioid safety in the perioperative period, and preoperative cessation of therapy removes these benefits when they may be most advantageous. A more nuanced strategy is to temporarily increase and/or divide buprenorphine or methadone dosing starting on the day of surgery to maximize pain control without increasing peak-related adverse effects. This has pharmacologic merit in that the analgesic duration of action for buprenorphine and methadone is far shorter than their active duration for reducing cravings [121,128].
For patients on buprenorphine doses exceeding 8–12 mg/day, some experts consider a preoperative reduction to 8–12 mg/day that is then continued throughout the perioperative period, in concert with the patient and buprenorphine prescriber [122,126,132] (see also Section 3.5.3). Data describing the impact of this strategy on patient-centered outcomes remains limited. An alternative option that has previously been proposed is transitioning the patient to a pure mu-opioid agonist (e.g., methadone) prior to surgery. This strategy creates challenges when converting back to buprenorphine postoperatively due to the risk of precipitous withdrawal and length of time (days) involved. Additionally, removing the protective effects of partial agonism to overdose risk likely makes this strategy less safe, and we discourage its use [123].
Preoperative discontinuation of buprenorphine is no longer recommended [18,119,120,122,126,132]. Complete buprenorphine cessation can lead to opioid withdrawal syndrome if sufficient alternative opioid agonists are not administered, and standard perioperative protocols may not be adequate for this purpose. While not life-threatening, opioid withdrawal is physically and psychologically taxing to the patient and is likely to contribute to increased perioperative opioid exposure, postoperative complications, prolonged hospital stays, and increased healthcare costs. In addition to necessitating increased doses of less safe opioids for adequate postoperative pain control, interruption of chronic buprenorphine therapy requires a subsequent opioid-free period prior to reinitiation. This is especially problematic in a population that may be experiencing opioid-induced hyperalgesia, uncontrolled pain, unmet psychosocial needs, continuity of care gaps, and access to non-prescribed opioids in the postoperative period. While clinical data is limited, expert opinion cites this dynamic as a key driver of postoperative opioid misuse and opioid use disorder development or relapse [74,119,120,122,123,126].
In short, buprenorphine is appropriately viewed as an effective basal analgesic therapy with possible protective effects against ORAEs, psychological destabilization, and relapse. Therapy interruption at the time of painful stimulus is likely to exacerbate the underlying indication for buprenorphine, opening the door to inadequate pain control, increased postoperative complications and costs, and opioid misuse. To this effect, a recent clinical practice advisory states, “it is almost always appropriate to continue buprenorphine at the preoperative dose; furthermore, it is rarely appropriate to reduce the buprenorphine dose” [119]. This is supported by current consensus statements and expert reviews [18,120,121,122,123,124,125,126,127,128]. Rigorous evidence on postoperative pain management in patients on MAT remains urgently needed to quantify these anecdotal benefits and to compare the effects of available perioperative strategies on patient-centered outcomes [115]. It is also important for healthcare providers to understand the role of buprenorphine coformulation with naloxone, and that continuing combination products (i.e., Suboxone®) poses no risk of opioid reversal when the dosage form is taken appropriately. The naloxone is only made bioavailable when the dosage form is altered in an attempt to inject it, and was developed as an abuse deterrent [126].
Conversely, naltrexone formulations must be discontinued in sufficient time to ensure complete wash-out prior to surgery to avoid iatrogenic pain crisis, since opioids are rendered largely ineffective during therapy [123,124]. Animal data suggest opioid therapies would need to be increased 10–20 times the standard clinical dose to achieve analgesia in patients on concomitant naltrexone [134], and human data is very limited [115,135]. Chronic naltrexone therapy induces opioid receptor up-regulation, however, so patients usually on naltrexone therapy may exhibit increased sensitivity to opioids after naltrexone discontinuation for surgery [117,136]. Postoperative planning for such patients should include maximal nonopioid therapies, opioid-naïve dosing for as-needed opioids, and increased monitoring for adverse events [117,124,128,135].
3.1.4. Perioperative Planning for the Patient with Active Substance Use
A thorough social history is imperative to proactively identifying other substance use that may have significant consequences for postoperative pain management. Patients who exhibit misuse of prescription and/or illicit opioids and also require surgery pose an exceptional challenge [137]. Providers should anticipate postoperative withdrawal symptoms and increased pain sensation in patients with active opioid use disorder (OUD) and ensure postoperative monitoring using validated measures [123,128,138]. Perioperative planning should include opioid withdrawal management and maximizing multimodal agents, including ketamine [104,123,139,140]. Medication-assisted treatment (MAT) initiation and optimization of psychiatric comorbidities should be attempted in the pre-admission phase when time and patient desire allow. If MAT initiation is not possible or desirable prior to surgery, planning for postoperative inpatient MAT initiation should be pursued, with patient consent. This should involve consultation with the inpatient addiction medicine consultant, who will also arrange outpatient follow-up and post-discharge resources for continued OUD management [123].
Patients with alcohol use disorder should be managed expectantly in the postoperative period using validated assessments [141,142]. While such patients do not demonstrate cross-tolerance requiring increased opioid doses to effectively treat pain, the concomitant use of benzodiazepines will confer an increased risk of respiratory depression and increased monitoring is needed. Likewise, patients using prescribed or illicit benzodiazepines should not be prescribed higher than routine opioids for postoperative pain, but are subject to increased postoperative respiratory risk [140,143]. Increased opioid tolerance has also not been observed in postoperative patients with baseline cocaine and/or amphetamine use, but stimulant withdrawal can occur upon cessation that may add to postoperative anxiety and discomfort [140].
Recreational and medicinal cannabinoid use is expanding, including various applications to chronic pain management, and may be replacing chronic opioid and other substance use in some patients [144,145,146]. Providers should actively engage patients in shared decision-making and education regarding the perioperative implications of chronic cannabinoid use (discussed comprehensively elsewhere [147,148]), including how postoperative pain is affected. Cannabinoid use is associated with significantly increased anesthetic requirements during surgery, higher postoperative pain scores, higher perioperative opioid consumption, and poorer postoperative sleep quality [149,150,151,152]. This may be due to cannabinoid receptor downregulation and the complex interactions of the endocannabinoid system with various neurotransmitters and pain modulation pathways [153,154]. Cannabinoids may also increase risks for perioperative medical complications and drug interactions, and so many practitioners are advising perioperative cessation [148]. Chronic cannabinoid users will experience an uncomfortable withdrawal syndrome after abrupt cessation, however, so preoperative down-titration and close postoperative monitoring may be considered [104,140,155]. High-quality evidence to guide perioperative management of active substance use remains elusive.
3.2. Preoperative Phase
The preoperative phase of surgical care begins at patient presentation to the preoperative area on the day of procedure (“postoperative day zero” or POD0). This onsite period, prior to the administration of sedatives or anxiolytics, is ideal to renew education and expectation-setting regarding perioperative analgesia. The patient and caregiver(s) should be engaged in shared decision-making to finalize the anesthetic plan and complete consent documentation.
Preoperative anxiety is common among patients and caregivers. Patient education is associated with decreased anxiety, and nonpharmacologic modalities improve relaxation and positive thinking as part of a multimodal approach to postoperative pain management [15]. While evidence is insufficient to strongly recommend specific strategies, perioperative cognitive-behavioral therapies including guided imagery and music therapy are noninvasive and unlikely to cause harm. Their positive effects on reducing anxiety may provide downstream benefits to narcotic avoidance and analgesia, but further study is needed [15,55,156,157,158,159,160]. Massage and physiotherapy have contributed to improved pain control in other settings and are being explored for perioperative applications [55]. Preoperative virtual reality technology has also been successfully employed to reduce perioperative anxiety and pain [161,162,163].
Most notably, the preoperative phase of care should be employed to administer preemptive analgesia. Preemptive analgesia refers to the administration of analgesics prior to a painful stimulus (i.e., surgical incision) to decrease subsequent pain response. A complex interplay between surgical incision and preexisting factors drives a cascade of central and peripheral sensitization, inflammation, and neuromodulation that intensifies and prolongs postoperative pain beyond the point of physical healing. Preemptive analgesia attenuates these processes to confer reduced postoperative pain, decreased opioid requirements, and potentially less-frequent development of persistent postsurgical pain across diverse procedures [15,53,164,165,166,167,168,169,170,171,172]. Preemptive analgesics can generally be administered orally with sips of water one to two hours prior to operating time. This strategy is expected to maximize efficacy by aligning pharmacokinetics with therapeutic goals and avoids the risks and costs of unnecessary intravenous agents, which are unlikely to confer meaningful benefit over their enteral counterparts [15,169,173,174,175,176]. Intravenous agents should be employed in patients with true contraindications to enteral administration or in those with significantly impaired enteral drug absorption.
While every surgical patient should be offered multimodal preemptive analgesia as a component of comprehensive perioperative analgesia and opioid stewardship, not every patient is an ideal candidate for each medication. Table 4 contains a sample preemptive analgesia protocol with applicable patient-specific exclusion criteria. The optimal pharmacologic agents and doses for preemptive analgesia are undetermined. Acetaminophen is frequently used alongside anti-inflammatory and neuropathic agents, and the combination of these three classes appears to provide the greatest opioid-sparing benefit [177]. Preemptive acetaminophen should be employed widely due to its favorable safety profile, including in patients with cirrhosis [178]. Preemptive opioids may be counterproductive, however, even in opioid-tolerant patients, and are not recommended preoperatively [15,18,106,179]. Preemptive opioids should be especially avoided in opioid-naïve patients due to the risk of increasing postoperative pain perception and opioid use [180].

Table 4.
Example Preemptive Analgesia Protocol.
The use of perioperative gabapentinoids has been increasingly controversial owing to conflicting evidence of analgesic benefit and risks of adverse effects, including dizziness and synergistic sedation with concomitant opioids [61,185,186,187,188,189,190]. The U.S. FDA has issued additional warnings regarding the risk of respiratory depression with gabapentinoids in patients who have respiratory risk factors, including the elderly, the renally impaired, those with chronic lung diseases, and those on concomitant sedatives [191]. This warning cited predominantly observational data and emphasized the need for patient-specific risk assessments. One of the reviewed studies suggested increased risk with preoperative gabapentin doses over 300 mg [61], while another did not identify any significantly increased risk when exposure was limited to a single preoperative dose [189]. A third retrospective analysis found preoperative gabapentin exposure was associated with a 47% increase in odds of experiencing a postoperative respiratory event, though the vast majority of the studied population were administered doses exceeding 300 mg [190,191]. Gabapentinoids exhibit dose-dependent propensity to increase postoperative pulmonary complications, though combination with other multimodal agents may negate this risk, and the absolute risk of adverse events with perioperative gabapentinoids appears low [177,192,193]. Hence, adverse event risks of gabapentinoids can be substantially mitigated by using conservative doses (i.e., 300 mg gabapentin preoperatively), avoiding postoperative use in patients experiencing or at risk for sedation or dizziness, and/or avoiding entirely in high-risk patients.
Despite these limitations, gabapentinoids have consistently demonstrated significant opioid-sparing benefits and reduced postoperative nausea [15,60,185,194,195,196,197,198,199]. A recent meta-analysis suggested minimal analgesic benefit to perioperative gabapentinoids in terms of patient-reported pain scores, yet found a significant opioid reduction of approximately 90 mg oral morphine over the first seventy-two postoperative hours [185]. Additionally, gabapentinoids may mitigate central sensitization and decrease the risk of persistent surgical pain, though further research is needed [53,172,200]. Opioid-tolerant patients may especially benefit [117]. Hence, gabapentinoids remain a valuable tool in the perioperative opioid stewardship arsenal for appropriate patients and are supported by multiple guidelines [15,18,197,201]. Ongoing controlled trials may further delineate the effectiveness, safety, and cost-effectiveness of perioperative gabapentinoids [202].
Some pharmacokinetic differences exist between gabapentin and pregabalin, though both are heavily renally eliminated. Pharmacokinetic profiling suggests an equipotent ratio of 6:1 for gabapentin:pregabalin doses [203]. Some have suggested that switching to pregabalin from gabapentin may reduce adverse events in the chronic neuropathic pain setting, but these benefits were not sustained or significantly different from patients who remained on gabapentin [204]. The relative safety profiles of the gabapentinoids in perioperative settings are therefore unlikely to differ when use is limited to short-term, low doses. Duloxetine, a serotonin- and norepinephrine-reuptake inhibitor with analgesic properties, has also been effective in perioperative multimodal regimens, representing a potential alternative to gabapentinoids [205,206,207,208,209,210].
Nonsteroidal anti-inflammatory drugs (NSAIDs) have long been shrouded in safety concerns of variable validity [183]. Bleeding risk has been of primary concern with perioperative NSAID exposure given the anti-platelet effects of cyclooxygenase-1 (COX-1) inhibition. Bleeding times and postoperative bleeding events do not appear significantly affected by NSAIDs at usual doses, and this risk may be further mitigated by using COX-2 selective agents [211,212,213,214,215,216]. Traditional dogma has suggested avoiding NSAIDs in spinal/orthopedic fusion surgeries because of the risk of nonunion. More recent and higher quality data suggests short-term NSAID use at normal doses does not affect spinal fusion rates and is valuable for postoperative analgesia and opioid minimization [60,167,217]. High-quality prospective studies are needed to definitively assess this risk. In gastrointestinal surgery, NSAID use has been associated with increased risk of anastomotic leak, but recent meta-analyses suggest this concern may be limited to non-selective NSAIDs [218,219,220].
Available literature suggests celecoxib, a selective COX-2 inhibitor, is not associated with the aforementioned concerns with NSAID use in spine and gastrointestinal surgery [60,218,219,220]. Celecoxib is the only NSAID specifically recommended for preoperative use in clinical practice guidelines for postoperative pain management, likely owing to the significant evidence in this setting and lower rates of some adverse effects [15,212]. While celecoxib could be viewed as the NSAID of choice for perioperative use in many surgical populations, it must be avoided in cardiac surgery, where selective COX-2 inhibitors have been associated with increased rates of major adverse cardiac events [201,221]. Increased rates of adverse cardiac events have not been demonstrated with nonselective NSAIDs in cardiac surgery, nor with selective COX-2 inhibitors in noncardiac surgery [183,222]. Caution may still be warranted with selective COX-2 inhibitors in noncardiac surgery patients with significant cardiovascular disease, but these risks may not be significant when exposure is limited to short-term perioperative use [183,212,223,224,225]. Patient-specific risk-benefit assessments regarding perioperative NSAID use are warranted and should include consideration of the risks of increased pain and opioid use in each given patient [183]. All perioperative NSAIDs are inadvisable in patients with preexisting renal disease or otherwise at high risk of postoperative acute kidney injury [226,227,228,229,230]. NSAIDs, including celecoxib, should not be withheld in patients with sulfa allergies, however [231,232,233]. Although chronic NSAID should be avoided in bariatric surgery patients, short-term perioperative use is considered safe and beneficial, and is recommended in this population per current guidelines [234,235,236]. Concomitant, temporary proton pump inhibitor therapy could be considered in patients with high gastrointestinal risk.
3.3. Intraoperative Phase
Anesthetists are crucial team members in optimizing perioperative pain management and opioid stewardship since these aspects, alongside many postoperative outcomes, hinge upon effective anesthesia. Anesthetic strategies include general, regional, and local modalities, as reviewed comprehensively elsewhere [237,238,239,240,241]. General anesthesia has progressed from its origins in deep, long-acting sedative-hypnotics to a more “balanced” strategy employing a combination of agents to create the anesthetized state while facilitating quicker recovery. Balanced general anesthesia now includes broader multimodal agents to mitigate surgical stress and decrease reliance on systemic opioids [242]. Regional anesthesia is divided into neuraxial and peripheral strategies, and various techniques within these strata are reviewed (Table 5). These ever-expanding anesthetic options have rendered controlled comparative efficacy studies challenging, limiting available guidance on optimal techniques for perioperative analgesia and opioid stewardship. Furthermore, the feasibility of anesthetic strategies varies widely by procedure type, anesthetist training, institutional capabilities, and patient-specific factors. Multiple professional collaboratives have generated quality procedure-specific reviews and recommendations to which perioperative teams should refer when developing anesthetic pathways at the institutional level [20,22].
3.3.1. Regional and Local Anesthesia
Regional anesthesia is a cornerstone of multimodal analgesia and opioid minimization, in addition to reducing perioperative morbidity and mortality. General anesthetics can be reduced or sometimes avoided with regional anesthesia, resulting in shorter recovery times and less adverse drug effects such as postoperative nausea and vomiting. Hence, regional anesthesia is integral to the enhanced recovery paradigm [23,62,63,243,244,245]. The benefits of regional anesthesia continue to be explored and include reduced cancer recurrence when used in oncologic surgeries, likely owing to the mitigation of inflammatory marker surges and other immunomodulatory effects [246,247]. While regional anesthesia is a foundational modality for perioperative analgesia and opioid stewardship, it requires input from patients, expertise from clinicians, and careful procedural assessment and institution-specific tailoring of anesthetic options [15,62,63,248]. Key components and considerations for regional and local anesthetic strategies are summarized in Table 5.
The main limitation of local anesthetics is their duration of action, which diminishes their ability to provide opioid-sparing analgesia for multiple postoperative days [249]. One strategy for extending clinical duration of regional anesthesia is the addition of pharmacologic adjuvants such as dexamethasone, clonidine or dexmedetomidine, and/or epinephrine [249,250,251,252,253,254]. While additives to local anesthetics may extend duration of peripheral nerve blockade by as much as 6–10 h and are supported by clinical practice guidelines, total duration of action for single-shot injections will still be limited to less than 24 h [15,249,252]. Additionally, despite considerable research, data remains of low quality and with conflicting results for common pharmacologic adjuvants to peripheral nerve blocks, and they may confer additional risks. These dynamics preclude strong recommendations or expert consensus regarding their use [251,252]. Alternatively, continuous catheters are effective strategies for extending local anesthetic analgesia, and are supported by clinical practice guidelines when the duration of analgesia is expected to exceed the capacity of single-injection nerve blocks [15,255,256]. Continuous catheters are not without limitations, however, including increased complexity to perform and maintain, catheter-related complications, and additional monitoring and follow-up requirements [249]. As such, controlled-release local anesthetic formulations have also been developed [257,258,259]. Liposomal bupivacaine has not demonstrated clinically meaningful benefits to postoperative pain control or opioid reduction when compared to conventional local anesthetics in local wound infiltration, periarticular injection, or peripheral nerve blockade [249,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275]. Potential benefits and cost-effectiveness of extended-release local anesthetic formulations are likely to vary significantly depending on injection technique, site, and type of surgical procedure, so institutions should consider surgery- and patient-specific use of these agents.
To ensure patient safety, it is imperative to have a standardized, collaborative assessment of the total local anesthetic exposure from all sources. Clinicians must remain vigilant to ensure toxic doses are not reached inadvertently when using multiple local anesthetics across anesthesia and surgical applications (i.e., peripheral nerve block in addition to periarticular injection in total knee arthroplasty). Furthermore, local anesthetic toxicity may be masked while a patient is under general anesthesia. To avoid cardiovascular collapse and death, local anesthetic systemic toxicity must be recognized and treated early [276,277]. Accordingly, current guidelines recommend against intravenous lidocaine within four hours of most local anesthetic-containing regional anesthetic strategies, though local anesthetic infusions through wound or epidural catheters may be started without boluses at thirty minutes after IV lidocaine has been stopped [26]. Additionally, local anesthetics must be used extremely carefully in patients with Brugada Syndrome due to potential arrhythmic effect [278].

Table 5.
Selected Attributes of Regional and Local Anesthetic Strategies for Pain Management and/or Opioid Stewardship.
Table 5.
Selected Attributes of Regional and Local Anesthetic Strategies for Pain Management and/or Opioid Stewardship.
| Category, General Considerations | Anesthetic Strategy | Application | Specific Clinical Considerations |
|---|---|---|---|
| Neuraxial Regional Anesthesia Provides motor, sensory, and sympathetic blockade Includes local anesthetics +/− opioids May serve as primary or adjunctive anesthetic or analgesic strategy Significantly improves pain control and decreases use of systemic narcotics May decrease postop morbidity and mortality Increases risks of urinary retention, hypotension Rare catastrophic complications Requires interruption and careful management of antithrombotics | |||
| Spinal (intrathecal) injections | Single injection of local anesthetic +/− opioid 1 into subarachnoid space; for surgeries below umbilicus | Hypotension, pruritus (if opioid used); Requires careful assessment and monitoring of postop narcotics if opioid used | |
| Epidural infusions | Continuous infusion +/− PCEA or PIEB of local anesthetic +/− opioid into posterior epidural space; wide range of procedures (thoracic, abdominal, lower extremity) | Infusion pumps and catheters require special monitoring; may complicate or delay postop mobility or pose other logistical challenges; require careful postop narcotic management if opioid used | |
| Para-vertebral blocks | Single/multiple injections or catheter placement for continuous local anesthetic infusion along vertebra near spinal nerve emergence; for thoracic or abdominal procedures | Effective blockade of complete hemithorax or hemiabdomen but technically difficult; modern practice generally favors fascial plane blocks or alternative neuraxial modalities | |
| Peripheral Regional Anesthesia Includes local anesthetic injections or infusions (CRA), +/− pharmacologic adjuvants Can limit/avoid need for general anesthesia for some procedures, or can be combined with anesthesia as analgesic strategy Fewer risks and contraindications than neuraxial techniques as most are IM injections Most do not provide sympathetic block Significantly improves analgesia, decreases narcotic requirements May decrease morbidity Rare risks of nerve injury, bleeding, infection, LAST Use of ultrasound guidance has increased safety and consistency | |||
| Plexus blocks | Brachial plexus blocks for unilateral upper extremity procedures; lumbar plexus blocks for hip or lower extremity | Requires significant clinician expertise of anatomy; proximal brachial plexus blockade risks hemidiaphragmatic paresis | |
| Peripheral nerve blocks | Provide targeted anesthesia and/or analgesia of specific nerve or nerve bundles for extremity procedures | Numb limb or distribution must be protected from inadvertent injury, such as thermal injuries, hyperextension, or falls | |
| Fascial plane blocks (e.g., TAP, ESPB, FIB, PECS-2) | Use higher volumes of dilute local anesthetics to target dermatomes/nerve planes; for thoracic, abdominal, spinal or extremity procedures | Provide unilateral, dermatomal, or regional analgesia; increasing use in modern practice due to safety, ease of administration and broad applications | |
| Intravenous blocks (IVRA) | Use high doses of short-acting local anesthetic injected into venous system of an exsanguinated distal extremity to provide anesthesia and analgesia | High doses of local anesthetic are used so dual tourniquets must be used and their release carefully timed to prevent LAST; use limited to procedures less than 1 h | |
| Local Anesthesia Mild sensory blockade of superficial/cutaneous nerves Minimal side effects Caution with type of local anesthetic, total exposure, and comorbid conditions (e.g., Reynaud) Avoid open wounds and compromised dermis with some techniques/products | |||
| Wound infiltration | SC and/or intradermal injection(s) by surgeon for incisional pain | Less effective if injected into areas of tissue infection | |
| Periarticular injections | Generally injected by surgeon without use of ultrasound guidance, such as in TKA | Provides effective postop analgesia, in some cases minimizing the need for peripheral nerve blockade | |
| Topical | Applied as sprays, creams, gels, patches, or oral rinses for superficial pain | Some can be safely self-administered by patient |
1 Routine intrathecal opioids are not recommended by some guidelines [188]. Abbreviations: CRA = continuous regional anesthesia, ESPB = erector spinae plane block, FIB = fascia iliaca block, IM = intramuscular, IV = intravenous, IVRA = intravenous regional anesthesia (e.g., Bier block), LAST = local anesthetic systemic toxicity, PECS-2 = pectoralis nerve block (2 injections), PCEA = patient-controlled epidural analgesia, PIEB = programmed intermittent epidural bolus, SC = subcutaneous, TAP = transversus abdominis plane block, TKA = total knee arthroplasty. References: [15,18,23,170,188,237,240,242,249,250,255,279,280,281,282,283,284,285,286,287].
3.3.2. Systemic Multimodal Adjuncts
Limitations to regional anesthesia include patient and systems factors. As such, systemic multimodal adjuncts should be implemented or used concurrently with regional anesthesia. These systemic therapies are usually started perioperatively and limited to the intraoperative phase of care or continued into the short-term recovery or postoperative phases. Table 6 summarizes dosing and clinical considerations for common intraoperative multimodal analgesics administered systemically.

Table 6.
Clinical Considerations for Intraoperative Systemic Multimodal Analgesics.
Lidocaine infusions are one adjunct that may be applied in the perioperative period. Data exist for lidocaine infusions as opioid-sparing modalities across multiple procedure types, though most literature is for intra-abdominal procedures. Multiple studies have suggested decreased pain scores, decreased 24-h postoperative opioid usage, possible decreased length of stay, and minimal adverse effects [15,18,26,281,288,289,290,291]. Studies vary widely regarding the dosing of lidocaine infusions, whether or not boluses are administered, and infusion duration [291,292,293,294]. Although lidocaine infusions are frequently started intraoperatively, some centers may instate or continue therapy in the postoperative period where supported by institutional protocols [290]. Lidocaine infusions have been used to provide analgesia outside of the surgical arena, such as in patients with traumatic rib fractures [295]. Current guidelines generally recommend a loading dose of no more than 1.5 mg/kg be given as an infusion over 10 min, followed by an infusion of no more than 1.5 mg/kg/h for no longer than 24 h [26]. All doses must be calculated based upon ideal body weight and should not exceed 120 mg/h in any patient. Doses should be substantially reduced in patients with mild renal or hepatic dysfunction, and avoided entirely in patients with moderate or significant end organ dysfunction and in those weighing less than 40 kg. Other relative contraindications should be evaluated prior to use, including cardiac disease, electrolyte disorders, seizure and other neurologic disorders, and pregnancy or breastfeeding. Serum lidocaine level monitoring is not generally warranted with short-term perioperative use but could be considered if toxicity concerns emerge. Extensive monitoring recommendations should be reviewed and standardized institutional protocols put in place for this modality [26,296].
Similarly, sub-anesthetic ketamine by bolus or infusion has been applied to perioperative and inpatient settings for nonopioid analgesia. Ketamine’s ability to improve analgesia and mitigate opioid tolerance and hyperalgesia stems from its antagonism at the NMDA receptor; however, ketamine has a complex receptor profile that likely informs multiple acute and chronic pain pathways. While ketamine may be appropriately considered for opioid-naïve patients undergoing painful procedures, it is especially beneficial to the opioid-tolerant population [15,18,25,117]. Professional consensus statements exist for both intravenous lidocaine and ketamine use for postoperative analgesia and should be consulted. Patient selection, monitoring, and systems implementation are imperative for safety and success with these agents [25,26].
Magnesium has been investigated for its role in attenuating acute and chronic pain. Proposed mechanisms include magnesium’s antagonism of the NMDA-receptor, similar to that of ketamine. NMDA-receptor antagonism may interrupt central sensitization of pain, therefore allaying the pathologic transition from acute to chronic pain. An additional potential mechanism is magnesium’s antagonistic effects on calcium, as elevated levels of calcium are involved in central sensitization [297,298,299,300].
Other systemic medications studied for nonopioid perioperative analgesia include the α2-adrenergic receptor agonists dexmedetomidine and clonidine. These medications provide central analgesia and decrease agitation and sympathetic tone without significant inhibition of respiratory drive. Dexmedetomidine is a highly selective agonist at the α2-2A receptor subtype, which mediates analgesia and sedation from multiple locations within the central nervous system. This central sympatholysis blunts surgical stress and decreases kidney injury, though evidence is limited [261,317,320,321]. Similarly, esmolol has been investigated as a synergistic analgesic intraoperatively. Esmolol may contribute to antinociception by blunting sympathetic arousal transmission through β-adrenergic receptor antagonism, but mechanisms and benefits are still being elucidated [324,325].
Systemic multimodal analgesics have been studied as additives to peripheral and/or neuraxial regional anesthetic strategies, including magnesium, α2-agonists, dexamethasone, and methadone. Limited comparative efficacy among routes of administration has emerged. This appears most true for dexamethasone, which confers similar benefits to pain control and opioid use when administered via either modality [259,327,328,329,330,333]. Although administering dexamethasone as a component of peripheral nerve blockade may avoid systemic side effects, perineural dexamethasone may have a local effect on nerve tissues that may be undesirable in some patient populations. While literature exists for individual additives to various regional anesthetic techniques, there is no widely accepted consensus regarding ideal drug selection and dosing and if/when systemic administration is preferred [15,250,254,259,300,331,332,341].
Methadone is a systemic multimodal agent explored with increasing interest. A unique opioid in kinetic and mechanistic properties, methadone can be administered once intravenously at procedure commencement to provide prolonged analgesia into the postoperative period. In addition to mu-opioid receptor agonism, methadone’s complex mechanism includes NMDA-receptor antagonism and inhibition of serotonin and norepinephrine uptake in the central nervous system. These actions confer benefit in the treatment of chronic neuropathic pain and may also inhibit surgical stress and central sensitization, thus reducing the risks of opioid-related hyperalgesia, tolerance, and persistent postoperative pain [335,336,337,339,342,343]. Appropriate monitoring and communication across transitions of care is important when the anesthetist administers methadone intraoperatively. Education and processes should be implemented to ensure reduced subsequent opioid use and minimization of ORAEs, especially the risk of respiratory depression with concomitant narcotics given during methadone’s prolonged and variable half-life. Alerts embedded in the medication administration record may be ideal, since a “once” dose of intraoperative methadone is likely to be missed by providers in subsequent phases of care, despite its ongoing medication effects in the patient. Still, methadone appears a viable option in the multimodal arsenal and likely a preferable alternative to some clinicians’ use of long-acting pure opioids (e.g., OxyContin®) in preemptive protocols.
Systemic multimodal agents available to the intraoperative phase of care are plentiful but remain underutilized. This phenomenon results from the lack of high-quality data to guide many patient care decisions, especially comparative efficacy to inform agent selection, dosing, combination, and contraindications. Institutions are encouraged to generate collaborative protocols and processes that support the safe use of these agents in appropriate patients, including pre-built order sets with recommended patient selection, drug dosing, and monitoring. Deciding and designing an institution-specific “menu” of supported intraoperative options with appropriate safeguards should increase practice utilization and research opportunities.
3.4. Recovery Phase
Ample research supports preoperative nerve blocks to facilitate quicker discharge from post-anesthesia care units (PACUs), owing to their opioid-sparing properties and associated reductions in ORAEs, especially postoperative nausea and vomiting. Patients who undergo surgical procedures with nerve blocks as their primary anesthetic may bypass PACU Phase I with a quicker discharge, enabling increased throughput and efficiency of care while maintaining patient safety and opioid stewardship [63,255,261,344,345].
Multimodal and opioid-sparing strategies should be continued while a patient is in the recovery phase. However, when continuing multimodal strategies, clinicians must be mindful of prior doses of similar agents administered in prior phases of care. When patients are sufficiently awake, providers should limit the intravenous route of opioid administration per current guidelines [15]. Oral administration facilitates longer analgesia with fewer peak-related adverse effects and risks as compared to intravenous routes. Sublingual administration of concentrated oral opioid preparations may be an advantageous strategy for increasing onset of analgesic action with fewer risks than the intravenous route, but this warrants additional study [346]. Additionally, nonpharmacologic analgesic and anxiolytic strategies should be reintroduced in the recovery phase to facilitate patient comfort without reliance on narcotics [158,159,160,347,348,349,350,351,352].
Deliberate opioid stewardship, avoidance of the IV route of administration, and maximal multimodal analgesics are also crucial for facilitating timely discharge from PACU for same-day surgical patients. Regional anesthesia and lighter levels of intraoperative sedation, combined with more minimally invasive surgical techniques, are allowing many previously inpatient procedures to be pursued in the ambulatory setting [353,354,355].
3.5. Postoperative Phase
Postoperative pain management should be individualized to the needs of each patient, noting goals and response to the prescribed approach. This requires the use of a validated pain assessment tool (e.g., numerical, verbal, or faces rating scales, or visual analog score) to assess pain intensity on a recurring basis in addition to functional assessments and evaluation for adverse events [15]. Additionally, pain assessment tools should be appropriate for the patient’s age, language, and cognitive ability [15]. The pain assessment should be made during movement as well as at rest, and must include location, onset and pattern, quality or type of pain (i.e., nociceptive, visceral, neuropathic, or inflammatory), aggravating factors, and response to treatment. Typically, assessments should be performed 15–30 min and 1–2 h after administration of parenteral and oral analgesics, respectively, and less frequently for patients with stable pain control. However, analgesic regimens should not be adjusted based on pain ratings alone, given their inherent limitations for predicting analgesic requirements and the increased risk for opioid overexposure [356,357,358,359]. Functional assessment of how pain is influencing the patient’s ability to achieve postoperative recovery goals should be integrated into a multidimensional approach to adjusting therapeutic regimens [360,361]. Providers should also use pain assessment interactions to reinforce realistic expectations and include the patient in treatment plans throughout the hospital stay. Providers should also be mindful of implicit bias risks when assessing and treating pain. Multiple analyses have found that lower amounts of analgesics are routinely prescribed to Black and other patients of color despite higher degrees of self-reported pain, and that race influences prescriber perceptions of risk for opioid misuse [362,363,364].
Many of the strategies discussed herein for inpatient postoperative patients may also be applied to various special populations, including trauma/emergent surgical patients, the elderly, the obese, obstetric populations, and pediatrics, as discussed in more detail elsewhere [293,300,365,366,367,368,369,370,371,372,373,374,375,376,377].
3.5.1. Postoperative Nonopioid Considerations
Postoperative pain management should continue to incorporate multiple treatment modalities to maximize therapeutic benefits and minimize complications, including nonpharmacologic strategies (Table 7) [15,55]. Physical modalities, including transcutaneous electrical nerve stimulation (TENS), acupuncture, massage, or cold therapy, alone or in combination with medications, may offer pain relief and reduce opioid use, though evidence is variable [15,55,158,160,347,350,378]. Preliminary evidence also suggests cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT), other mindfulness-based psychotherapy and music may reduce postoperative pain intensity and disability [15,79,379,380,381]. Surgery centers should devote due resources to making a variety of nonpharmacologic therapies standardly available to postoperative patients, as strongly supported by current guidelines and regulatory requirements [15,18,36].
To provide effective multimodal and opioid-sparing analgesia, clinicians should standardly provide around-the-clock nonopioid medications after surgery [15,18,33]. Acetaminophen, NSAIDs, and gabapentinoids are commonly prescribed nonopioids in postoperative settings. When used in combination, they are more effective in reducing pain and minimizing opioids compared with monotherapy [177,382,383,384]. Around-the-clock oral acetaminophen should be the backbone of postoperative pain regimens because of its safety and low cost, in the absence of acute decompensated liver disease [178,385]. Compared with the oral route, intravenous acetaminophen administration may offer faster onset and better analgesia thirty minutes after administration, but overall drug exposure after repeated doses and general clinical benefits are not significantly different [176,386,387,388]. Additionally, the intravenous formulation may impose financial toxicity without additional benefit in patients with functional gastrointestinal tracts as discussed previously [389,390,391].

Table 7.
Nonpharmacologic Interventions for Postoperative Analgesia and Comfort.
Table 7.
Nonpharmacologic Interventions for Postoperative Analgesia and Comfort.
| Category | Examples |
|---|---|
| Behavioral/cognitive | Progressive muscle relaxation, mindfulness meditation, art therapy, guided imagery/audio-visual distraction |
| Psychological | Cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT), locus of control assessment |
| Environmental | Music, lighting, comfort items, sleep hygiene (e.g., ear plugs, eye shield), personal hygiene (e.g., shower, hair or nail care) |
| Physical | Heat, ice/cooling, physical therapy, repositioning, acupuncture, massage, osteopathic manipulation, tai chi, yoga, nutrition counseling, healing touch therapy, reiki |
| Activities | Hobbies/leisure (e.g., playing cards, magazines/books, puzzles, games, journaling, knitting), relaxation (e.g., stress ball, television), pet visitation |
| Spiritual | Religious literature & services, onsite spiritual counseling |
References: [55,163,347,378,380,392].
Selective COX-2 inhibitors or other NSAIDs should be incorporated into most postoperative pain regimens with consideration of the type of surgery, renal function, and cardiovascular risk factors (see Section 3.2). Since inflammation is a key driver of pain after surgery, early anti-inflammatories may be the most effective postoperative analgesic strategies, as evidenced by their superior performance over opioids in analyses of randomized controlled studies [164,393,394,395,396]. Novel intravenous formulations of ibuprofen and diclofenac currently have limited roles in therapy due to a lack of demonstrated superiority to ketorolac and significantly higher cost [214,215]. Escalating doses of ketorolac greater than 10–15 mg per dose and ibuprofen greater than 400 mg per dose may offer additional analgesic benefit, and the duration of ketorolac therapy should generally be limited to no more than 5 days [212,397,398,399,400]. Gabapentin or pregabalin should be considered for patients with neuropathic pain and may help reduce postoperative opioid use in select patients (see Section 3.2). If initiating postoperative gabapentinoids, dose reductions and close monitoring should be provided for the elderly, those with impaired renal or lung function, and those on multiple narcotic medications [191]. Genetic phenotypes at multiple metabolic enzymes contribute to variation in patient response to NSAID and other nonopioid analgesics, and emerging guidelines provide therapeutic recommendations [184,401].
Other nonopioid agents including cannabinoids, muscle relaxants, and tricyclic antidepressants cannot be recommended for routine postoperative use based on available data but may have roles in select surgical populations (e.g., chronic pain, spinal surgery) [144,217,402,403]. Analyses of the endocannabinoid system suggest certain cannabinoid receptors mediate pain sensitization and hyperalgesia, possibly increasing risk of acute pain conversion to chronic pain. Cannabinoids may therefore be detrimental in the acute pain setting despite being beneficial in chronic pain management [150,153,154,404].
3.5.2. Postoperative Opioid Considerations
In addition to nonopioid analgesia, many patients undergoing major painful procedures may benefit from short-term postoperative opioid therapy. Table 8 provides a comprehensive example of postoperative opioid and nonopioid medication orders. As with nonopioid agents, oral opioids should be used preferentially over intravenous agents for patients who can utilize oral administration. The intravenous route does not confer superior efficacy and carries greater risk for adverse events, and should therefore be reserved for patients unable to use the oral route or patients with severe pain that is refractory to increased doses of oral agents [15,38,405]. When the intravenous route is intermittently warranted for severe breakthrough pain, healthcare provider administration of opioid doses according to patient-reported and functional pain assessments is typically adequate, especially for opioid-naïve inpatients. The sublingual and subcutaneous routes are also reasonable, but the intramuscular route should be avoided due to delayed and erratic absorption [15]. One single-center retrospective cohort study suggests sublingual opioids can be utilized for postoperative breakthrough pain with comparable efficacy as the intravenous route, and the sublingual route was associated with reduced opioid-related respiratory depression [346].

Table 8.
Example of Postoperative Inpatient Pain Management Orders.
When complete reliance on the intravenous route is considered necessary due to severe gastrointestinal dysfunction or surgical need for strict bowel rest, patient-controlled analgesia (PCA) is recommended over intermittent bolus by healthcare providers by some guidelines [24,403]. This notion is increasingly challenged by enhanced recovery practice, however, especially in minimally invasive colorectal surgery [24,406,407]. Providers may consider reserving use of PCA for patients with acute on chronic pain or otherwise requiring significant amounts of intermittent IV opioids, and only until other routes can be used. Maximizing multimodal therapies in earlier phases of care, especially regional anesthesia or lidocaine infusions, may allow for avoidance of PCA in routine patients undergoing colorectal surgery [24]. The use of intraoperative methadone (see Section 3.3.2) or the sublingual route of administration for postoperative opioids are also promising modalities that could be explored for reducing reliance on PCAs. Medication and patient safety issues abound with PCAs [408,409]. Accordingly, average duration of PCA use has been discussed as a quality indicator of hospital opioid stewardship practices [38]. Use of PCAs should be guided by institutional order sets with pre-built doses stratified for opioid-naïvety and risk for opioid-related respiratory depression, and continuous infusions should generally be avoided in opioid-naïve patients [15,71,408,409].
Empiric opioid selection should align with generally preferred agents, patient-specific pharmacologic needs, and the oral route of administration. Oxycodone, hydrocodone, and hydromorphone should be used preferentially due to their decreased propensities for active metabolites, accumulation in end organ dysfunction, drug-drug interactions, and histamine release (Table 9) [410,411,412,413,414]. Morphine, tramadol, and codeine are significantly metabolized to active metabolites and heavily renally eliminated, increasing the risk of adverse effects in some patient populations [410,415]. Codeine and tramadol have limited roles in postoperative pain management due to well-documented interindividual variability in efficacy and safety [416,417]. Polymorphisms at CYP2D6 and drug-drug interactions significantly affect codeine bioactivation to morphine, the pathway most responsible for analgesic efficacy. Likewise, tramadol is metabolized by CYP2D6 into an active metabolite more potent than the parent drug. Patients possessing increased metabolic variants at CYP2D6 (1.5–9.5% of the worldwide population) are at heightened risk of adverse effects from these agents due to greater conversion to active metabolites, and patients with poor metabolizer phenotypes (25.3–70.3% of the worldwide population) may report decreased efficacy from reduced bioactivation [410,411,412,417,418]. These medications should be avoided in most patients since phenotype testing is not routinely performed before prescribing and since multiple agents with more favorable safety and efficacy profiles exist.
Individual patient response to preferred opioids still varies substantially. Genetic polymorphisms affecting opioid metabolism are not uncommon, so rotation to an agent utilizing an alternative metabolic pathway should be considered in patients with unexplained lack of response and/or significant intolerance (e.g., extreme nausea and vomiting with or without insufficient analgesia from oxycodone may be remedied by change to hydrocodone or hydromorphone) (Table 9) [414,418,419]. Newer opioid agonists can also be considered. Oxymorphone may be advantageous in cases of persistent opioid overexposure related to altered metabolism from phase I enzymatic alterations and/or significant renal impairment. Tapentadol is unique in pharmacologic and pharmacokinetic profiles and can be a valuable option in cases of significant widespread opioid intolerance, but is completely reliant on renal function for excretion. While tramadol is also sometimes considered in patients with intolerance to preferred opioids, its diverse receptor profile confers increased adverse event risks that are especially undesirable in the postoperative period, in addition to previously discussed risks related to its metabolic pathways [417,420,421,422,423,424,425,426,427,428]. Pharmacists can also assess medication regimens for clinically significant drug-drug pharmacokinetic interactions, especially in patients on antiepileptic medications, azole antifungals, or rifampin [413,429,430]. The interprofessional team should also evaluate for pharmacodynamic interactions affecting the patient’s response, such as additive toxicity risk with concomitant sedatives or anticholinergics.
While allergic reactions to opioids are frequently reported, true IgE-mediated hypersensitivity is rare. Only 15% of patients referred for drug provocation testing due to concern with anaphylactic opioid reactions were diagnosed with opioid allergy in one analysis, and opioids are believed to be implicated in less than 2% of all cases of intraoperative anaphylaxis [431,432]. Angioedema and hemodynamic instability are more likely to indicate true hypersensitivity than other reactions [431,433]. In cases of true opioid hypersensitivity, opioids of different structural classes are unlikely to demonstrate cross-allergenicity, though this risk remains uncertain. The majority of opioid reactions are not mediated by IgE but by mast cell degranulation, however, and may present as hives, hypotension, urticaria, pruritus, and/or severe anaphylactoid responses. More synthetic opioids exhibit decreasing rates of opioid-mediated histamine release, so should be considered in cases of pseudoallergy [431,432,433,434].
Clinicians should adjust the empiric postoperative pain management plan in cases for efficacy and tolerability, taking into account the duration and intensity of expected pain for the specific surgical procedure [15]. The use of “may repeat” doses and separate orders only for breakthrough pain can usually allow for a workable escalation pathway for uncontrolled pain within standardized postoperative order sets, as displayed in Table 8. Incomplete analgesic response precluding usual postoperative functional progress despite these orders should prompt a 25–50% increase to the first-line opioid order dose, based on severity of ongoing pain and in the absence of dose-limiting adverse effects. Breakthrough pain regimens should generally be limited to the first 24 postoperative hours, with acceptable pain control maintained by adjusting oral doses if needed. Adjusting opioid regimens in longer-term pain and in cancer-related pain is discussed extensively elsewhere [71,435]. Patients with adequate analgesia but experiencing ORAEs should be assessed for opioid dose reductions, and all opioids should be tapered after surgery as acute postoperative pain improves. If usual surgical recovery is inhibited by unsuccessful functional pain management and/or unacceptable adverse effects despite appropriate multimodal therapies and patient-specific opioid optimization, postoperative pain management specialty consultation is advised. Acute and transitional pain services for surgical patients are evolving, and have been associated with reduced opioid use and length of stay [113,436,437,438,439,440,441].

Table 9.
Opioid Properties to Consider When Selecting or Modifying Postoperative Regimens.
Table 9.
Opioid Properties to Consider When Selecting or Modifying Postoperative Regimens.
| Opioid (Structural Class) | Major Metabolic Pathways | Active Metabolites | Effects of End Organ Function 1 |
|---|---|---|---|
| Phenanthrene opium alkaloids–highest rate of histamine release | |||
| Morphine, Codeine (after bioactivation) 2 | UGT2B7 (phase II metabolism) | Extensive production of active metabolites | Renal impairment significantly increases exposure |
| Semisynthetic phenanthrene derivatives of opium alkaloids–cross-reactivity possible between agents | |||
| Oxycodone | CYP3A4 (primary), CYP2D6 (minor) | Produces small amounts of oxymorphone and other active metabolites | Renal impairment mildly increases exposure |
| Hydrocodone | CYP3A4 (primary), CYP2D6 (minor) | Produces small amount of hydromorphone and other active metabolites | Not significantly altered by renal impairment |
| Hydromorphone | UGT2B7 (phase II metabolism) | Multiple active metabolites but clinically unimportant | Not significantly altered by renal impairment |
| Oxymorphone | UGT2B7 (phase II metabolism) | Metabolites have little activity | Not significantly altered by renal impairment |
| Synthetic phenylpropylamine derivatives of opioid alkaloids–cross-reactivity with phenanthrenes unlikely | |||
| Tapentadol | Unspecified glucuronidation | No active metabolites | Renal impairment significantly increases exposure |
| Tramadol | CYP2D6, CYP3A4 | Extensive production of active metabolites by CYP2D6 | Renal impairment increases exposure |
1 All listed opioids should be reduced in cases of significant hepatic impairment. 2 Codeine is a prodrug of morphine (activated by CYP2D6) and is not recommended for postoperative pain management; see text. Abbreviations: CYP = cytochrome P450 enzyme superfamily, i.e., hepatic enzymes responsible for phase I metabolism. References: [178,410,411,412,414,415,423,425,426,429,430].
Despite employing opioid minimization and evidence-based opioid selection when treating postoperative pain, the interprofessional team should actively anticipate and mitigate opioid-related adverse events (ORAEs, Table 10). Nausea/vomiting, constipation, pruritus, respiratory depression, sedation, and delirium continue to be common adverse effects negatively affecting postoperative outcomes and costs of care [6,7,8]. Sedation and respiratory depression are the most concerning ORAEs and should be actively mitigated through institutional monitoring protocols based on current practice guidelines and published literature. Protocols should include the use of the Pasero Opioid-Induced Sedation Scale (POSS) and capnography monitoring in addition to conventional respiratory parameters and nursing assessments [15,442,443,444,445,446]. Avoiding concomitant sedatives, especially benzodiazepines, to all feasible extent is also an important modifiable risk for postoperative respiratory depression, sedation, and delirium. This is crucial in patients with higher baseline risks for this complications, including the elderly, obese, and those with preexisting lung disease [38,143,190,447,448,449,450,451,452]. Specialized monitoring for patients receiving perioperative neuraxial opioids must be standardly executed and supported by institutional order sets as outlined elsewhere [15,453]. Some enhanced recovery guidelines recommend against routine intrathecal opioids as this strategy may not have a positive benefit-risk profile in this setting [188].

Table 10.
Recommended Monitoring and Mitigation Strategies for Postoperative ORAEs.
Patients prescribed opioids should also receive scheduled stimulant bowel regimens to avoid opioid-induced constipation and progression to ileus, a risk that is heightened in the postoperative period (Table 10). Standard preventative use of a stimulant laxative such as senna or bisacodyl is generally effective in preventing opioid-induced constipation in opioid-naive patients, and available evidence does not suggest a superior agent [454,455,456,457,458]. The addition of stool softeners (i.e., docusate) and/or laxatives of alternative classes (e.g., osmotic agents like polyethylene glycol or magnesium oxide) may be added if needed postoperatively, but sugar-based strategies such as lactulose or sorbitol should be avoided due to adverse event risks [454,455]. Unique considerations exist in major colorectal surgery and are discussed in enhanced recovery guidelines [281]. Peripherally acting opioid antagonists have been developed to combat opioid-induced constipation with mixed results for clinical outcomes and cost-effectiveness related to postoperative ileus [459,460,461,462]. Naloxegol and alvimopan may have comparable efficacy in the postoperative period [463]. An alternative agent used in chronic constipation, lubiprostone, does not appear to have superior efficacy over senna in the postoperative setting [464].
3.5.3. Postoperative Considerations in the Opioid-Tolerant and/or Substance Use Disorder Populations
Postoperative pain management in patients with preexisting opioid tolerance and/or substance use disorders is more complicated and high-risk than that of opioid-naïve counterparts, and specialist consultation is strongly advised [15,18,36]. Nonopioid medications and nonpharmacologic options are especially important in this population due to significant opioid receptor up-regulation. In the opioid-tolerant surgical patient, multimodal analgesia may help limit opioid dose escalation, reduce the incidence of adverse events, and facilitate faster postoperative opioid weaning. Stronger consideration should be given to postoperative use of gabapentinoids, ketamine, and regional anesthesia than what may be used in opioid-naïve patients.
Empiric as-needed opioid regimens should be dosed with consideration to baseline opioid use and closely monitored, recognizing that higher doses and/or longer tapers may be warranted. Patients on preoperative opioids have increased risk for suffering if undertreated and increased rates of ORAEs if overexposed. Still, opioids should be utilized only after first-line administration of nonopioids and used at the lowest effective dose, avoiding persistent dose escalations in the postoperative period [18]. To this end, opioid-exposed patients (i.e., those with preoperative opioid use below 60 MED) can usually be prescribed routine postoperative opioid orders as for opioid-naïve patients, with increased monitoring and adjustment for efficacy as needed. Truly opioid-tolerant patients (i.e., those with preoperative opioid use ≥60 MED) should be interviewed to discern their precise preoperative daily utilization to inform a patient-specific postoperative opioid regimen. Postoperative opioids should not be dosed solely upon prescription drug monitoring program (PDMP) data to avoid unnecessary narcotic exposure in patients taking less than maximum quantities prescribed. Opioid-tolerant patients undergoing minor procedures may only warrant routine as-needed opioid dose orders (e.g., oxycodone 5 mg q4h PRN, may repeat within 1 h if ineffective) in addition to their baseline opioid exposure.
After major painful procedures, opioid-tolerant patients often warrant opioid exposure equivalent to a 50–100% increase from their baseline MED to achieve adequate analgesia and functional outcomes in the immediate postoperative period. Some literature suggests postoperative opioid requirements up to four times that of opioid-naïve patients may be necessary after the same procedure, and little published guidance exists on how best to accomplish this [18,117,128]. Chronic opioid requirements may be maintained by modestly increasing the patient’s usual as-needed opioid dose at the same dosing interval, with additional orders as-needed for breakthrough pain. Alternatively, opioid doses could be scheduled throughout daytime hours to provide the patient’s baseline MED, with additional as-needed doses to allow for adequate control of postoperative pain. A third option may be to order the patient’s usual as-needed opioid dose at a shorter dosing interval (e.g., every 3 h as needed instead of every 4 h) with a breakthrough pain option. To illustrate, a patient regularly taking oxycodone 10 mg every 4 h throughout the day prior to admission (i.e., 60–75 MED baseline use) could be ordered one of the following sets of empiric opioid orders upon postoperative inpatient admission after a major painful procedure, assuming the oral route of administration for primary analgesia and the sublingual route for breakthrough pain:
- (a)
- oxycodone 10 mg PO q4hr PRN moderate-to-severe pain, may repeat 5 mg dose within 1 h if pain unrelieved; oxycodone 5 mg SL q4hr PRN moderate-to-severe breakthrough pain × 24 h
- (b)
- oxycodone 10 mg PO q4hr scheduled while awake; oxycodone 5 mg PO q4hr PRN moderate-to-severe pain; oxycodone 5 mg SL q4hr PRN moderate-to-severe breakthrough pain × 24 h
- (c)
- oxycodone 10 mg q3hr PRN moderate-to-severe pain; oxycodone 5 mg SL q4hr PRN moderate-to-severe breakthrough pain × 24 h.
All initial opioid options are in addition to maximal scheduled nonopioid and nonpharmacologic orders, and accompanied by close monitoring for any appropriate adjustments. Orders for opioids as-needed for breakthrough pain should generally still be limited to the immediate postoperative period (i.e., order should automatically expire after the first 24 h of inpatient ward admission). Ongoing need for breakthrough pain opioid doses should prompt evaluation for nonsurgical causes of pain, further optimization nonopioid therapies, and an increase to the primary as-needed opioid order on a patient-specific basis.
Patients with chronic pain and/or opioid use disorders may benefit from a patient-controlled analgesia (PCA) modality when pain is very difficult to control or when the oral route cannot be used [15,117,128,468]. Empiric reliance on intravenous opioids via PCA is increasingly falling out of favor, however, and should not be viewed as routinely necessary in colorectal surgery when enhanced recovery and multimodal analgesia modalities are maximized [24,406]. Experts are increasingly finding this to be true even in opioid-tolerant patients, and opioid-free intraoperative analgesia is even being explored in this population [18]. If PCAs are employed for opioid-tolerant patients, dosing should be patient-specific after assessment of baseline opioid use, as discussed in detail elsewhere [71,117,128,469].
Continuation of chronic long-acting pain medication regimens is recommended, in consultation with the patient’s outpatient prescriber (see Section 3.1.3). Chronic buprenorphine or methadone therapy should be continued either at baseline dosing regimens or by dividing the total daily dose throughout the day to maximize their analgesic activity (see Section 3.1.3). The patient’s usual total daily dose, or a slightly increased total daily dose, is divided into 2 to 4 doses throughout the day starting on the day of surgery. The patient can then be discharged on their usual preoperative regimen without therapy interruption [121,125,128]. Alternatively, some have advocated for a buprenorphine dose reduction in the perioperative period if the patient is on higher chronic doses and/or is experiencing inadequate pain relief despite appropriately dosed as-needed opioids, citing the dose-dependent mu opioid receptor antagonism of buprenorphine [119,122,126,132]. Patients on maintenance buprenorphine or methadone must also be ordered as-needed opioids at tolerant doses (see examples provided earlier in this section) to effectively treat postoperative pain in addition to the continued buprenorphine/methadone regimen, regardless of the dosing strategy employed for them.
Despite available evidence and guidance, healthcare providers may carry prejudices that result in under-treatment of postoperative pain in the opioid-tolerant and/or opioid use disorder populations. Such misconceptions often include that maintenance therapy with buprenorphine or methadone alone provides sufficient postoperative analgesia, that additional opioids for analgesia may cause addiction relapse or undue respiratory depression risk, or that the use of patient-controlled analgesia (PCA) may exacerbate these risks. In actuality, receptor up-regulation and the pharmacology of these agents confer the need for additional short-acting opioids at opioid-tolerant doses in order to provide equipotent analgesia to that provided to opioid-naïve patients. Available evidence does not support that this strategy exacerbates substance use disorders or increases risk for respiratory depression when appropriate dosing and monitoring are employed. Conversely, under-treated pain is likely a more significant risk factor for opioid misuse, ORAEs, and relapse [74,128,470].
3.6. Discharge Phase
Discharge opioid prescribing following surgery has significantly contributed to the ongoing U.S. opioid epidemic [29]. Collaborative discussions surrounding discharge opioid prescribing are imperative to minimize the risks of dependency and misuse, and should include all analgesics that are to be continued after discharge. Enhanced recovery programs that integrate standardized opioid-sparing analgesic regimens have significantly reduced or eliminated opioid use in the postoperative setting [13]. Opioid-sparing analgesics should therefore be optimized during the inpatient stay and continued at discharge. Postdischarge multimodal analgesia has been associated with decreased outpatient opioid consumption after major procedures [471]. Duration of opioid-sparing analgesics after hospital discharge should be tailored to the individual needs of the patient and the anticipated length of pain expected after surgery. To mitigate adverse effects and dependence, prescriptions for NSAIDs and gabapentinoids should generally be limited to 1–2 weeks postdischarge. If refills are to be prescribed, an evaluation from a prescriber should be conducted to assess etiology of ongoing pain and appropriateness of continued therapies [472].
Until recently, evidence-based guidelines on postoperative opioid prescribing were not readily available. Variable and often excessive opioid quantities have been prescribed after surgery, especially in the U.S. [4,473]. In 2016, the Michigan Opioid Prescribing Engagement Network (OPEN) released procedure-specific guidelines to help reduce overprescribing of opioids after surgery. These guidelines are adjusted regularly using expert opinion, patient claims data, and evidence-based literature, and are only intended for patients who are considered opioid-naïve [32]. Since implementation at 43 hospitals, there has been a significant reduction in the quantity of opioids prescribed after surgery and a corresponding reduction in opioid consumption by patients [474]. Subsequently, multiple other collaboratives have also published postoperative opioid prescribing guidelines for adults [30,31,475,476] and for children [477].
These guidelines should be used as a foundation to inform procedure-specific institutional practices for opioid prescribing at the point of hospital discharge after surgery. However, opioid prescribing must be individualized within this framework. The patient’s pain control and opioid use in the 12–24 h preceding discharge should be evaluated before prescribing discharge analgesics [478]. Patients undergoing minor procedures, those experiencing minimal pain, or patients who are opioid-naïve may not require opioid prescriptions at discharge. When opioids are prescribed to the opioid-naïve patient population, it is best practice to minimize the duration of supply to three days or less for procedures associated with rapid recovery from severe pain, seven days or less for medium term recovery procedures, and fourteen days or less for expected longer term recovery procedures [31]. Long-acting opioids should not be prescribed for the management of acute postoperative pain after discharge and should be especially avoided in patients who were previously opioid-naïve [15,32]. Opioid-tolerant patients generally have higher opioid requirements than opioid-naïve patients and prescribing a postdischarge opioid taper for this patient population is recommended. Typically, tapering the opioid dose by 20–25% every one to two days is tolerated by most patients as their pain is improving [15]. Detailed postoperative opioid taper examples are presented elsewhere [478]. Additionally, prescription drug monitoring programs (PDMPs) should be reviewed prior to prescribing opioids at discharge to chronic opioid users. This allows for review of the patient’s current home supply and prevents overprescribing of unnecessary opioids at discharge [478].
Despite successful institutional efforts to decrease inpatient opioid prescribing, this has not necessarily translated into reduced opioid quantities prescribed at hospital discharge [479]. Discharge analgesic prescriptions are therefore unlikely to correlate with inpatient orders unless enhanced recovery pathways also have effective transitions of care procedures in place. This should include multidisciplinary communication informing patient-specific prescriptions as opposed to “per protocol” discharge opioid prescriptions for a given procedure. Additionally, data is emerging that shared decision-making, where patients are able to play a role in the amount of opioids they are prescribed at discharge, in conjunction with patient discharge education, can reduce the number of pills prescribed [480]. When considering reduced opioid quantities at discharge, a common concern among surgeons is an increase in office calls from patients requesting opioid prescription refills. Ample evidence supports that a large portion of opioids prescribed at discharge after surgery go unused, however, and initiatives to limit discharge opioid prescription quantities have successfully reduced opioid exposure without adversely affecting pain management or refill requests [42,44,45,46,93,473,476,481,482,483,484,485,486,487,488,489,490]. Maximizing nonopioid therapies and developing patient-specific plans are essential to the success and safety of such practice changes.
Pain management exit plans (PMEP) are an excellent resource for all postoperative patients, especially those with high opioid requirements [478]. Exit plans provide a detailed summary of the analgesics prescribed at discharge, including how each medication should be taken, common side effects, and appropriate disposal techniques (Figure 2). Exit plans focus on multimodal analgesia with an emphasis on nonopioids as the mainstay of therapy. If opioids are prescribed, a taper is developed and outlined in the PMEP using the lowest effective dose. Attention to tablet size and formulation should be considered for those given a taper in order to improve patient compliance. Note that splitting tablets can be challenging for some and use of whole tablets may be preferred for those undergoing a taper. Combination opioid products (e.g., oxycodone/acetaminophen) should be avoided in discharge opioid prescriptions since they limit the ability to safely maximize opioid-sparing analgesia throughout the recovery phase.
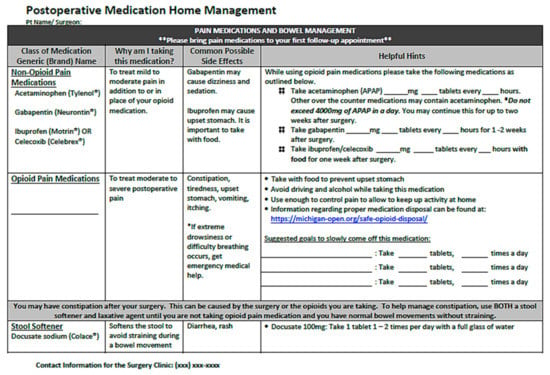
Figure 2.
Example of a Pain Management Exit Plan (PMEP) to be used at postoperative hospital Discharge.
Discharge counseling, with an emphasis on nonopioid analgesics as first line therapy, is essential for safe and successful postoperative pain control [15,101,478]. Discharge counseling should be pursued in conjunction with a PMEP or other standardized educational tool and may be completed by a pharmacist, pharmacy or medical student, advanced practice provider, or physician. Patients being discharged with opioid prescriptions should be educated about proper opioid storage and disposal. Opioids should be kept in a locked cabinet, away from children, pets and friends or family. Storing opioids appropriately can reduce accidental overdoses and decrease opioid diversion, since a majority of people who misuse opioids obtain them from a friend or family member [491]. Providers may consider involving a family member to secure and administer the medication to provide accountability and reduce temptation for opioid misuse or diversion in at-risk patients. If able, facilities dispensing opioid prescriptions should provide safe, at-home medication disposal systems to encourage appropriate and prompt disposal of unused opioids [480,492,493,494]. Other disposal methods include medication collecting bins, often found in hospitals, pharmacies, or police stations, and community medication take back events. As a last resort, patients may consider mixing unused medications in a plastic bag with coffee grounds or cat litter and disposing of them in the household trash. Flushing unwanted medications down the toilet should be discouraged as this leads to pharmaceutical contamination of the water supply [27,100,478,495,496].
Careful attention to the quantity of opioids prescribed at discharge to patients planning to resume medical marijuana or other illicit substances, such as heroin, is vital. In 2018, 67,367 drug overdoses were reported in the U.S., with 69.5% involving opioids [497]. Incidence of opioid overdose after postoperative discharge is greatest in the early period, and estimated to be 26.3 events per person-year during the first thirty postoperative days [498]. Co-prescribing of naloxone, a rapid-acting opioid antagonist, should therefore be considered at the point of postoperative discharge for patients at risk of opioid overdose. These patients may include those prescribed more than 50 MED per day, patients prescribed concomitant benzodiazepines, and patients with a history of respiratory disease, substance use disorder, or mental health disorders [54,499,500]. Naloxone may also be prescribed to patients if they are concerned about opioid misuse in their household.
While acute pain management prescribing is the responsibility of the surgical team, collaboration with chronic pain prescribers and/or addiction medicine specialists is crucial for successful postoperative pain control and mitigation of adverse events in these high-risk populations. This communication can help prevent relapse in those with a history of substance use disorder and promote a smooth transition to maintenance medication regimens; hence, the outpatient provider should be engaged before surgery and as soon as feasible after discharge [104,119]. For patients on chronic buprenorphine, therapy should almost always be continued perioperatively, including at the point of hospital discharge, in addition to a short-acting full mu-opioid agonist prescription for acute pain management where usually indicated [119,126,132]. Surgical providers should ensure the patient has enough buprenorphine to last until they can see their buprenorphine prescriber, contacting the prescriber to troubleshoot any foreseeable gaps. Ideally, this appointment should be within 3 days of discharge. As an alternative to the “bridge prescription,” patients can return to the emergency department for administration of buprenorphine for up to 72 h after discharge. For methadone, if the patient’s home dose was decreased or split during the perioperative period, the dose should generally be returned to home dosing at discharge. Arrangements must be made for the patient on methadone to go to their clinic the following day to receive their medication. It is imperative to discontinue chronic naltrexone products at discharge and to defer their reinitiation to the outpatient prescriber after the patient has been off of opioids (see also Section 3.1.3) [117,124].
3.7. Follow-Up Phase
Development of persistent opioid use is a risk when prescribing opioids for the treatment of acute pain. This risk is amplified by increased doses, additional days supplied, and duration of use. The likelihood of long-term opioid use significantly increases after five days of opioid therapy [501]. For this reason, patient follow-up should ideally take place within five days of discharge, particularly for those who were prescribed opioids. Follow-up may be conducted in person or via telemedicine. A mobile phone app, downloaded by the patient prior to hospital admission, has been shown to effectively monitor patient pain and opioid requirements after surgery. The patient answers daily mobile phone app questions that include pain assessment. These data are reviewed and pain management revisions are implemented at an in-person or telemedicine clinic visit within 4–7 days after discharge [502].
Follow-up assessments should evaluate ongoing postoperative pain, opioid and nonopioid use, and the status of unused opioids. The pain evaluation should assess pain trajectory, which includes pain intensity as well as time to resolution of pain. Patients identified as having an abnormal pain trajectory (e.g., those experiencing numeric pain scores greater than four on postoperative days three-seven) have been found to have a higher risk of developing persistent postoperative pain and should be monitored closely [503]. Closer follow up may also be warranted in those with a history of substance use disorder or those with mental health comorbidities.
Patients identified as having difficulty with postoperative pain control should receive education about proactive pain management. By taking scheduled doses of nonopioid medications, patients are able to “stay ahead” of their pain and prevent severe pain breakthroughs. For those struggling to wean off of opioids, providers should further optimize nonopioid medications, reiterate nonpharmacologic modalities, and encourage opioid tapers whenever possible. Pain management exit plans can be employed as they are at hospital discharge or updated in the outpatient setting, and should be strongly considered in this patient population [478]. The need for additional opioid prescriptions should be limited and assessed on a case-by-case basis, e.g., in opioid-tolerant patients requiring longer tapers. Coordination with the patient’s other outpatient providers is important, and opioid refills from both surgical and nonsurgical providers should be accounted for [504].
For patients with unused opioids, medication disposal education should be reiterated. Providing patients with local medication take-back locations or safe disposal devices can facilitate appropriate narcotic disposal and limit redistribution within the community [492,493,494].
4. Interprofessional Collaboration in Sustaining Perioperative Performance Measures Related to Pain Management and Opioid Prescribing
4.1. From the Surgical Institution Perspective
Pain assessment and management metrics have been critical focus areas for healthcare institutions in recent decades, sometimes with deleterious effects. In 2001, as part of a national effort to address the widespread underassessment and undertreatment of pain, The Joint Commission (formerly The Joint Commission on the Accreditation of Healthcare Organizations or JCAHO) introduced pain management standards for healthcare organizations [505]. While well-intended, the standards were also informed by an unfortunately misguided understanding of the addictive potential of opioids at the time [3,506]. This practice movement ultimately resulted in the elevation of pain as the “fifth vital sign”, giving pain equal status with blood pressure, heart rate, respiratory rate, and temperature. Nurses were required to assess pain as an objective sign, instead of as a subjective symptom of surgical recovery [507,508,509]. Hospitals have also been incentivized to improve patient satisfaction with pain management via the Centers for Medicare & Medicaid Services (CMS) Value-Based Purchasing (VBP) program, which adjusts each hospital inpatient payment according to its performance on quality measures [510]. One tool used to evaluate quality measures within VBP is the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. This survey is administered to patients after hospital discharge and previously asked patients how often hospital providers did, “everything in their power to control your pain” [505]. By directly linking patient satisfaction with pain management to hospital compensation, the survey may have incentivized opioid overprescribing [511].
The Joint Commission and other organizations have since recognized a need to modify standards to mitigate unintended consequences in the wake of the ensuing opioid epidemic [3,359,508,509,512]. The Joint Commission revised their pain standards to include an emphasis on patient safety and the promotion of multimodal analgesia in 2018 [3,36]. Additionally, the revised pain management-related HCAHPS questions shifted from a focus on the perceived quality of pain management efforts to quality of communication about pain management [513]. Furthermore, many U.S. states have enacted opioid prescribing restrictions affecting surgical providers [35]. These revised standards and a shifting paradigm to reduce opioid prescribing are driving surgery centers to reevaluate their approach to perioperative pain management. The requirement by many states to review prescription drug monitoring programs (PDMPs) when prescribing opioids has been linked with a reduced rate of opioid prescriptions in hospitals [514]. Additionally, The Joint Commission requires hospitals to collect and analyze data to monitor their ability to safely prescribe opioids, an important step in the effort to demonstrate reductions in perioperative opioid prescribing without negatively impacting the quality of pain management [67].
In addition to reimbursement-driving quality metrics and legal pressures, healthcare institutions are motivated by increased transparency of their patients’ pain management-related outcomes. Tools such as the CMS Hospital Compare websites and Leapfrog Hospital Safety Grade are available online to consumers [515,516,517]. These quality data are influenced by subjective patient satisfaction indicators in addition to objective outcome metrics. Evaluations of elective surgical programs, such as those providing hip and knee replacements, are therefore only an internet search away from prospective patients. Evidence suggests that an institution’s reputation for postoperative pain management has an important influence on potential healthcare consumers. A recent study assessed the preferences of hip and knee arthroplasty patients regarding publicly available quality metrics. This discrete choice experiment yielded that patients are willing to accept suboptimal hospital ratings and facility cleanliness in exchange for better postoperative pain management and complication rates [518].
Some institutions have implemented opioid stewardship programs (OSPs) to achieve these goals. Core pillars of OSPs include interprofessional collaboration on protocols and services related to multimodal pain management, education on opioid prescribing and stewardship to staff and providers, education to patients, caregivers and community members on safe opioid use and disposal, opioid-related risk reduction, and data analysis and reporting of related quality metrics [38,66,68,519,520,521,522]. An expert panel has proposed quality indicators for measuring opioid stewardship interventions in hospital and emergency settings. These nineteen measures assess quality of inpatient pain management, opioid prescribing practices, ORAE prevention, and transitions of care [38,523].
Although current quality standards and market incentives better align with shared goals by patients, providers, and institutions, the cost of nonopioid medications can pose a barrier for institutions to implement multimodal analgesia throughout perioperative care. Intravenous acetaminophen (pending the widespread availability of this formulation from generic manufacturers in early 2021), intravenous NSAID formulations, and liposomal bupivacaine represent newer nonopioid interventions that drive analgesics to rank among the most expensive therapeutic drug categories [524]. The substantial cost of these agents relative to conventional generic medications may contribute to overreliance on cheap, widely available opioid medications in the perioperative setting [391]. Fortunately, collaborative investigator-initiated research has provided comparative efficacy data to inform cost–benefit comparisons between some of these high-cost agents and their conventional counterparts [176,268,270]. Interprofessional stewardship efforts have demonstrated success in mitigating the potential financial toxicity of perioperative multimodal analgesia by limiting such high-cost agents to populations unable to achieve the same degree of benefit from conventional alternatives [390,525].
It has long been recognized that successful perioperative care involves interdisciplinary collaboration among surgeons, anesthetists, medicine physicians, nurses, and physical therapy providers. Perhaps historically underrecognized has been the value of the clinical pharmacist in improving perioperative patient outcomes and efficiencies [526]. Despite well-supported benefits to diverse patient outcomes and care teams, pharmacists may be underutilized in postoperative pain management. As pharmacotherapy experts with a longitudinal view of the perioperative care continuum, pharmacists are well-poised to perform or oversee many important functions to optimize surgical patient analgesia and institutional opioid stewardship efforts [27,478,527]. These may include completing pre-admission medication reconciliation, advising on preoperative optimization and planning for perioperative management of chronic pain therapies, developing standardized preemptive analgesic protocols with appropriate patient-specific adjustments, supporting intraoperative multimodal analgesic use through protocol development, education, and operationalization, managing postoperative analgesic therapies, advising on discharge opioid and nonopioid prescribing, developing patient educational materials and providing discharge counseling, and assessing patients at follow-up to optimize opioid tapers and screen for postoperative complications [68,478,528,529]. One pre- and post-intervention study spanning 6 years evaluated the impact of a pharmacy-directed pain management service that performed both consult-based and stewardship functions at a large public hospital. The service was associated with decreased total institutional opioid use, increased nonopioid analgesic use, fewer opioid-related respiratory depression events, and ongoing improvement in pain-related HCAHPS patient survey domains [530]. Similarly, a pharmacist-led post-discharge opioid deescalation service was implemented at a major tertiary institution for orthopedic surgery patients recently discharged from the institution’s acute pain service. In the published evaluation of this service, the post-intervention group realized similar pain intensity ratings with significantly lowered opioid doses and incidence of constipation [437]. Healthcare institutions may therefore consider investment in pharmacy services to help drive quality improvement and cost-savings initiatives related to postoperative pain management and opioid stewardship.
4.2. From the Surgeon Perspective
The surgeon perspective of best-practices evidence-based perioperative performance is a team approach within standardized enhanced recovery pathways. Each member of the perioperative interdisciplinary team provides valuable knowledge that contributes to opioid stewardship efforts. Where resources are available, perioperative pain management and opioid stewardship is ideally pharmacist-led, from preoperative evaluation through the inpatient stay and postdischarge follow-up [531]. Described below is an example of the teamwork required in a colorectal enhanced recovery pathway to minimize opioid use while effectively treating postoperative pain.
Nonopioid pain management options are optimized throughout the care continuum for all patients on the surgical service. Through preadmission screening, an enhanced recovery nurse navigator may identify patients with a history of chronic opioid use. This allows the pharmacist to contact the patient and develop a focused perioperative pain management plan. Anesthetists are other important enhanced recovery collaborators. Their expertise in perioperative pain management and postoperative nausea and vomiting (PONV) prevention assist with minimizing the need for opioids. Enhanced recovery patients without complications typically receive transversus abdominis plane (TAP) blocks in the preoperative suite from the anesthetist. Postoperative patients are never “nothing by mouth” after surgery when awake and alert, therefore, enhanced recovery postoperative orders should not routinely include intravenous opioids. The pharmacist leads the multimodal pain management strategy at daily inpatient interdisciplinary rounds that include surgeon, resident surgeon, physician assistant, case manager, social worker, enterostomal nursing, and patient care unit nursing staff. Knowledgeable patient care nurses, well-informed in pain management goals and providing consistent care plan messages to patients, are an integral component of standardized perioperative pain control.
Surgeon opioid and nonopioid discharge prescriptions are written in consultation with the enhanced recovery team pharmacist and are based on inpatient pain control and opioid needs in the 12–24 h leading up to discharge. Pain management exit plans are developed by the pharmacist and provided to those with high opioid requirements. Patients receiving an exit plan are seen by pharmacy and educated about the importance of multimodal analgesia and opioid tapers. One study showed that a pharmacist-led enhanced recovery pain management plan resulted in less than 50% of patients requiring opioid prescriptions at the time of discharge for patients having robotic colorectal surgery. The average number of 5 mg oxycodone tablets prescribed in those who received prescriptions was 6 to 8 while the average number used was 2.5 to 3 tablets. Only 0.5% to 0.75% of patients required opioid prescription refills [531].
Perioperative pain management and opioid stewardship continues after patient discharge in the surgeon clinic. One study showed that enhanced recovery pharmacist participation in an early post-discharge clinic where all postoperative patients are seen within 4–7 days of discharge maximized assessment of pain management and reinforcement of nonopioids as the primary pain management option. Additionally, overall readmission rates were significantly decreased, especially with postoperative pain as a readmission diagnosis [502]. In addition to improved patient outcomes, longitudinal involvement of clinical pharmacists in perioperative pain management has been associated with surgical provider satisfaction [528]. Pharmacists may therefore be valuable to optimizing patient care and in maximizing surgeon resources.
Pain management in enhanced recovery is therefore a dynamic, collaborative, interprofessional effort that requires reassessment and evidence-based changes. A prospectively maintained database allows real-time collection and evaluation of enhanced recovery data that includes opioid and nonopioid information [65]. Implementation of an opioid stewardship program is applicable to all surgical specialties and should be incorporated into enhanced recovery pathways.
4.3. From the Patient Perspective
Patient-centered outcomes and the surgical patient experience should remain the focus of collaborative care and process improvement. Clinical practice guidelines endorse an individualized approach to all aspects of postoperative pain management based on patient needs and preferences, and echo the need to engage patients in shared decision-making throughout this process [15]. Available evidence suggests clinical pharmacists can positively impact patient experience indicators related to postoperative pain management. The incorporation of clinical pharmacists into patient education prior to joint arthroplasty was associated with modest increases in pain-related domains of the HCAHPS satisfaction survey [532]. A comprehensive clinical pharmacy service in a total joint arthroplasty population at another institution included preoperative education, postoperative pain management optimization, and discharge counseling interventions. This service was associated with improved patient understanding of discharge medications and patients indicated a high degree of satisfaction with pharmacist interactions [529].
To illustrate the importance of postoperative pain management and opioid stewardship to the patient perspective, the following account was authored by a colorectal surgical patient of two of the authors and published with his permission (edited only for brevity):
4.3.1. Preparing for Surgery
“For patients, fully understanding how surgery will affect them physically and emotionally and what type of pain management practices will be employed both before and after is a critical first step if they are to take charge of their own health care. Surgery is a scary proposition for the patient. If you add in the anticipated discomfort and pain it only escalates the unknown, elevating fear and anxiety. Speaking from experience, this quickly takes center stage in a patient’s mind. With my three surgeries, I found it essential to take ownership and control and learn as much as I could about these surgeries and my recovery. Fully understanding possible surgical risks and complications, as well as the overall goal and expected positive outcomes, was vital if I was going to gain mental control of a challenging health situation.
Most patients do not realize the power exists within themselves to take better control of their surgical outcomes. Deciding on my frame of mind and focusing on the positives, rather than the negatives, immediately put me in a better position to reach my recovery goals. As I saw it, I had two choices: (1) I could worry about the possible complications associated with surgery. If I took that route, I was sure to be miserable, anxious, and not fully connected with my end goal; or (2) I could prepare by becoming knowledgeable about my surgery, perceiving it as another life challenge that would enable me to continue living and to improve my quality of life. For me, surgical challenges are a lot like flying a kite. If you run your kite before the wind, you cannot take off and fly. You have to turn into the wind and face it head-on. The challenge you push against is the very force that lifts you. Therefore, it was clear to me I had to face the headwinds.”
4.3.2. The Enhanced Recovery Program, Phone Applications, and Opioid Use
“My three surgeries would involve perioperative pain control, with transverse abdominis plane (TAP) or epidural pain blocks and a combination of oral pain medications including acetaminophen, ibuprofen, gabapentin, and oxycodone. Today, I’m a veteran when it comes to pain medication, but the real inspiration that empowered me and gave me reassurance that I could make a significant contribution to my recovery was the Enhanced Recovery Program (ERP) offered by my health provider. Along with that, I was able to use a phone application when I returned home. This application allowed me to have morning check-ins with my health-care team, if needed. My health provider also offered an informative class several weeks before my surgery that gave me valuable and concise information to help me understand my upcoming procedure and how to prepare for it and my hospital stay. The class also gave me important information about my postoperative care and recovery at home.
The ERP gave me the reassurance and tools needed to control my health care, creating a solid foundation for a good outcome. After attending this class, I realized that I had the power to actively engage as a patient who can contribute, participate, and determine outcomes. I was no longer a bystander but a player in this game. This significantly reduced my anxiety, replacing it with positive energy. I honestly believe this shortened my recovery time for all three surgeries.
In the ERP class, a nurse navigator and a pharmacist addressed the most concerning aspect of my surgery: How to control pain? I realized I am afraid of pain. I honestly believe all of us are. However, getting preoperative education on pain management led to the insight that I needed to take control of my pain rather than let it control me. Understanding opioid risks and benefits gave me the confidence and courage to set a goal to get them out of my life after surgery as soon as reasonably possible. Most of us are keenly aware of the opioid crisis still raging both worldwide and in the United States. I was initially concerned that this might eventually be me.
Well, it could have been me. All of us can be throttled by addictions when we least expect it. However, the underlying key to my success was the preoperative and postoperative education I received. What I did learn, and benefit from was the powerful combination of ibuprofen and acetaminophen and how they work together very well to relieve surgical pain. After stopping opioids, I was continued on a regimen of these over-the-counter pain relievers and quickly discovered my pain was being managed without the use of narcotics. This alternate step was presented and outlined in my ERP class. This was an enabler for me, and I was able to be more mentally alert, have less constipation issues and feel comfortable enough to go home. The ERP umbrella provided an open and honest conversation through clear and straightforward directions about what must be done before and after surgery. ERP and the medical staff gave me realistic and attainable goals for my recovery. I was a partner in my own health-care decisions, and I took ownership for my successful recovery. The well-trained medical staff promptly addressed my concerns. The addition of the phone application, which I found to be an excellent communication tool, provided me much needed emotional reassurance and support before, during, and after surgery.”
4.3.3. Lessons Learned
“As a frequent-flyer patient with lots of surgeries, treatments, and narcotics use, I can report that I landed safely back in my everyday life. Additionally, this was mainly because of the expert care as well as the comprehensive education I received from the medical staff, doctors, pharmacists, and nurses. In all cases, my ERP experience gave me the solid foundation I needed to empower myself and focus on the win, not the illness. I discovered journaling every day with accompanying photos, audio, and video. I now have five solid years of life experience, good and bad, that I can look back on.
All of us will eventually face fragility and mortality. However, for this patient, my medical experiences and the numerous medical staff who helped me during trying times have given me the gift of life. I am grateful that I was forced to confront an often inevitable part of being alive and to now fully understand that we as patients can take ownership of and apply direction to our recoveries.”
5. Conclusions and Future Directions
While myriad multimodal strategies exist, ongoing comparative assessments of analgesic combinations and anesthetic approaches within enhanced recovery practice are warranted to further understand and optimize perioperative patient care. Novel analgesic agents and modalities continue to be developed, and their place in therapy should be thoughtfully studied [56,286,533,534,535,536]. Pharmacogenomic assessments show promise in elucidating precision pain management [537,538]. Additional evaluation of the influence of perioperative analgesic strategies on the development of persistent postoperative pain and opioid use would be an invaluable contribution to the literature [2,50,539]. Implementation studies describing successful opioid stewardship programs should be pursued to address practice challenges and increase universal adoption [38,68,540].
Effective perioperative pain management requires a multifaceted team-based approach that begins prior to admission and continues after discharge. Healthcare providers must collaborate throughout institutional practice and process improvement with the shared goals of providing optimal patient care while minimizing opioid exposure. Standardized perioperative pathways should maximize nonpharmacologic therapies and multimodal analgesics, provide decision-support for the judicious use of opioids, and include mitigation strategies for ORAEs and postsurgical opioid dependence. Collaborative practice models should ensure appropriate patient-specific application of available strategies to high-risk and/or opioid-tolerant surgical populations. Pain and addiction medicine specialist consultation, transitional pain services, and opioid stewardship programs should be appropriately resourced across healthcare systems and surgery centers. Incorporating evidence-based pain management and opioid stewardship strategies into a standardized perioperative program will support safe, high-quality, and consistent surgical patient care.
Author Contributions
Conceptualization, S.J.H.; methodology, S.J.H., K.K.B., W.R.V.; writing—original draft preparation, S.J.H., K.K.B., W.R.V., N.Z.S., M.M.L., M.J.H., R.K.C.; writing—review and editing, S.J.H., K.K.B., W.R.V., N.Z.S., M.M.L., M.J.H., R.K.C.; visualization, S.J.H.; supervision, S.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully acknowledge the support and mentorship of Cheryl K. Genord, RPh, BSPharm and Richard H. Parrish II, PhD, FCCP. Additionally, we are honored to have had the support of Robert H. Miller, who lended his voice to this manuscript from the patient perspective. We appreciate his willingness to share his story with us and with the world so that providers everywhere may better understand the patient experience regarding perioperative pain management and opioid stewardship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef]
- Baker, D.W. History of The Joint Commission’s Pain Standards. JAMA 2017, 317, 1117–1118. [Google Scholar] [CrossRef]
- El Moheb, M.; Mokhtari, A.; Han, K.; Van Erp, I.; Kongkaewpaisan, N.; Jia, Z.; Rodriguez, G.; Kongwibulwut, M.; Kaafarani, H.M.; Sakran, J.V.; et al. Pain or No Pain, We Will Give You Opioids: Relationship Between Number of Opioid Pills Prescribed and Severity of Pain after Operation in US vs Non-US Patients. J. Am. Coll. Surg. 2020, 231, 639–648. [Google Scholar] [CrossRef]
- Loh, F.E.; Herzig, S.J. Pain in the United States: Time for a Culture Shift in Expectations, Messaging, and Management. J. Hosp. Med. 2019, 14, 787–788. [Google Scholar] [CrossRef]
- Oderda, G.M.; Senagore, A.J.; Morland, K.; Iqbal, S.U.; Kugel, M.; Liu, S.; Habib, A.S. Opioid-related respiratory and gastrointestinal adverse events in patients with acute postoperative pain: Prevalence, predictors, and burden. J. Pain Palliat. Care Pharmacother. 2019, 33, 82–97. [Google Scholar] [CrossRef]
- Kane-Gill, S.L.; Rubin, E.C.; Smithburger, P.L.; Buckley, M.S.; Dasta, J.F. The Cost of Opioid-Related Adverse Drug Events. J. Pain Palliat. Care Pharmacother. 2014, 28, 282–293. [Google Scholar] [CrossRef]
- Oderda, G.M.; Said, Q.; Evans, R.S.; Stoddard, G.J.; Lloyd, J.; Jackson, K.; Rublee, D.; Samore, M.H. Opioid-Related Adverse Drug Events in Surgical Hospitalizations: Impact on Costs and Length of Stay. Ann. Pharmacother. 2007, 41, 400–407. [Google Scholar] [CrossRef]
- Brat, G.A.; Agniel, D.; Beam, A.; Yorkgitis, B.; Bicket, M.; Homer, M.; Fox, K.P.; Knecht, D.B.; McMahill-Walraven, C.N.; Palmer, N.; et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: Retrospective cohort study. BMJ 2018, 360, j5790. [Google Scholar] [CrossRef]
- Brummett, C.M.; Waljee, J.F.; Goesling, J.; Moser, S.; Lin, P.; Englesbe, M.J.; Bohnert, A.S.B.; Kheterpal, S.; Nallamothu, B.K. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017, 152, e170504. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Brunt, L.M. Perioperative Opioids and Public Health. Anesthesiology 2016, 124, 960–965. [Google Scholar] [CrossRef]
- Kaafarani, H.M.A.; Han, K.; El Moheb, M.; Kongkaewpaisan, N.; Jia, Z.; El Hechi, M.W.; Van Wijck, S.; Breen, K.; Eid, A.; Rodriguez, G.; et al. Opioids After Surgery in the United States Versus the Rest of the World. Ann. Surg. 2020, 272, 879–886. [Google Scholar] [CrossRef]
- Echeverria-Villalobos, M.; Stoicea, N.; Todeschini, A.B.; Fiorda-Diaz, J.; Uribe, A.A.; Weaver, T.; Bergese, S.D. Enhanced Recovery After Surgery (ERAS). Clin. J. Pain 2020, 36, 219–226. [Google Scholar] [CrossRef]
- Ladha, K.S.; Neuman, M.D.; Broms, G.; Bethell, J.; Bateman, B.T.; Wijeysundera, D.N.; Bell, M.; Hallqvist, L.; Svensson, T.; Newcomb, C.W.; et al. Opioid Prescribing After Surgery in the United States, Canada, and Sweden. JAMA Netw. Open 2019, 2, e1910734. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Ansari, A.; Rizk, D.; Whinney, C. The Society of Hospital Medicine. The Society of Hospital Medicine’s (SHM’s) Multimodal Pain Strategies Guide for Postoperative Pain Management. 2017. Available online: https://www.hospitalmedicine.org/globalassets/clinical-topics/clinical-pdf/ctr-17-0004-multi-model-pain-project-pdf-version-m1.pdf (accessed on 14 September 2020).
- American Society of Anesthesiologists Task Force on Acute Pain. Management Practice Guidelines for Acute Pain Management in the Perioperative Setting. Anesthesiology 2012, 116, 248–273. [Google Scholar] [CrossRef]
- Edwards, D.A.; Hedrick, T.L.; Jayaram, J.; Argoff, C.; Gulur, P.; Holubar, S.D.; Gan, T.J.; Mythen, M.G.; Miller, T.E.; Shaw, A.D.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Perioperative Management of Patients on Preoperative Opioid Therapy. Anesth. Analg. 2019, 129, 553–566. [Google Scholar] [CrossRef]
- McEvoy, M.D.; Scott, M.J.; Gordon, D.B.; Grant, S.A.; Thacker, J.K.; Wu, C.L.; Gan, T.J.; Mythen, M.G.; Shaw, A.D.; Miller, T.E.; et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: Part 1—From the preoperative period to PACU. Perioper. Med. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Joshi, G.P.; Van De Velde, M.; Kehlet, H.; Pogatzki-Zahn, E.; Schug, S.; Bonnet, F.; Rawal, N.; Delbos, A.; Lavand’Homme, P.; Beloeil, H.; et al. Development of evidence-based recommendations for procedure-specific pain management: PROSPECT methodology. Anaesthesia 2019, 74, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- European Society for Regional Anesthesia & Pain Therapy. Procedure Specific Postoperative Pain Management (PROSPECT) Guidelines. Available online: http://postoppain.org/ (accessed on 14 September 2020).
- List of Guidelines. Enhanced Recovery After Surgery (ERAS) (R) Society. Available online: https://erassociety.org/guidelines/list-of-guidelines/ (accessed on 14 September 2020).
- Memtsoudis, S.G.; Cozowicz, C.; Bekeris, J.; Bekere, D.; Liu, J.; Soffin, E.M.; Mariano, E.R.; Johnson, R.L.; Hargett, M.J.; Lee, B.H.; et al. Anaesthetic care of patients undergoing primary hip and knee arthroplasty: Consensus recommendations from the International Consensus on Anaesthesia-Related Outcomes after Surgery group (ICAROS) based on a systematic review and meta-analysis. Br. J. Anaesth. 2019, 123, 269–287. [Google Scholar] [CrossRef]
- McEvoy, M.D.; Scott, M.J.; Gordon, D.B.; Grant, S.A.; Thacker, J.K.; Wu, C.L.; Gan, T.J.; Mythen, M.G.; Shaw, A.D.; Miller, T.E.; et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) Joint Consensus Statement on Optimal Analgesia within an Enhanced Recovery Pathway for Colorectal Surgery: Part 2—From PACU to the Transition Home. Perioper. Med. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Schwenk, E.S.; Viscusi, E.R.; Buvanendran, A.; Hurley, R.W.; Wasan, A.D.; Narouze, S.; Bhatia, A.; Davis, F.N.; Hooten, W.M.; Cohen, S.P. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 2018, 43, 456–466. [Google Scholar] [CrossRef]
- Foo, I.; Macfarlane, A.J.R.; Srivastava, D.; Bhaskar, A.; Barker, H.; Knaggs, R.; Eipe, N.; Smith, A.F. The use of intravenous lidocaine for postoperative pain and recovery: International consensus statement on efficacy and safety. Anaesthesia 2021, 76, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Bicket, M.C.; Brat, G.A.; Hutfless, S.; Wu, C.L.; Nesbit, S.A.; Alexander, G.C. Optimizing opioid prescribing and pain treatment for surgery: Review and conceptual framework. Am. J. Health-Syst. Pharm. 2019, 76, 1403–1412. [Google Scholar] [CrossRef]
- Yorkgitis, B.K.; Brat, G.A. Postoperative opioid prescribing: Getting it RIGHTT. Am. J. Surg. 2018, 215, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Varley, P.R.; Zuckerbraun, B.S. Opioid Stewardship and the Surgeon. JAMA Surg. 2018, 153, e174875. [Google Scholar] [CrossRef]
- Overton, H.N.; Hanna, M.N.; Bruhn, W.E.; Hutfless, S.; Bicket, M.C.; Makary, M.A.; Matlaga, B.; Johnson, C.; Sheffield, J.; Shechter, R.; et al. Opioid-Prescribing Guidelines for Common Surgical Procedures: An Expert Panel Consensus. J. Am. Coll. Surg. 2018, 227, 411–418. [Google Scholar] [CrossRef]
- Dr. Robert Bree Collaborative and Washington State Agency Medical Directors’ Group. Prescribing Opioids for Postoperative Pain—Supplemental Guidance. July 2018. Available online: http://www.agencymeddirectors.wa.gov/Files/FinalSupBreeAMDGPostopPain091318wcover.pdf (accessed on 14 September 2020).
- Michigan OPEN. Prescribing Recommendations. Available online: https://michigan-open.org/prescribing-recommendations/ (accessed on 14 September 2020).
- Wu, C.L.; King, A.B.; Geiger, T.M.; Grant, M.C.; Grocott, M.P.W.; Gupta, R.; Hah, J.M.; Miller, T.E.; Shaw, A.D.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Perioperative Opioid Minimization in Opioid-Naïve Patients. Anesth. Analg. 2019, 129, 567–577. [Google Scholar] [CrossRef]
- Kent, M.L.; Hurley, R.W.; Oderda, G.M.; Gordon, D.B.; Sun, E.; Mythen, M.; Miller, T.E.; Shaw, A.D.; Gan, T.J.; Thacker, J.K.M.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative-4 Joint Consensus Statement on Persistent Postoperative Opioid Use. Anesth. Analg. 2019, 129, 543–552. [Google Scholar] [CrossRef]
- Pharmacy Times. Opioid Prescribing Limits Across the States. Available online: https://www.pharmacytimes.com/contributor/marilyn-bulloch-pharmd-bcps/2019/02/opioid-prescribing-limits-across-the-states (accessed on 14 September 2020).
- The Joint Commission. R3 Report: Pain Assessment and Management Standards for Hospitals. 2017 Aug. Report No.: Issue 11. Available online: https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_report_issue_11_2_11_19_rev.pdf (accessed on 14 September 2020).
- Meissner, W.; Huygen, F.; Neugebauer, E.A.; Osterbrink, J.; Benhamou, D.; Betteridge, N.; Coluzzi, F.; De Andres, J.; Fawcett, W.; Fletcher, D.; et al. Management of acute pain in the postoperative setting: The importance of quality indicators. Curr. Med. Res. Opin. 2017, 34, 187–196. [Google Scholar] [CrossRef]
- Rizk, E.; Swan, J.T.; Cheon, O.; Colavecchia, A.C.; Bui, L.N.; Kash, B.A.; Chokshi, S.P.; Chen, H.; Johnson, M.L.; Liebl, M.G.; et al. Quality indicators to measure the effect of opioid stewardship interventions in hospital and emergency department settings. Am. J. Health Pharm. 2019, 76, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Habib, A.S.; Miller, T.E.; White, W.; Apfelbaum, J.L. Incidence, patient satisfaction, and perceptions of post-surgical pain: Results from a US national survey. Curr. Med. Res. Opin. 2014, 30, 149–160. [Google Scholar] [CrossRef]
- Ladha, M.K.S.; Patorno, M.E.; Huybrechts, M.K.F.; Liu, M.J.; Rathmell, M.J.P.; Bateman, M.B.T. Variations in the Use of Perioperative Multimodal Analgesic Therapy. Anesthesiology 2016, 124, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Collinsworth, A.W.; Copeland, L.A.; Ogola, G.O.; Qiu, T.; Kouznetsova, M.; Liao, I.-C.; Mears, N.; Pham, A.T.; Wan, G.J.; et al. Association of Opioid-Related Adverse Drug Events With Clinical and Cost Outcomes Among Surgical Patients in a Large Integrated Health Care Delivery System. JAMA Surg. 2018, 153, 757–763. [Google Scholar] [CrossRef]
- Bicket, M.C.; White, E.; Pronovost, P.J.; Wu, C.L.; Yaster, M.; Alexander, G.C. Opioid Oversupply After Joint and Spine Surgery. Anesth. Analg. 2019, 128, 358–364. [Google Scholar] [CrossRef]
- Neuman, M.D.; Bateman, B.T.; Wunsch, H. Inappropriate opioid prescription after surgery. Lancet 2019, 393, 1547–1557. [Google Scholar] [CrossRef]
- Huang, P.S.; Copp, S.N. Oral Opioids Are Overprescribed in the Opiate-Naive Patient Undergoing Total Joint Arthroplasty. J. Am. Acad. Orthop. Surg. 2019, 27, e702–e708. [Google Scholar] [CrossRef]
- Saini, S.; McDonald, E.L.; Shakked, R.; Nicholson, K.; Rogero, R.; Chapter, M.; Winters, B.S.; Pedowitz, D.I.; Raikin, S.M.; Daniel, J.N. Prospective Evaluation of Utilization Patterns and Prescribing Guidelines of Opioid Consumption Following Orthopedic Foot and Ankle Surgery. Foot Ankle Int. 2018, 39, 1257–1265. [Google Scholar] [CrossRef]
- Bicket, M.C.; Long, J.J.; Pronovost, P.J.; Alexander, G.C.; Wu, C.L. Prescription Opioid Analgesics Commonly Unused After Surgery. JAMA Surg. 2017, 152, 1066–1071. [Google Scholar] [CrossRef]
- Kim, N.; Matzon, J.L.; Abboudi, J.; Jones, C.; Kirkpatrick, W.; Leinberry, C.F.; Liss, F.E.; Lutsky, K.F.; Wang, M.L.; Maltenfort, M.; et al. A Prospective Evaluation of Opioid Utilization After Upper-Extremity Surgical Procedures: Identifying Consumption Patterns and Determining Prescribing Guidelines. J. Bone Jt. Surg. Am. Vol. 2016, 98, e89. [Google Scholar] [CrossRef]
- Jones, C.M. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013, 132, 95–100. [Google Scholar] [CrossRef]
- Lipari, R.N.; Hughes, A. How People Obtain the Prescription Pain Relievers They Misuse. The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US). 2017. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28252901 (accessed on 14 September 2020).
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar] [CrossRef]
- Núñez-Cortés, R.; Chamorro, C.; Ortega-Palavecinos, M.; Mattar, G.; Paredes, O.; Besoaín-Saldaña, Á.; Cruz-Montecinos, C. Social determinants associated to chronic pain after total knee arthroplasty. Int. Orthop. 2019, 43, 2767–2771. [Google Scholar] [CrossRef]
- Weinrib, A.Z.; Azam, M.A.; Birnie, K.A.; Burns, L.C.; Clarke, H.; Katz, J. The psychology of chronic post-surgical pain: New frontiers in risk factor identification, prevention and management. Br. J. Pain 2017, 11, 169–177. [Google Scholar] [CrossRef]
- Ravindran, D. Chronic Postsurgical Pain: Prevention and Management. J. Pain Palliat. Care Pharmacother. 2014, 28, 51–53. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Kaye, A.D.; Granier, A.L.; Garcia, A.J.; Carlson, S.F.; Fuller, M.C.; Haroldson, A.R.; White, S.W.; Krueger, O.L.; Novitch, M.B.; Cornett, E.M. Non-Opioid Perioperative Pain Strategies for the Clinician: A Narrative Review. Pain Ther. 2020, 9, 25–39. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Kamdar, B.B.; Cata, J.P. Optimizing Perioperative Use of Opioids: A Multimodal Approach. Curr. Anesthesiol. Rep. 2020, 10, 404–415. [Google Scholar] [CrossRef]
- Wick, E.C.; Grant, M.C.; Wu, C.L. Postoperative Multimodal Analgesia Pain Management with Nonopioid Analgesics and Techniques. JAMA Surg. 2017, 152, 691–697. [Google Scholar] [CrossRef]
- Ogura, Y.; Gum, J.L.; Steele, P.; Iii, C.H.C.; Djurasovic, M.; Ii, R.K.O.; Laratta, J.L.; Davis, E.; Brown, M.; Daniels, C.; et al. Multi-modal pain control regimen for anterior lumbar fusion drastically reduces in-hospital opioid consumption. J. Spine Surg. 2020, 6, 681–687. [Google Scholar] [CrossRef]
- Hajewski, C.J.; Westermann, R.W.; Holte, A.; Shamrock, A.; Bollier, M.; Wolf, B.R. Impact of a Standardized Multimodal Analgesia Protocol on Opioid Prescriptions After Common Arthroscopic Procedures. Orthop. J. Sports Med. 2019, 7. [Google Scholar] [CrossRef]
- Kurd, M.F.; Kreitz, T.; Schroeder, G.; Vaccaro, A.R. The Role of Multimodal Analgesia in Spine Surgery. J. Am. Acad. Orthop. Surg. 2017, 25, 260–268. [Google Scholar] [CrossRef]
- Weingarten, T.N.; Jacob, A.K.; Njathi, C.W.; Wilson, G.A.; Sprung, J. Multimodal Analgesic Protocol and Postanesthesia Respiratory Depression During Phase I Recovery After Total Joint Arthroplasty. Reg. Anesth. Pain Med. 2015, 40, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, W.J.; Manning, M.W. Enhanced Recovery After Surgery and Multimodal Strategies for Analgesia. Surg. Clin. North Am. 2018, 98, 1171–1184. [Google Scholar] [CrossRef]
- Beverly, A.; Kaye, A.D.; Ljungqvist, O.; Urman, R.D. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol. Clin. 2017, 35, e115–e143. [Google Scholar] [CrossRef]
- Kaye, A.D.; Urman, R.D.; Rappaport, Y.; Siddaiah, H.; Cornett, E.M.; Belani, K.; Salinas, O.J.; Fox, C.J. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S40–S45. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, W.; Levy, N.; Scott, M.; Ljunqvist, O.; Lobo, D. The ERAS® society’s 2018 survey on post-operative opioid stewardship. Clin. Nutr. ESPEN 2019, 31, 122. [Google Scholar] [CrossRef]
- Frazee, R.; Garmon, E.; Isbell, C.; Bird, E.; Papaconstantinou, H. Postoperative Opioid Prescription Reduction Strategy in a Regional Healthcare System. J. Am. Coll. Surg. 2020, 230, 631–635. [Google Scholar] [CrossRef]
- Uritsky, T.J.; Busch, M.E.; Chae, S.G.; Genord, C. Opioid Stewardship: Building on Antibiotic Stewardship Principles. J. Pain Palliat. Care Pharmacother. 2020, 1–3. [Google Scholar] [CrossRef]
- Agency Medical Directors’ Group. AMDG—Interagency Guidelines. Available online: http://www.agencymeddirectors.wa.gov/guidelines.asp (accessed on 1 December 2020).
- CDC Guideline for Prescribing Opioids for Chronic Pain. 28 August 2019. Available online: https://www.cdc.gov/drugoverdose/prescribing/guideline.html (accessed on 1 December 2020).
- McPherson, M.L. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing, 2nd ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2019; Available online: https://play.google.com/store/books/details?id=1g9uDwAAQBAJ (accessed on 14 September 2020).
- Von Korff, M.; Saunders, K.; Ray, G.T.; Boudreau, D.; Campbell, C.; Merrill, J.; Sullivan, M.D.; Rutter, C.M.; Silverberg, M.J.; Banta-Green, C.; et al. De Facto Long-term Opioid Therapy for Noncancer Pain. Clin. J. Pain 2008, 24, 521–527. [Google Scholar] [CrossRef]
- American Society of Regional Anesthesia and Pain Medicine. Advisories & Guidelines. Available online: https://www.asra.com/advisory-guidelines (accessed on 28 December 2020).
- Huxtable, C.A.; Roberts, L.J.; Somogyi, A.A.; MacIntyre, P.E. Acute Pain Management in Opioid-Tolerant Patients: A Growing Challenge. Anaesth. Intensiv. Care 2011, 39, 804–823. [Google Scholar] [CrossRef]
- Doan, L.V.; Blitz, J. Preoperative Assessment and Management of Patients with Pain and Anxiety Disorders. Curr. Anesthesiol. Rep. 2020, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Banning, L.B.; El Moumni, M.; Visser, L.; van Leeuwen, B.L.; Zeebregts, C.J.; Pol, R.A. Frailty leads to poor long-term survival in patients undergoing elective vascular surgery. J. Vasc. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Attwood, K.; Arya, S.; Hall, D.E.; Johanning, J.M.; Gabriel, E.; Visioni, A.; Nurkin, S.; Kukar, M.; Hochwald, S.; et al. Association of Frailty With Failure to Rescue After Low-Risk and High-Risk Inpatient Surgery. JAMA Surg. 2018, 153, e180214. [Google Scholar] [CrossRef]
- Feldman, L.S.; Carli, F. From Preoperative Assessment to Preoperative Optimization of Frailty. JAMA Surg. 2018, 153, e180213. [Google Scholar] [CrossRef]
- Dindo, L.; Zimmerman, M.B.; Hadlandsmyth, K.; StMarie, B.; Embree, J.; Marchman, J.; Tripp-Reimer, T.; Rakel, B. Acceptance and Commitment Therapy for Prevention of Chronic Postsurgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. J. Pain 2018, 19, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Duncan, P.W.; Groban, L.; Segal, H.; Abbott, R.M.; Williamson, J.D. Patient-Reported Outcome Measures (PROM) as A Preoperative Assessment Tool. J. Anesth. Perioper. Med. 2017, 4, 274–281. [Google Scholar] [CrossRef][Green Version]
- Nyman, M.H.; Nilsson, U.; Dahlberg, K.; Jaensson, M. Association Between Functional Health Literacy and Postoperative Recovery, Health Care Contacts, and Health-Related Quality of Life Among Patients Undergoing Day Surgery. JAMA Surg. 2018, 153, 738–745. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Errea, M.; Bialek, J.; Kendall, M.C.; McCarthy, R.J. The impact of health literacy on shared decision making before elective surgery: A propensity matched case control analysis. BMC Health Serv. Res. 2018, 18, 958. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; McCarthy, R.J.; Wolf, M.S.; Holl, J.L. The impact of health literacy in the care of surgical patients: A qualitative systematic review. BMC Surg. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Roy, M.; Corkum, J.P.; Urbach, D.R.; Novak, C.B.; Von Schroeder, H.P.; McCabe, S.J.; Okrainec, K. Health Literacy Among Surgical Patients: A Systematic Review and Meta-analysis. World J. Surg. 2019, 43, 96–106. [Google Scholar] [CrossRef]
- Chang, M.E.; Baker, S.J.; Marques, I.C.D.S.; Liwo, A.N.; Chung, S.K.; Richman, J.S.; Knight, S.J.; Fouad, M.N.; Gakumo, C.A.; Davis, T.C.; et al. Health Literacy in Surgery. HLRP Health Lit. Res. Prract. 2020, 4, e46–e65. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.D.; Mays, M.Z.; Martz, W.; Castro, K.M.; DeWalt, D.A.; Pignone, M.P.; Mockbee, J.; Hale, F.A. Quick Assessment of Literacy in Primary Care: The Newest Vital Sign. Ann. Fam. Med. 2005, 3, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.D.; Bradley, K.A.; Boyko, E.J. Brief questions to identify patients with inadequate health literacy. Health 2004, 11, 12. [Google Scholar]
- Wolmeister, A.S.; Schiavo, C.L.; Nazário, K.C.K.; Castro, S.M.D.J.; De Souza, A.; Caetani, R.P.; Caumo, W.; Stefani, L.C. The Brief Measure of Emotional Preoperative Stress (B-MEPS) as a new predictive tool for postoperative pain: A prospective observational cohort study. PLoS ONE 2020, 15, e0227441. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Riva-Cambrin, J. Prediction tools for postoperative pain. PAIN Rep. 2021, 6, e875. [Google Scholar] [CrossRef]
- Braun, M.; Bello, C.; Riva, T.; Hönemann, C.; Doll, D.; Urman, R.D.; Luedi, M.M. Quantitative Sensory Testing to Predict Postoperative Pain. Curr. Pain Headache Rep. 2021, 25, 1–8. [Google Scholar] [CrossRef]
- Palanisami, D.R.; Reddy, D.A.; Huggi, V.; Rajasekaran, R.B.; Natesan, R.; Shanmuganathan, R. Assessing Preoperative Pain Sensitivity Predicts the Postoperative Analgesic Requirement and Recovery after Total Knee Arthroplasty: A Prospective Study of 178 Patients. J. Arthroplast. 2020, 35, 3545–3553. [Google Scholar] [CrossRef]
- Horn, A.; Kaneshiro, K.; Tsui, B.C.H. Preemptive and Preventive Pain Psychoeducation and Its Potential Application as a Multimodal Perioperative Pain Control Option. Anesth. Analg. 2020, 130, 559–573. [Google Scholar] [CrossRef]
- Bohan, P.M.K.; Chick, R.C.; Wall, M.E.; Hale, D.F.; Tzeng, C.-W.D.; Peoples, G.E.; Vreeland, T.J.; Clifton, G.T. An Educational Intervention Reduces Opioids Prescribed Following General Surgery Procedures. J. Surg. Res. 2021, 257, 399–405. [Google Scholar] [CrossRef]
- Khorfan, R.; Shallcross, M.L.; Yu, B.; Sanchez, N.; Parilla, S.; Coughlin, J.M.; Johnson, J.K.; Bilimoria, K.Y.; Stulberg, J.J. Preoperative patient education and patient preparedness are associated with less postoperative use of opioids. Surgery 2020, 167, 852–858. [Google Scholar] [CrossRef]
- Rucinski, K.; Cook, J.L. Effects of preoperative opioid education on postoperative opioid use and pain management in orthopaedics: A systematic review. J. Orthop. 2020, 20, 154–159. [Google Scholar] [CrossRef]
- Rief, W.; Shedden-Mora, M.C.; Laferton, J.A.C.; Auer, C.; Petrie, K.J.; Salzmann, S.; Schedlowski, M.; Moosdorf, R. Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: Results of the randomized controlled PSY-HEART trial. BMC Med. 2017, 15, 1–13. [Google Scholar] [CrossRef]
- Martin, L.A.; Finlayson, S.R.G.; Brooke, B.S. Patient Preparation for Transitions of Surgical Care: Is Failing to Prepare Surgical Patients Preparing Them to Fail? World J. Surg. 2017, 41, 1447–1453. [Google Scholar] [CrossRef]
- Poland, F.; Spalding, N.; Gregory, S.; McCulloch, J.; Sargen, K.; Vicary, P. Developing patient education to enhance recovery after colorectal surgery through action research: A qualitative study. BMJ Open 2017, 7, e013498. [Google Scholar] [CrossRef]
- Liebner, L.T. I Can’t Read That! Improving Perioperative Literacy for Ambulatory Surgical Patients. AORN J. 2015, 101, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Michigan OPEN. Patient Education. Available online: https://michigan-open.org/wp-content/uploads/2019/07/POP-education.7.01.19.pdf (accessed on 21 December 2020).
- Michigan OPEN. Patient Counseling. Available online: https://michigan-open.org/prescribing-recommendations/patient-counseling/ (accessed on 22 December 2020).
- Northwestern Medicine. Prescription Opioids—What You Need to Know. Available online: https://www.surgjournal.com/cms/10.1016/j.surg.2020.01.002/attachment/49637b3a-9996-4d9b-a612-935c44f0923f/mmc1.pdf (accessed on 22 December 2020).
- Patient Information. Enhanced Recovery After Surgery (ERAS) (R) Society. Available online: https://erassociety.org/patient-information/ (accessed on 21 December 2020).
- MacIntyre, P.E.; Roberts, L.J.; Huxtable, C.A. Management of Opioid-Tolerant Patients with Acute Pain: Approaching the Challenges. Drugs 2019, 80, 9–21. [Google Scholar] [CrossRef] [PubMed]
- McAnally, H.B.; Freeman, L.W.; Darnall, B. Preoperative Optimization of the Chronic Pain Patient: Enhanced Recovery Before Surgery; Oxford University Press: Oxford, UK, 2019; Available online: https://play.google.com/store/books/details?id=jpCqDwAAQBAJ (accessed on 14 September 2020).
- Hannon, C.P.; Fillingham, Y.A.; Nam, D.; Courtney, P.M.; Curtin, B.M.; Vigdorchik, J.M.; Buvanendran, A.; Hamilton, W.G.; Della Valle, C.J.; Deen, J.T.; et al. Opioids in Total Joint Arthroplasty: The Clinical Practice Guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J. Arthroplast. 2020, 35, 2709–2714. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-C.L.; Sing, D.C.; Bozic, K.J. Preoperative Reduction of Opioid Use Before Total Joint Arthroplasty. J. Arthroplast. 2016, 31, 282–287. [Google Scholar] [CrossRef]
- McAnally, H. Rationale for and approach to preoperative opioid weaning: A preoperative optimization protocol. Perioper. Med. 2017, 6, 19. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Varrassi, G.; Paladini, A.; LeQuang, J. Stopping or Decreasing Opioid Therapy in Patients on Chronic Opioid Therapy. Pain Ther. 2019, 8, 163–176. [Google Scholar] [CrossRef]
- Opioid Taper Decision Tool. Veterans Affairs. Available online: https://www.pbm.va.gov/AcademicDetailingService/Documents/Pain_Opioid_Taper_Tool_IB_10_939_P96820.pdf (accessed on 23 December 2020).
- Buys, M.J. Multidisciplinary Transitional Pain Service for the Veteran Population. Fed. Pract. 2020, 37, 472–478. [Google Scholar] [CrossRef]
- Vetter, T.R.; Kain, Z.N. Role of the Perioperative Surgical Home in Optimizing the Perioperative Use of Opioids. Anesth. Analg. 2017, 125, 1653–1657. [Google Scholar] [CrossRef]
- Katz, J.; Weinrib, A.; Fashler, S.R.; Katznelson, R.; Shah, B.R.; Ladak, S.S.; Jiang, J.; Li, Q.; McMillan, K.; Mina, D.S.; et al. The Toronto General Hospital Transitional Pain Service: Development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J. Pain Res. 2015, 8, 695–702. [Google Scholar] [CrossRef]
- Montbriand, J.J.; Weinrib, A.Z.; Azam, M.A.; Ladak, S.S.J.; Shah, B.R.; Jiang, J.; McRae, K.; Tamir, D.; Lyn, S.; Katznelson, R.; et al. Smoking, Pain Intensity, and Opioid Consumption 1–3 Months After Major Surgery: A Retrospective Study in a Hospital-Based Transitional Pain Service. Nicotine Tob. Res. 2018, 20, 1144–1151. [Google Scholar] [CrossRef]
- Veazie, S.; Mackey, K.; Peterson, K.; Bourne, D. Managing Acute Pain in Patients Taking Medication for Opioid Use Disorder: A Rapid Review. J. Gen. Intern. Med. 2020, 35, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Compton, P. Acute Pain Management for Patients Receiving Medication-Assisted Therapy. AACN Adv. Crit. Care 2019, 30, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.; Jackson, M. Perioperative management of opioid-tolerant patients. BJA Educ. 2016, 17, 124–128. [Google Scholar] [CrossRef]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia—When is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef]
- Goel, A.; Azargive, S.; Weissman, J.S.; Shanthanna, H.; Hanlon, J.G.; Samman, B.; Dominicis, M.; Ladha, K.S.; Lamba, W.; Duggan, S.; et al. Perioperative Pain and Addiction Interdisciplinary Network (PAIN) clinical practice advisory for perioperative management of buprenorphine: Results of a modified Delphi process. Br. J. Anaesth. 2019, 123, e333–e342. [Google Scholar] [CrossRef]
- Mehta, D.; Thomas, V.; Johnson, J.; Scott, B.; Cortina, S.; Berger, L. Continuation of Buprenorphine to Facilitate Postoperative Pain Management for Patients on Buprenorphine Opioid Agonist Therapy. Pain Physician 2020, 23, E163–E174. [Google Scholar] [PubMed]
- Buresh, M.; Ratner, J.; Zgierska, A.; Gordin, V.; Alvanzo, A. Treating Perioperative and Acute Pain in Patients on Buprenorphine: Narrative Literature Review and Practice Recommendations. J. Gen. Intern. Med. 2020, 35, 3635–3643. [Google Scholar] [CrossRef]
- Lembke, A.; Ottestad, E.; Schmiesing, C. Patients Maintained on Buprenorphine for Opioid Use Disorder Should Continue Buprenorphine Through the Perioperative Period. Pain Med. 2019, 20, 425–428. [Google Scholar] [CrossRef]
- Ward, E.N.; Quaye, A.N.-A.; Wilens, T.E. Opioid use disorders: Perioperative management of a special population. Anesth. Analg. 2018, 127, 539–547. [Google Scholar] [CrossRef]
- Harrison, T.K.; Kornfeld, H.; Aggarwal, A.K.; Lembke, A. Perioperative Considerations for the Patient with Opioid Use Disorder on Buprenorphine, Methadone, or Naltrexone Maintenance Therapy. Anesthesiol. Clin. 2018, 36, 345–359. [Google Scholar] [CrossRef]
- Sritapan, Y.; Clifford, S.; Bautista, A. Perioperative Management of Patients on Buprenorphine and Methadone: A Narrative Review. Balk. Med. J. 2020, 37, 247–252. [Google Scholar] [CrossRef]
- Quaye, A.N.-A.; Zhang, Y. Perioperative Management of Buprenorphine: Solving the Conundrum. Pain Med. 2018, 20, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Jonan, A.B.; Kaye, A.D.; Urman, R.D. Buprenorphine Formulations: Clinical Best Practice Strategies Recommendations for Perioperative Management of Patients Undergoing Surgical or Interventional Pain Procedures. Pain Physician 2018, 21, e1–e12. [Google Scholar] [PubMed]
- Coluzzi, F.; Bifulco, F.; Cuomo, A.; Dauri, M.; Leonardi, C.; Melotti, R.M.; Natoli, S.; Romualdi, P.; Savoia, G.; Corcione, A. The challenge of perioperative pain management in opioid-tolerant patients. Ther. Clin. Risk Manag. 2017, 13, 1163–1173. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Aloisi, A.M.; Dahan, A.; Filitz, J.; Langford, R.; Likar, R.; Mercadante, S.; Morlion, B.; Raffa, R.B.; Sabatowski, R.; et al. Current Knowledge of Buprenorphine and Its Unique Pharmacological Profile. Pain Pract. 2010, 10, 428–450. [Google Scholar] [CrossRef]
- Dahan, A.; Yassen, A.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br. J. Anaesth. 2006, 96, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.G.; Raymond, B.L. Lack of Evidence for Ceiling Effect for Buprenorphine Analgesia in Humans. Anesth. Analg. 2018, 127, 310–311. [Google Scholar] [CrossRef]
- Warner, N.S.; Warner, M.A.; Cunningham, J.L.; Gazelka, H.M.; Hooten, W.M.; Kolla, B.P.; Warner, D.O. A Practical Approach for the Management of the Mixed Opioid Agonist-Antagonist Buprenorphine During Acute Pain and Surgery. Mayo Clin. Proc. 2020, 95, 1253–1267. [Google Scholar] [CrossRef]
- MacIntyre, P.E.; Russell, R.A.; Usher, K.A.N.; Gaughwin, M.; Huxtable, C.A. Pain Relief and Opioid Requirements in the First 24 Hours after Surgery in Patients Taking Buprenorphine and Methadone Opioid Substitution Therapy. Anaesth. Intensive Care 2013, 41, 222–230. [Google Scholar] [CrossRef]
- Dean, R.L.; Todtenkopf, M.S.; Deaver, D.R.; Arastu, M.F.; Dong, N.; Reitano, K.; O’Driscoll, K.; Kriksciukaite, K.; Gastfriend, D.R. Overriding the blockade of antinociceptive actions of opioids in rats treated with extended-release naltrexone. Pharmacol. Biochem. Behav. 2008, 89, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Petri, C.R.; Richards, J.B. Management of Sedation and Analgesia in Critically Ill Patients Receiving Long-Acting Naltrexone Therapy for Opioid Use Disorder. Ann. Am. Thorac. Soc. 2020, 17, 1352–1357. [Google Scholar] [CrossRef]
- Yoburn, B.C.; Sierra, V.; Lutfy, K. Chronic opioid antagonist treatment: Assessment of receptor upregulation. Eur. J. Pharmacol. 1989, 170, 193–200. [Google Scholar] [CrossRef]
- Menendez, M.E.; Ring, D.; Bateman, B.T. Preoperative Opioid Misuse is Associated With Increased Morbidity and Mortality After Elective Orthopaedic Surgery. Clin. Orthop. Relat. Res. 2015, 473, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.R.; Ling, W. The Clinical Opiate Withdrawal Scale (COWS). J. Psychoact. Drugs 2003, 35, 253–259. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, V.; Zhu, R.; Hines, R. Pain Management of Patients with Substance Abuse in the Ambulatory Setting. Curr. Pain Headache Rep. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, F.F.J.; Cox, F.F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017, 2, e611. [Google Scholar] [CrossRef] [PubMed]
- Makdissi, R.; Stewart, S.H. Care for hospitalized patients with unhealthy alcohol use: A narrative review. Addict. Sci. Clin. Pract. 2013, 8, 11. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Sykora, K.; Schneiderman, J.; Naranjo, C.A.; Sellers, E.M. Assessment of Alcohol Withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br. J. Addict. 1989, 84, 1353–1357. [Google Scholar] [CrossRef]
- Babalonis, S.; Walsh, S.L. Warnings Unheeded:The Risks of Co-Prescribing Opioids and Benzodiazepines. Pain Clin. Updates. 2015, 23, 1–7. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33343182 (accessed on 14 September 2020).
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.; Boyd, S.; Milloy, M.-J.; Walsh, Z. Cannabis Significantly Reduces the Use of Prescription Opioids and Improves Quality of Life in Authorized Patients: Results of a Large Prospective Study. Pain Med. 2020, pnaa396. [Google Scholar] [CrossRef]
- Lucas, P.; Baron, E.P.; Jikomes, N. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduct. J. 2019, 16, 9. [Google Scholar] [CrossRef]
- Alexander, J.C.; Joshi, G.P. A review of the anesthetic implications of marijuana use. Bayl. Univ. Med. Cent. Proc. 2019, 32, 364–371. [Google Scholar] [CrossRef]
- Goel, A.; McGuinness, B.; Jivraj, N.K.; Wijeysundera, D.N.; Mittleman, M.A.; Bateman, B.T.; Clarke, H.; Kotra, L.P.; Ladha, K.S. Cannabis Use Disorder and Perioperative Outcomes in Major Elective Surgeries. Anesthesiology 2020, 132, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Bhatia, A.; Buzon-Tan, A.; Walker, S.; Ilangomaran, D.; Kara, J.; Venkatraghavan, L.; Prabhu, A.J. Weeding Out the Problem. Anesth. Analg. 2019, 129, 874–881. [Google Scholar] [CrossRef]
- Salottolo, K.; Peck, L.; Ii, A.T.; Carrick, M.M.; Madayag, R.; McGuire, E.; Bar-Or, D. The grass is not always greener: A multi-institutional pilot study of marijuana use and acute pain management following traumatic injury. Patient Saf. Surg. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Cannabis and Postoperative Pain. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2020/10/cannabis-and-postoperative-pain (accessed on 10 January 2021).
- Twardowski, M.A.; Link, M.M.; Twardowski, N.M. Effects of Cannabis Use on Sedation Requirements for Endoscopic Procedures. J. Am. Osteopat. Assoc. 2019, 119, 307. [Google Scholar] [CrossRef]
- Paulsen, R.T.; Burrell, B.D. Comparative studies of endocannabinoid modulation of pain. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190279. [Google Scholar] [CrossRef]
- Pernía-Andrade, A.J.; Kato, A.; Witschi, R.; Nyilas, R.; Katona, I.; Freund, T.F.; Watanabe, M.; Filitz, J.; Koppert, W.; Schüttler, J.; et al. Spinal Endocannabinoids and CB1 Receptors Mediate C-Fiber-Induced Heterosynaptic Pain Sensitization. Science 2009, 325, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U.; Preuss, U.W. The cannabis withdrawal syndrome: Current insights. Subst. Abus. Rehabil. 2017, 8, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Bradt, J.; Dileo, C.; Shim, M. Music interventions for preoperative anxiety. Cochrane Database Syst. Rev. 2013, CD006908. [Google Scholar] [CrossRef] [PubMed]
- Gasti, V.; Kurdi, M.S. Intraoperative meditation music as an adjunct to subarachnoid block for the improvement of postoperative outcomes following cesarean section: A randomized placebo-controlled comparative study. Anesth. Essays Res. 2018, 12, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Matsota, P.; Christodoulopoulou, T.; Smyrnioti, M.E.; Pandazi, A.; Kanellopoulos, I.; Koursoumi, E.; Karamanis, P.; Kostopanagiotou, G. Music’s Use for Anesthesia and Analgesia. J. Altern. Complement. Med. 2013, 19, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kühlmann, A.Y.R.; de Rooij, A.; Kroese, L.F.; van Dijk, M.; Hunink, M.G.M.; Jeekel, J. Meta-analysis evaluating music interventions for anxiety and pain in surgery. BJS 2018, 105, 773–783. [Google Scholar] [CrossRef]
- Poulsen, M.J.; Coto, J. Nursing Music Protocol and Postoperative Pain. Pain Manag. Nurs. 2018, 19, 172–176. [Google Scholar] [CrossRef]
- Koo, C.-H.; Park, J.-W.; Ryu, J.-H.; Han, S.-H. The Effect of Virtual Reality on Preoperative Anxiety: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 3151. [Google Scholar] [CrossRef]
- Eijlers, R.; Dierckx, B.; Staals, L.M.; Berghmans, J.M.; Van Der Schroeff, M.P.; Strabbing, E.M.; Wijnen, R.M.; Hillegers, M.H.; Legerstee, J.S.; Utens, E.M. Virtual reality exposure before elective day care surgery to reduce anxiety and pain in children. Eur. J. Anaesthesiol. 2019, 36, 728–737. [Google Scholar] [CrossRef]
- Ding, L.; Hua, H.; Zhu, H.; Zhu, S.; Lu, J.; Zhao, K.; Xu, Q. Effects of virtual reality on relieving postoperative pain in surgical patients: A systematic review and meta-analysis. Int. J. Surg. 2020, 82, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain—From mechanisms to treatment. Pain Rep. 2017, 2, e588. [Google Scholar] [CrossRef] [PubMed]
- Urman, R.D.; Vadivelu, N.; Mitra, S.; Kodumudi, V.; Kaye, A.D.; Schermer, E. Preventive analgesia for postoperative pain control: A broader concept. Local Reg. Anesth. 2014, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Dilip, C.R.S.; Shetty, A.P.; Subramanian, B.; Kanna, R.M.; Rajasekaran, S. A prospective randomized study to analyze the efficacy of balanced pre-emptive analgesia in spine surgery. Spine J. 2019, 19, 569–577. [Google Scholar] [CrossRef]
- Haffner, M.; Saiz, A.M.; Nathe, R.; Hwang, J.; Migdal, C.; Klineberg, E.; Roberto, R. Preoperative multimodal analgesia decreases 24-hour postoperative narcotic consumption in elective spinal fusion patients. Spine J. 2019, 19, 1753–1763. [Google Scholar] [CrossRef]
- Nir, R.-R.; Nahman-Averbuch, H.; Moont, R.; Sprecher, E.; Yarnitsky, D. Preoperative preemptive drug administration for acute postoperative pain: A systematic review and meta-analysis. Eur. J. Pain 2016, 20, 1025–1043. [Google Scholar] [CrossRef]
- Barker, J.C.; DiBartola, K.; Wee, C.; Andonian, N.; Abdel-Rasoul, M.; Lowery, D.; Janis, J.E. Preoperative Multimodal Analgesia Decreases Postanesthesia Care Unit Narcotic Use and Pain Scores in Outpatient Breast Surgery. Plast. Reconstr. Surg. 2018, 142, 443e–450e. [Google Scholar] [CrossRef]
- Moucha, C.S.; Weiser, M.C.; Levin, E.J. Current Strategies in Anesthesia and Analgesia for Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2016, 24, 60–73. [Google Scholar] [CrossRef]
- Doleman, B.; Read, D.; Lund, J.N.; Williams, J.P. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery. Reg. Anesth. Pain Med. 2015, 40, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Bonin, R.P.; Orser, B.A.; Englesakis, M.; Wijeysundera, D.N.; Katz, J. The Prevention of Chronic Postsurgical Pain Using Gabapentin and Pregabalin. Anesth. Analg. 2012, 115, 428–442. [Google Scholar] [CrossRef]
- Cain, K.E.; Iniesta, M.D.; Fellman, B.M.; Suki, T.S.; Siverand, A.; Corzo, C.; Lasala, J.D.; Cata, J.P.; Mena, G.E.; Meyer, L.A.; et al. Effect of preoperative intravenous vs oral acetaminophen on postoperative opioid consumption in an enhanced recovery after surgery (ERAS) program in patients undergoing open gynecologic oncology surgery. Gynecol. Oncol. 2021, 160, 464–468. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nguyen, D.K.; Acosta, J.M.; O’Brien, A.L.; Doyle, P.D.; Medina-Rivera, G. Intravenous Versus Oral Acetaminophen in Ambulatory Surgical Center Laparoscopic Cholecystectomies: A Retrospective Analysis. PT Peer-Rev. J. Formul. Manag. 2019, 44, 359–363. [Google Scholar]
- Westrich, G.H.; Birch, G.A.; Muskat, A.R.; Padgett, D.E.; Goytizolo, E.A.; Bostrom, M.P.; Mayman, D.J.; Lin, Y.; YaDeau, J.T. Intravenous vs Oral Acetaminophen as a Component of Multimodal Analgesia After Total Hip Arthroplasty: A Randomized, Blinded Trial. J. Arthroplast. 2019, 34, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.R.; Mathieson, K.M.; Bradford, L.M.; Garman, C.D.; Gregg, R.W.; Lukens, D.W. Randomized trial of oral versus intravenous acetaminophen for postoperative pain control. Am. J. Health Pharm. 2018, 75, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Raghunathan, K.; Ellis, A.R.; Whittle, J.; Pyati, S.; Bryan, W.E.; Pepin, M.J.; Bartz, R.R.; Krishnamoorthy, V. Effects of Acetaminophen, NSAIDs, Gabapentinoids, and Their Combinations on Postoperative Pulmonary Complications After Total Hip or Knee Arthroplasty. Pain Med. 2020, 21, 2385–2393. [Google Scholar] [CrossRef]
- Dwyer, J.P.; Jayasekera, C.; Nicoll, A. Analgesia for the cirrhotic patient: A literature review and recommendations. J. Gastroenterol. Hepatol. 2014, 29, 1356–1360. [Google Scholar] [CrossRef]
- Doleman, B.; Leonardi-Bee, J.; Heinink, T.P.; Bhattacharjee, D.; Lund, J.N.; Williams, J.P. Pre-emptive and preventive opioids for postoperative pain in adults undergoing all types of surgery. Cochrane Database Syst. Rev. 2018, 12, CD012624. [Google Scholar] [CrossRef]
- Cooper, H.J.; Lakra, A.; Maniker, R.B.; Hickernell, T.R.; Shah, R.P.; Geller, J.A. Preemptive Analgesia with Oxycodone Is Associated With More Pain Following Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 2878–2883. [Google Scholar] [CrossRef]
- Ong, C.K.-S.; Lirk, P.; Seymour, R.A.; Jenkins, B.J. The Efficacy of Preemptive Analgesia for Acute Postoperative Pain Management: A Meta-Analysis. Anesth. Analg. 2005, 100, 757–773. [Google Scholar] [CrossRef]
- Kim, M.P.; Godoy, C.; Nguyen, D.T.; Meisenbach, L.M.; Chihara, R.; Chan, E.Y.; Graviss, E.A. Preemptive pain-management program is associated with reduction of opioid prescriptions after benign minimally invasive foregut surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 734–744.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.W.; Tompkins, D.M.; Cohn, S.M. Are NSAIDs Safe? Assessing the Risk-Benefit Profile of Nonsteroidal Anti-inflammatory Drug Use in Postoperative Pain Management. Am. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Verret, M.; Lauzier, F.; Zarychanski, R.; Perron, C.; Savard, X.; Pinard, A.-M.; Leblanc, G.; Cossi, M.-J.; Neveu, X.; Turgeon, A.F.; et al. Perioperative Use of Gabapentinoids for the Management of Postoperative Acute Pain. Anesthesiology 2020, 133, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhao, Z.; Lv, J.; Sun, L.; Lu, B.; Dong, B.; Ma, J.; Ma, X. The efficacy of perioperative gabapentin for the treatment of postoperative pain following total knee and hip arthroplasty: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Clark, J.D.; Kheterpal, S. Perioperative Gabapentinoids. Anesthesiology 2020, 133, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, T.W.; Gill, M.; McDonald, D.A.; Middleton, R.G.; Reed, M.; Sahota, O.; Yates, P.; Ljungqvist, O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020, 91, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Deljou, A.; Hedrick, S.; Portner, E.; Schroeder, D.; Hooten, W.; Sprung, J.; Weingarten, T. Pattern of perioperative gabapentinoid use and risk for postoperative naloxone administration. Br. J. Anaesth. 2018, 120, 798–806. [Google Scholar] [CrossRef]
- Cavalcante, A.N.; Sprung, J.; Schroeder, D.R.; Weingarten, T.N. Multimodal Analgesic Therapy With Gabapentin and Its Association With Postoperative Respiratory Depression. Anesth. Analg. 2017, 125, 141–146. [Google Scholar] [CrossRef]
- Center for Drug Evaluation, Research. Serious Breathing Difficulties with Gabapentin and Pregabalin. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-breathing-problems-seizure-and-nerve-pain-medicines-gabapentin-neurontin (accessed on 5 January 2021).
- Ohnuma, T.; Raghunathan, K.; Moore, S.; Setoguchi, S.; Ellis, A.R.; Fuller, M.; Whittle, J.; Pyati, S.; Bryan, W.E.; Pepin, M.J.; et al. Dose-Dependent Association of Gabapentinoids with Pulmonary Complications After Total Hip and Knee Arthroplasties. J. Bone Jt. Surg. Am. Vol. 2019, 102, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bykov, K.; Bateman, B.T.; Franklin, J.M.; Vine, S.M.; Patorno, E. Association of Gabapentinoids With the Risk of Opioid-Related Adverse Events in Surgical Patients in the United States. JAMA Netw. Open 2020, 3, e2031647. [Google Scholar] [CrossRef]
- Liu, B.; Liu, R.; Wang, L. A meta-analysis of the preoperative use of gabapentinoids for the treatment of acute postoperative pain following spinal surgery. Medicine 2017, 96, e8031. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Kuang, M.-J.; Ma, J.-X.; Ma, X.-L. The Efficacy of Preoperative Gabapentin in Spinal Surgery: A Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, 649–661. [Google Scholar] [PubMed]
- Mao, Y.; Wu, L.; Ding, W. The efficacy of preoperative administration of gabapentin/pregabalin in improving pain after total hip arthroplasty: A meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hannon, C.P.; Fillingham, Y.A.; Browne, J.A.; Schemitsch, E.H.; Buvanendran, A.; Hamilton, W.G.; Della Valle, C.J.; Deen, J.T.; Erens, G.A.; Lonner, J.H.; et al. Gabapentinoids in Total Joint Arthroplasty: The Clinical Practice Guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J. Arthroplast. 2020, 35, 2700–2703. [Google Scholar] [CrossRef]
- Eipe, N.; Penning, J.; Yazdi, F.; Mallick, R.; Turner, L.; Ahmadzai, N.; Ansari, M.T. Perioperative use of pregabalin for acute pain—A systematic review and meta-analysis. Pain 2015, 156, 1284–1300. [Google Scholar] [CrossRef]
- Doleman, B.; Heinink, T.P.; Read, D.J.; Faleiro, R.J.; Lund, J.N.; Williams, J.P. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 2015, 70, 1186–1204. [Google Scholar] [CrossRef]
- Chaparro, L.E.; Smith, S.A.; Moore, R.A.; Wiffen, P.J.; Gilron, I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst. Rev. 2013, 2013, CD008307. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery. JAMA Surg. 2019, 154, 755. [Google Scholar] [CrossRef]
- Baos, S.; Rogers, C.A.; Abbadi, R.; Alzetani, A.; Casali, G.; Chauhan, N.; Collett, L.; Culliford, L.; De Jesus, S.E.; Edwards, M.; et al. Effectiveness, cost-effectiveness and safety of gabapentin versus placebo as an adjunct to multimodal pain regimens in surgical patients: Protocol of a placebo controlled randomised controlled trial with blinding (GAP study). BMJ Open 2020, 10, e041176. [Google Scholar] [CrossRef]
- Chincholkar, M. Gabapentinoids: Pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br. J. Pain 2020, 14, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Toth, C. Substitution of Gabapentin Therapy with Pregabalin Therapy in Neuropathic Pain due to Peripheral Neuropathy. Pain Med. 2010, 11, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Branton, M.W.; Hopkins, T.J.; Nemec, E.C. Duloxetine for the reduction of opioid use in elective orthopedic surgery: A systematic review and meta-analysis. Int. J. Clin. Pharm. 2021, 1–10. [Google Scholar] [CrossRef]
- Zorrilla-Vaca, A.; Stone, A.; Caballero-Lozada, A.F.; Paredes, S.; Grant, M.C. Perioperative duloxetine for acute postoperative analgesia: A meta-analysis of randomized trials. Reg. Anesth. Pain Med. 2019, 44, 959–965. [Google Scholar] [CrossRef]
- Koh, I.J.; Kim, M.S.; Sohn, S.; Song, K.Y.; Choi, N.Y.; In, Y. Duloxetine Reduces Pain and Improves Quality of Recovery Following Total Knee Arthroplasty in Centrally Sensitized Patients. J. Bone Jt. Surg.-Am. Vol. 2019, 101, 64–73. [Google Scholar] [CrossRef] [PubMed]
- YaDeau, J.T.; Brummett, C.M.; Mayman, D.J.; Lin, Y.; Goytizolo, E.A.; Padgett, D.E.; Alexiades, M.M.; Kahn, R.L.; Jules-Elysee, K.M.; Fields, K.G.; et al. Duloxetine and Subacute Pain after Knee Arthroplasty when Added to a Multimodal Analgesic Regimen. Anesthesiology 2016, 125, 561–572. [Google Scholar] [CrossRef]
- Castro-Alves, L.J.; De Medeiros, A.C.P.O.; Neves, S.P.; De Albuquerque, C.L.C.; Modolo, N.S.; De Azevedo, V.L.; De Oliveira, G.S. Perioperative Duloxetine to Improve Postoperative Recovery After Abdominal Hysterectomy. Anesth. Analg. 2016, 122, 98–104. [Google Scholar] [CrossRef]
- Nasr, D. Efficacy of perioperative duloxetine on acute and chronic postmastectomy pain. Ain-Shams J. Anaesthesiol. 2014, 7, 129. [Google Scholar] [CrossRef]
- Sheth, K.R.; Bernthal, N.M.; Ho, H.S.; Bergese, S.D.; Apfel, C.C.; Stoicea, N.; Jahr, J.S. Perioperative bleeding and non-steroidal anti-inflammatory drugs. Medicine 2020, 99, e20042. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Hannon, C.P.; Roberts, K.C.; Hamilton, W.G.; Della Valle, C.J.; Deen, J.T.; Erens, G.A.; Lonner, J.H.; Pour, A.E.; Sterling, R.S. Nonsteroidal Anti-Inflammatory Drugs in Total Joint Arthroplasty: The Clinical Practice Guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J. Arthroplast. 2020, 35, 2704–2708. [Google Scholar] [CrossRef]
- Martinez, L.; Ekman, E.; Nakhla, N. Perioperative Opioid-sparing Strategies: Utility of Conventional NSAIDs in Adults. Clin. Ther. 2019, 41, 2612–2628. [Google Scholar] [CrossRef] [PubMed]
- Maslin, B.; Lipana, L.; Roth, B.; Kodumudi, G.; Vadivelu, N. Safety Considerations in the Use of Ketorolac for Postoperative Pain. Curr. Drug Saf. 2017, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gobble, R.M.; Hoang, H.L.T.; Kachniarz, B.; Orgill, D.P. Ketorolac Does Not Increase Perioperative Bleeding. Plast. Reconstr. Surg. 2014, 133, 741–755. [Google Scholar] [CrossRef]
- Cassinelli, E.H.; Dean, C.L.; Garcia, R.M.; Furey, C.G.; Bohlman, H.H. Ketorolac Use for Postoperative Pain Management Following Lumbar Decompression Surgery. Spine 2008, 33, 1313–1317. [Google Scholar] [CrossRef]
- Devin, C.J.; McGirt, M.J. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef]
- Jamjittrong, S.; Matsuda, A.; Matsumoto, S.; Kamonvarapitak, T.; Sakurazawa, N.; Kawano, Y.; Yamada, T.; Suzuki, H.; Miyashita, M.; Yoshida, H. Postoperative non-steroidal anti-inflammatory drugs and anastomotic leakage after gastrointestinal anastomoses: Systematic review and meta-analysis. Ann. Gastroenterol. Surg. 2020, 4, 64–75. [Google Scholar] [CrossRef]
- Modasi, A.; Pace, D.; Godwin, M.; Smith, C.; Curtis, B. NSAID administration post colorectal surgery increases anastomotic leak rate: Systematic review/meta-analysis. Surg. Endosc. 2019, 33, 879–885. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, S.R.; Young, C.J. Nonsteroidal anti-inflammatory drugs and anastomotic dehiscence after colorectal surgery: A meta-analysis. ANZ J. Surg. 2017, 88, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Nussmeier, N.A.; Whelton, A.A.; Brown, M.T.; Langford, R.M.; Hoeft, A.; Parlow, J.L.; Boyce, S.W.; Verburg, K.M. Complications of the COX-2 Inhibitors Parecoxib and Valdecoxib after Cardiac Surgery. N. Engl. J. Med. 2005, 352, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Joshi, G.P.; Camu, F.; Pan, S.; Cheung, R. Cardiovascular Safety of the Cyclooxygenase-2 Selective Inhibitors Parecoxib and Valdecoxib in the Postoperative Setting: An Analysis of Integrated Data. Anesth. Analg. 2009, 108, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Schug, A.S.; Parsons, B.; Li, C.; Xia, F. The safety profile of parecoxib for the treatment of postoperative pain: A pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data. J. Pain Res. 2017, 10, 2451–2459. [Google Scholar] [CrossRef]
- Fanelli, A.; Ghisi, D.; Aprile, P.L.; Lapi, F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: Latest evidence and clinical implications. Ther. Adv. Drug Saf. 2017, 8, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M.; Hindley, C.E. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin. Ther. 2010, 32, 667–677. [Google Scholar] [CrossRef]
- Massoth, C.; Zarbock, A.; Meersch, M. Risk Stratification for Targeted AKI Prevention After Surgery: Biomarkers and Bundled Interventions. Semin. Nephrol. 2019, 39, 454–461. [Google Scholar] [CrossRef]
- Goren, O.; Matot, I. Perioperative acute kidney injury. Br. J. Anaesth. 2015, 115, ii3–ii14. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Zarbock, A. Perioperative Acute Kidney Injury. Anesth. Analg. 2017, 125, 1223–1232. [Google Scholar] [CrossRef]
- Zarbock, A.; Koyner, J.L.; Hoste, E.A.J.; Kellum, J.A. Update on Perioperative Acute Kidney Injury. Anesth. Analg. 2018, 127, 1236–1245. [Google Scholar] [CrossRef]
- Bihorac, A. Acute Kidney Injury in the Surgical Patient: Recognition and Attribution. Nephron 2015, 131, 118–122. [Google Scholar] [CrossRef]
- Khan, D.A.; Knowles, S.R.; Shear, N.H. Sulfonamide Hypersensitivity: Fact and Fiction. J. Allergy Clin. Immunol. Prract. 2019, 7, 2116–2123. [Google Scholar] [CrossRef]
- Wulf, N.R.; Matuszewski, K.A. Sulfonamide cross-reactivity: Is there evidence to support broad cross-allergenicity? Am. J. Health Pharm. 2013, 70, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Brackett, C.C. Sulfonamide allergy and cross-reactivity. Curr. Allergy Asthma Rep. 2007, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yska, J.P.; Gertsen, S.; Flapper, G.; Emous, M.; Wilffert, B.; Van Roon, E.N. NSAID Use after Bariatric Surgery: A Randomized Controlled Intervention Study. Obes. Surg. 2016, 26, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Zeid, H.A.; Kallab, R.; Najm, M.A.; Jabbour, H.; Noun, R.; Sleilati, F.; Chucri, S.; Dagher, C.; Sleilaty, G.; Naccache, N. Safety and Efficacy of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Used for Analgesia After Bariatric Surgery: A Retrospective Case-Control Study. Obes. Surg. 2018, 29, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Anesthesia 101: Types of Anesthesia. Available online: https://www.asahq.org/whensecondscount/anesthesia-101/types-of-anesthesia/ (accessed on 21 September 2020).
- Lee, J.H. Anesthesia for ambulatory surgery. Korean J. Anesthesiol. 2017, 70, 398–406. [Google Scholar] [CrossRef]
- New York Society of Regional Anesthesia (NYSORA). Neuraxial Techniques. Available online: https://www.nysora.com/techniques/neuraxial-and-perineuraxial-techniques/ (accessed on 21 September 2020).
- American Society of Regional Anesthesia, Pain Medicine. Regional Anesthesia for Surgery. Available online: https://www.asra.com/page/41/regional-anesthesia-for-surgery (accessed on 21 September 2020).
- Urban, B.W.; Bleckwenn, M. Concepts and correlations relevant to general anaesthesia. Br. J. Anaesth. 2002, 89, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal general anesthesia: Theory and practice. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.C.; Memtsoudis, S.G.; Mariano, E.R. Regional Anesthesia. Anesthesiology 2019, 131, 1205–1206. [Google Scholar] [CrossRef]
- Smith, L.M.; Cozowicz, C.; Uda, Y.; Memtsoudis, S.G.; Barrington, M.J. Neuraxial and Combined Neuraxial/General Anesthesia Compared to General Anesthesia for Major Truncal and Lower Limb Surgery. Anesth. Analg. 2017, 125, 1931–1945. [Google Scholar] [CrossRef]
- Guay, J.; Choi, P.; Suresh, S.; Albert, N.; Kopp, S.; Pace, N.L. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2014, 2014, CD010108. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, O.; Cuéllar-Guzmán, L.F.; Soliz, J.; Cata, J.P. Impact of Regional Anesthesia on Recurrence, Metastasis, and Immune Response in Breast Cancer Surgery. Reg. Anesth. Pain Med. 2017, 42, 751–756. [Google Scholar] [CrossRef]
- Le-Wendling, L.; Nin, O.; Capdevila, X. Cancer Recurrence and Regional Anesthesia: The Theories, the Data, and the Future in Outcomes. Pain Med. 2016, 17, pme12893-75. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Kehlet, H.; Beloeil, H.; Bonnet, F.; Fischer, B.; Hill, A.; Lavandhomme, P.; Lirk, P.; Pogatzki-Zhan, E.; Raeder, J.; et al. Guidelines for perioperative pain management: Need for re-evaluation. Br. J. Anaesth. 2017, 119, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Chin, K.J. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia 2020, 75, e101–e110. [Google Scholar] [CrossRef]
- Emelife, P.I.; Eng, M.R.; Menard, B.L.; Meyers, A.S.; Cornett, E.M.; Urman, R.D.; Kaye, A.D. Adjunct medications for peripheral and neuraxial anesthesia. Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 83–99. [Google Scholar] [CrossRef]
- Desai, N.; Kirkham, K.R.; Albrecht, E. Local anaesthetic adjuncts for peripheral regional anaesthesia: A narrative review. Anaesthesia 2021, 76, 100–109. [Google Scholar] [CrossRef]
- Ranganath, Y.S.; Seering, M.S.; Marian, A.A. American Society of Regional Anesthesia News. Curb Your Enthusiasm: Local Anesthetic Adjuvants for Peripheral Nerve Blocks. 2020. Available online: https://www.asra.com/asra-news/article/301/curb-your-enthusiasm-local-anesthetic-ad (accessed on 23 November 2020).
- Gola, W.; Zając, M.; Cugowski, A. Adjuvants in peripheral nerve blocks—The current state of knowledge. Anestezjol. Intensywna Ter. 2020, 52, 323–329. [Google Scholar] [CrossRef]
- Bailard, N.S.; Ortiz, J.; Flores, R.A. Additives to local anesthetics for peripheral nerve blocks: Evidence, limitations, and recommendations. Am. J. Health Pharm. 2014, 71, 373–385. [Google Scholar] [CrossRef]
- Joshi, G.; Gandhi, K.; Shah, N.; Gadsden, J.; Corman, S.L. Peripheral nerve blocks in the management of postoperative pain: Challenges and opportunities. J. Clin. Anesth. 2016, 35, 524–529. [Google Scholar] [CrossRef]
- Suksompong, S.; Von Bormann, S.; Von Bormann, B. Regional Catheters for Postoperative Pain Control: Review and Observational Data. Anesthesiol. Pain Med. 2020, 10, e99745. [Google Scholar] [CrossRef]
- Prabhakar, A.; Ward, C.T.; Watson, M.; Sanford, J.; Fiza, B.; Moll, V.; Kaye, R.J.; Hall, O.M.; Cornett, E.M.; Urman, R.D.; et al. Liposomal bupivacaine and novel local anesthetic formulations. Best Pract Res. Clin. Anaesthesiol. 2019, 33, 425–432. [Google Scholar] [CrossRef]
- New York Society of Regional Anesthesia (NYSORA). Controlled-Release Local Anesthetics. 8 June 2018. Available online: https://www.nysora.com/foundations-of-regional-anesthesia/pharmacology/controlled-release-local-anesthetics/ (accessed on 21 September 2020).
- Orebaugh, S.L.; Dewasurendra, A. Has the future arrived? Liposomal bupivacaine versus perineural catheters and additives for interscalene brachial plexus block. Curr. Opin. Anaesthesiol. 2020, 33, 704–709. [Google Scholar] [CrossRef]
- Onwochei, D.N.; West, S.; Pawa, A. If Wishes Were Horses, Beggars Would Ride. Reg. Anesth. Pain Med. 2017, 42, 546. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Swisher, M.W.; Sztain, J.F.; Furnish, T.J.; Ilfeld, B.M.; Said, E.T. State of the art opioid-sparing strategies for post-operative pain in adult surgical patients. Expert Opin. Pharmacother. 2019, 20, 949–961. [Google Scholar] [CrossRef]
- McCann, M.E. Liposomal Bupivacaine. Anesthesiology 2021, 134, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.W.; Athanassoglou, V.; Mellon, S.; Strickland, L.H.H.; Trivella, M.; Murray, D.; Pandit, H.G. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst. Rev. 2017, 2, CD011419. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Gabriel, R.A.; Eisenach, J.C. Liposomal Bupivacaine Infiltration for Knee Arthroplasty. Anesthesiology 2018, 129, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-C.; Liu, B.-G.; Wang, Z.-H. Efficacy of liposomal bupivacaine vs. traditional anaesthetic infiltration for pain management in total hip arthroplasty: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11305–11314. [Google Scholar]
- Schwarzkopf, R.; Drexler, M.; Ma, M.W.; Schultz, V.M.; Le, K.T.; Rutenberg, T.F.; Rinehart, J.B. Is There a Benefit for Liposomal Bupivacaine Compared to a Traditional Periarticular Injection in Total Knee Arthroplasty Patients with a History of Chronic Opioid Use? J. Arthroplast. 2016, 31, 1702–1705. [Google Scholar] [CrossRef]
- Kuang, M.-J.; Du, Y.; Ma, J.-X.; He, W.; Fu, L.; Ma, X.-L. The Efficacy of Liposomal Bupivacaine Using Periarticular Injection in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2017, 32, 1395–1402. [Google Scholar] [CrossRef]
- Hyland, S.J.; Deliberato, D.G.; Fada, R.A.; Romanelli, M.J.; Collins, C.L.; Wasielewski, R.C. Liposomal Bupivacaine Versus Standard Periarticular Injection in Total Knee Arthroplasty with Regional Anesthesia: A Prospective Randomized Controlled Trial. J. Arthroplast. 2019, 34, 488–494. [Google Scholar] [CrossRef]
- Abildgaard, J.T.; Chung, A.S.; Tokish, J.M.; Hattrup, S.J. Clinical Efficacy of Liposomal Bupivacaine. JBJS Rev. 2019, 7, e8. [Google Scholar] [CrossRef] [PubMed]
- Bravin, L.N.; Ernest, E.P.; Dietz, M.J.; Frye, B.M. Liposomal Bupivacaine Offers No Benefit Over Ropivacaine for Multimodal Periarticular Injection in Total Knee Arthroplasty. Orthopedics 2019, 43, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Brull, R.; Sheehy, B.T.; Kushelev, M.; Essandoh, M.K.; Abdallah, F.W. The mornings after—Periarticular liposomal bupivacaine infiltration does not improve analgesic outcomes beyond 24 hours following total knee arthroplasty: A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2021, 46, 61–72. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Eisenach, J.C.; Gabriel, R.A. Clinical Effectiveness of Liposomal Bupivacaine Administered by Infiltration or Peripheral Nerve Block to Treat Postoperative Pain. Anesthesiology 2021, 134, 283–344. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, C.; Kuroda, M.; Witvrouw, R.; Anne, L.; Bellemans, J.; Corten, K.; Vanelderen, P.; Mesotten, D.; Leunen, I.; Heylen, M.; et al. Addition of Liposome Bupivacaine to Bupivacaine HCl Versus Bupivacaine HCl Alone for Interscalene Brachial Plexus Block in Patients Having Major Shoulder Surgery. Reg. Anesth. Pain Med. 2017, 42, 334–341. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, C.; Ma, C.; Sun, G.; Yuan, L.; Hei, Z.; Guo, C.; Yao, W. Which is the best analgesia treatment for total knee arthroplasty: Adductor canal block, periarticular infiltration, or liposomal bupivacaine? A network meta-analysis. J. Clin. Anesth. 2021, 68, 110098. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Brull, R.; Sheehy, B.; Essandoh, M.K.; Stahl, D.L.; Weaver, T.E.; Abdallah, F.W. Perineural Liposomal Bupivacaine Is Not Superior to Nonliposomal Bupivacaine for Peripheral Nerve Block Analgesia. Anesthesiology 2021, 134, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M.; Barrington, M.J.; Fettiplace, M.R.; Gitman, M.; Memtsoudis, S.G.; Mörwald, E.E.; Rubin, D.S.; Weinberg, G. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity. Reg. Anesth. Pain Med. 2018, 43, 113–123. [Google Scholar] [CrossRef] [PubMed]
- New York Society of Regional Anesthesia (NYSORA). Local Anesthetic Systemic Toxicity. 24 June 2018. Available online: https://www.nysora.com/foundations-of-regional-anesthesia/complications/local-anesthetic-systemic-toxicity/ (accessed on 21 September 2020).
- BrugadaDrugs.org. Drugs Preferably Avoided by Brugada Syndrome Patients. Available online: https://www.brugadadrugs.org/pref_avoid/ (accessed on 28 February 2020).
- New York Society of Regional Anesthesia (NYSORA). Home—NYSORA. Available online: http://www.nysora.com (accessed on 21 September 2020).
- American Society of Regional Anesthesia, Pain Medicine. Resources. Available online: https://www.asra.com/education (accessed on 29 December 2020).
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q.; Salinas, F.V.; Benzon, H.T.; Neal, J.M. Lower extremity regional anesthesia: Essentials of our current understanding. Reg. Anesth. Pain Med. 2019, 44, 143–180. [Google Scholar] [CrossRef] [PubMed]
- Neilio, J.; Kunze, L.; Drew, J.M. Contemporary Perioperative Analgesia in Total Knee Arthroplasty: Multimodal Protocols, Regional Anesthesia, and Peripheral Nerve Blockade. J. Knee Surg. 2018, 31, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Ariza, F.; Rodriguez-Mayoral, H.; Villarreal, K. Epidural analgesia in abdominal major surgery. Colomb. J. Anesthesiol. 2018, 46, 175–176. [Google Scholar] [CrossRef]
- Elsharkawy, H.; Pawa, A.; Mariano, E.R. Interfascial Plane Blocks. Reg. Anesth. Pain Med. 2018, 43, 341–346. [Google Scholar] [CrossRef]
- Ilfeld, B.M. Continuous Peripheral Nerve Blocks. Anesth. Analg. 2017, 124, 308–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S. Recent advances in topical anesthesia. J. Dent. Anesth. Pain Med. 2016, 16, 237–244. [Google Scholar] [CrossRef]
- Dunn, L.K.; Durieux, M.E. Perioperative Use of Intravenous Lidocaine. Anesthesiology 2017, 126, 729–737. [Google Scholar] [CrossRef]
- Earls, B.; Bellil, L. Systemic Lidocaine: An Effective and Safe Modality for Postoperative Pain Management and Early Recovery—Anesthesia Patient Safety Foundation. Anesthesia Patient Safety Foundation Newsletter. 2018. Available online: https://www.apsf.org/article/systemic-lidocaine-an-effective-and-safe-modality-for-postoperative-pain-management-and-early-recovery/ (accessed on 14 September 2020).
- American Society of Regional Anesthesia, Pain Medicine. Clinical Implications of IV Lidocaine Infusion in Preoperative/Acute Pain Settings. Available online: https://www.asra.com/asra-news/article/114/clinical-implications-of-iv-lidocaine-in (accessed on 21 September 2020).
- Weibel, S.; Jelting, Y.; Pace, N.L.; Helf, A.; Eberhart, L.H.; Hahnenkamp, K.; Hollmann, M.W.; Poepping, D.M.; Schnabel, A.; Kranke, P. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst. Rev. 2018, 6, CD009642. [Google Scholar] [CrossRef]
- Khan, J.S.; Yousuf, M.; Victor, J.C.; Sharma, A.; Siddiqui, N. An estimation for an appropriate end time for an intraoperative intravenous lidocaine infusion in bowel surgery: A comparative meta-analysis. J. Clin. Anesth. 2016, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, F.; Yang, L.; Zhu, T. Effects of perioperative intravenous lidocaine infusion for postoperative pain and recovery in elderly patients undergoing surgery: A systematic review and meta-analysis of randomized controlled trials. BMC. in process. [CrossRef]
- Dewinter, G.; Moens, P.; Fieuws, S.; Vanaudenaerde, B.; Van De Velde, M.; Rex, S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br. J. Anaesth. 2017, 118, 576–585. [Google Scholar] [CrossRef]
- Lii, T.R.; Aggarwal, A.K. Comparison of intravenous lidocaine versus epidural anesthesia for traumatic rib fracture pain: A retrospective cohort study. Reg. Anesth. Pain Med. 2020, 45, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.; Nimmo, S.; Paterson, H.; Homer, N.; Foo, I. Intravenous lidocaine infusion as a component of multimodal analgesia for colorectal surgery—Measurement of plasma levels. Perioper. Med. 2019, 8, 1–5. [Google Scholar] [CrossRef]
- Oh, T.K.; Chung, S.H.; Park, J.; Shin, H.; Chang, C.B.; Kim, T.K.; Do, S.-H. Effects of Perioperative Magnesium Sulfate Administration on Postoperative Chronic Knee Pain in Patients Undergoing Total Knee Arthroplasty: A Retrospective Evaluation. J. Clin. Med. 2019, 8, 2231. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, E.-Y.; Na, H.-S.; Kim, T.; Kim, M.-H.; Do, S.-H. Magnesium sulphate attenuates acute postoperative pain and increased pain intensity after surgical injury in staged bilateral total knee arthroplasty: A randomized, double-blinded, placebo-controlled trial. Br. J. Anaesth. 2016, 117, 497–503. [Google Scholar] [CrossRef]
- Pockett, S. Spinal Cord Synaptic Plasticity and Chronic Pain. Anesth. Analg. 1995, 80, 173–179. [Google Scholar] [CrossRef]
- Zhu, A.; Benzon, H.A.; Anderson, T.A. Evidence for the Efficacy of Systemic Opioid-Sparing Analgesics in Pediatric Surgical Populations. Anesth. Analg. 2017, 125, 1569–1587. [Google Scholar] [CrossRef] [PubMed]
- Beaussier, M.; Delbos, A.; Maurice-Szamburski, A.; Ecoffey, C.; Mercadal, L. Perioperative Use of Intravenous Lidocaine. Drugs 2018, 78, 1229–1246. [Google Scholar] [CrossRef]
- Daykin, H. The efficacy and safety of intravenous lidocaine for analgesia in the older adult: A literature review. Br. J. Pain 2016, 11, 23–31. [Google Scholar] [CrossRef]
- Eipe, M.N.; Gupta, F.S.; Penning, F.J. Intravenous lidocaine for acute pain: An evidence-based clinical update. BJA Educ. 2016, 16, 292–298. [Google Scholar] [CrossRef]
- Cooke, C.; Kennedy, E.D.; Foo, I.; Nimmo, S.; Speake, D.; Paterson, H.M.; Ventham, N.T. Meta-analysis of the effect of perioperative intravenous lidocaine on return of gastrointestinal function after colorectal surgery. Tech. Coloproctol. 2019, 23, 15–24. [Google Scholar] [CrossRef]
- Missair, A.; Cata, J.P.; Votta-Velis, G.; Johnson, M.; Borgeat, A.; Tiouririne, M.; Gottumukkala, V.; Buggy, D.; Vallejo, R.; De Marrero, E.B.; et al. Impact of perioperative pain management on cancer recurrence: An ASRA/ESRA special article. Reg. Anesth. Pain Med. 2019, 44, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jiang, R.; Su, W.; Wang, M.; Liu, Y.; Zuo, Y. Impact of perioperative intravenous lidocaine infusion on postoperative pain and rapid recovery of patients undergoing gastrointestinal tumor surgery: A randomized, double-blind trial. J. Gastrointest. Oncol. 2020, 11, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.K.; Kaya, M.; Aksu, B.; Ünver, S. The Effect of Perioperative Lidocaine Infusion on Postoperative Pain and Postsurgical Recovery Parameters in Gynecologic Cancer Surgery. Clin. J. Pain 2021, 37, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.D.; Smyth, D.; Vasilopoulos, T.; Friedman, J.; Sappenfield, J.W.; Alex, G. Ketamine infusion reduces narcotic requirements following gastric bypass surgery: A randomized controlled trial. Surg. Obes. Relat. Dis. 2020. [Google Scholar] [CrossRef]
- Helander, E.M.; Menard, B.L.; Harmon, C.M.; Homra, B.K.; Allain, A.V.; Bordelon, G.J.; Wyche, M.Q.; Padnos, I.W.; Lavrova, A.; Kaye, A.D. Multimodal Analgesia, Current Concepts, and Acute Pain Considerations. Curr. Pain Headache Rep. 2017, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, B.M.; Shah, K.; Parikh, A.; Sifonios, A.N.; Le, V.; Eloy, J.D. Role of Ketamine in Acute Postoperative Pain Management: A Narrative Review. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Nava, E.; Del Pozo, J.S.G.; Jordán, J. Influence of the perioperative administration of magnesium sulfate on the total dose of anesthetics during general anesthesia. A systematic review and meta-analysis. J. Clin. Anesth. 2017, 39, 129–138. [Google Scholar] [CrossRef]
- Park, J.-Y.; Hong, J.H.; Kim, D.-H.; Yu, J.; Hwang, J.-H.; Kim, Y.-K. Magnesium and Bladder Discomfort after Transurethral Resection of Bladder Tumor. Anesthesiology 2020, 133, 64–77. [Google Scholar] [CrossRef]
- Kim, R.K.; Hwang, J.H.; Tsui, B.C. Utilization of Magnesium in Opioid-Free Anesthesia for Peroral Endoscopic Myotomy: A Case Report. A&A Pract. 2021, 15, e01372. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative Systemic Magnesium to Minimize Postoperative Pain. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, A.; Zhou, K.; Chen, Q.; Zhang, M.; Liu, J. Impact of Dexmedetomidine Infusion on Postoperative Acute Kidney Injury in Elderly Patients Undergoing Major Joint Replacement: A Retrospective Cohort Study. Drug Des. Dev. Ther. 2020, 14, 4695–4701. [Google Scholar] [CrossRef]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Gottschalk, A.; Whitman, G.; Lawton, J.S.; Dodd, O.J.; Barodka, V. Opioid-Sparing Cardiac Anesthesia. Anesth. Analg. 2020, 131, 1852–1861. [Google Scholar] [CrossRef]
- Wieruszewski, P.M.; Wittwer, E.D. It’s All in the Details: Dexmedetomidine and Acute Kidney Injury After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2549. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Naik, B.I.; Nemergut, E.C.; Kazemi, A.; Fernández, L.; Cederholm, S.K.; McMurry, T.L.; Durieux, M.E. The Effect of Dexmedetomidine on Postoperative Opioid Consumption and Pain After Major Spine Surgery. Anesth. Analg. 2016, 122, 1646–1653. [Google Scholar] [CrossRef]
- Lundorf, L.J.; Nedergaard, H.K.; Møller, A.M. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst. Rev. 2016, 2, CD010358. [Google Scholar] [CrossRef]
- Ge, D.-J.; Qi, B.; Tang, G.; Li, J.-Y. Intraoperative Dexmedetomidine Promotes Postoperative Analgesia and Recovery in Patients after Abdominal Hysterectomy: A Double-Blind, Randomized Clinical Trial. Sci. Rep. 2016, 6, 21514. [Google Scholar] [CrossRef]
- Cheung, C.W.; Qiu, Q.; Ying, A.C.L.; Choi, S.W.; Law, W.L.; Irwin, M. The effects of intra-operative dexmedetomidine on postoperative pain, side-effects and recovery in colorectal surgery. Anaesthesia 2014, 69, 1214–1221. [Google Scholar] [CrossRef]
- Bajracharya, J.L.; Subedi, A.; Pokharel, K.; Bhattarai, B. The effect of intraoperative lidocaine versus esmolol infusion on postoperative analgesia in laparoscopic cholecystectomy: A randomized clinical trial. BMC Anesthesiol. 2019, 19, 198–199. [Google Scholar] [CrossRef]
- Bahr, M.P.; Williams, B.A. Esmolol, Antinociception, and Its Potential Opioid-Sparing Role in Routine Anesthesia Care. Reg. Anesth. Pain Med. 2018, 43, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Gelineau, A.M.; King, M.R.; Ladha, K.S.; Burns, S.M.; Houle, T.; Anderson, T.A. Intraoperative Esmolol as an Adjunct for Perioperative Opioid and Postoperative Pain Reduction. Anesth. Analg. 2018, 126, 1035–1049. [Google Scholar] [CrossRef]
- Klag, E.A.; Kuhlmann, N.A.; Tramer, J.S.; Franovic, S.; Muh, S.J. Dexamethasone decreases postoperative opioid and antiemetic use in shoulder arthroplasty patients: A prospective, randomized controlled trial. J. Shoulder Elb. Surg. 2021. [Google Scholar] [CrossRef]
- McHardy, P.G.; Singer, O.; Awad, I.T.; Safa, B.; Henry, P.D.; Kiss, A.; Au, S.K.; Kaustov, L.; Choi, S. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: A randomised equivalence trial. Br. J. Anaesth. 2020, 124, 84–91. [Google Scholar] [CrossRef]
- Cortés-Flores, A.; Jiménez-Tornero, J.; Morgan-Villela, G.; Delgado-Gómez, M.; Del Valle, C.J.Z.-F.; García-Rentería, J.; Rendón-Félix, J.; Fuentes-Orozco, C.; Macías-Amezcua, M.; Ambriz-González, G.; et al. Effects of preoperative dexamethasone on postoperative pain, nausea, vomiting and respiratory function in women undergoing conservative breast surgery for cancer: Results of a controlled clinical trial. Eur. J. Cancer Care 2017, 27, e12686. [Google Scholar] [CrossRef]
- Kahn, R.L.; Cheng, J.; Gadulov, Y.; Fields, K.G.; YaDeau, J.T.; Gulotta, L.V. Perineural Low-Dose Dexamethasone Prolongs Interscalene Block Analgesia with Bupivacaine Compared With Systemic Dexamethasone. Reg. Anesth. Pain Med. 2018, 43, 572–579. [Google Scholar] [CrossRef]
- Holland, D.; Amadeo, R.J.J.; Wolfe, S.; Girling, L.; Funk, F.; Collister, M.; Czaplinski, E.; Ferguson, C.; Leiter, J.; Old, J.; et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: A randomized controlled trial. Can. J. Anaesth. 2017, 65, 34–45. [Google Scholar] [CrossRef]
- Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Optimal Dose of Perineural Dexamethasone to Prolong Analgesia After Brachial Plexus Blockade. Anesth. Analg. 2018, 126, 270–279. [Google Scholar] [CrossRef]
- Pehora, C.; Pearson, A.M.; Kaushal, A.; Crawford, M.W.; Johnston, B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst. Rev. 2017, 11, CD011770. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.W.; Johnson, J.; Chan, V.; Murgatroyd, H.; Ghafari, M.; Ami, N.; Jin, R.; Brull, R. Intravenous Dexamethasone and Perineural Dexamethasone Similarly Prolong the Duration of Analgesia After Supraclavicular Brachial Plexus Block. Reg. Anesth. Pain Med. 2015, 40, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; Palmeira, C.C.D.A.; Torres, J.N.L.; Vieira, J.E.; Ashmawi, H.A. Intraoperative use of methadone improves control of postoperative pain in morbidly obese patients: A randomized controlled study. J. Pain Res. 2018, 11, 2123–2129. [Google Scholar] [CrossRef]
- Murphy, G.S.; Wu, C.L.; Mascha, E.J. Methadone. Anesth. Analg. 2019, 129, 1456–1458. [Google Scholar] [CrossRef]
- Komen, H.; Brunt, L.M.; Deych, E.; Blood, J.; Kharasch, E.D. Intraoperative Methadone in Same-Day Ambulatory Surgery. Anesth. Analg. 2019, 128, 802–810. [Google Scholar] [CrossRef]
- Machado, F.C.; Vieira, J.E.; De Orange, F.A.; Ashmawi, H.A. Intraoperative Methadone Reduces Pain and Opioid Consumption in Acute Postoperative Pain. Anesth. Analg. 2019, 129, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Marymont, J.H.; Shear, T.; Parikh, K.N.; Patel, S.S.; Gupta, D.K. Intraoperative Methadone for the Prevention of Postoperative PainA Randomized, Double-blinded Clinical Trial in Cardiac Surgical Patients. Anesthesiology 2015, 122, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Shear, T.D.; Deshur, M.A.; Vender, J.S.; Benson, J.; Newmark, R.L. Clinical Effectiveness and Safety of Intraoperative Methadone in Patients Undergoing Posterior Spinal Fusion Surgery. Anesthesiology 2017, 126, 822–833. [Google Scholar] [CrossRef]
- Gottschalk, A.; Durieux, M.E.; Nemergut, E.C. Intraoperative Methadone Improves Postoperative Pain Control in Patients Undergoing Complex Spine Surgery. Anesth. Analg. 2011, 112, 218–223. [Google Scholar] [CrossRef]
- Hussain, N.; Grzywacz, V.P.; Ferreri, C.A.; Atrey, A.; Banfield, L.; Shaparin, N.; Vydyanathan, A. Investigating the Efficacy of Dexmedetomidine as an Adjuvant to Local Anesthesia in Brachial Plexus Block. Reg. Anesth. Pain Med. 2017, 42, 184–196. [Google Scholar] [CrossRef]
- Hanna, V.; Senderovich, H. Methadone in Pain Management: A Systematic Review. J. Pain 2020. [Google Scholar] [CrossRef]
- McNicol, E.D.; Ferguson, M.C.; Schumann, R. Methadone for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 5, 012499. [Google Scholar] [CrossRef]
- Tripathi, S.; Hunter, J. Neuromuscular blocking drugs in the critically ill. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 119–123. [Google Scholar] [CrossRef]
- Treanor, N.; Vezina, V.; Lui, A. ESRA19-0218 ‘Fast-track’ patients to phase II recovery and decrease pacu duration in ambulatory arthroscopic shoulder surgery with combined peripheral nerve block and monitored anesthesia care. E-Poster Discuss. 2019, 44, A136. [Google Scholar] [CrossRef]
- Wood, C. Effect of sublingual versus intravenous opioid administration on total opioid administration in patients following total knee arthroplasty. In Proceedings of the Great Lakes Pharmacy Residency Conference, Pursue University, West Lafayette, IN, USA, 25 April 2019. [Google Scholar]
- Fan, M.; Chen, Z. A systematic review of non-pharmacological interventions used for pain relief after orthopedic surgical procedures. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.W.; Rosenkilde, C.; Lauridsen, J.; Hasfeldt, D. Effects of Nonpharmacologic Distraction Methods on Children’s Postoperative Pain—A Nonmatched Case-Control Study. J. PeriAnesth. Nurs. 2020, 35, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, N.; Zhang, X.; Shang, Y.; Yan, L.; Chu, J.; Sun, R.; Xu, Y. Music for reducing the anxiety and pain of patients undergoing a biopsy: A meta-analysis. J. Adv. Nurs. 2017, 74, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Rafer, L.; Austin, F.; Frey, J.; Mulvey, C.; Vaida, S.; Prozesky, J. Effects of jazz on postoperative pain and stress in patients undergoing elective hysterectomy. Adv. Mind Body Med. 2015, 29, 6–11. [Google Scholar]
- Liu, Y.; Petrini, M.A. Effects of music therapy on pain, anxiety, and vital signs in patients after thoracic surgery. Complement. Ther. Med. 2015, 23, 714–718. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Guo, Q.; Liu, J.; Liu, X.; Luo, J.; Yang, W. Effects of Intravenous Patient-Controlled Sufentanil Analgesia and Music Therapy on Pain and Hemodynamics After Surgery for Lung Cancer: A Randomized Parallel Study. J. Altern. Complement. Med. 2015, 21, 667–672. [Google Scholar] [CrossRef]
- Ardon, A.E.; Prasad, A.; McClain, R.L.; Melton, M.S.; Nielsen, K.C.; Greengrass, R. Regional Anesthesia for Ambulatory Anesthesiologists. Anesthesiol. Clin. 2019, 37, 265–287. [Google Scholar] [CrossRef]
- Amundson, A.W.; Panchamia, J.K.; Jacob, A.K. Anesthesia for Same-Day Total Joint Replacement. Anesthesiol. Clin. 2019, 37, 251–264. [Google Scholar] [CrossRef]
- Beaussier, M.; Sciard, D.; Sautet, A. New modalities of pain treatment after outpatient orthopaedic surgery. Orthop. Traumatol. Surg. Res. 2016, 102, S121–S124. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C.; Quinlan-Colwell, A.; Rae, D.; Broglio, K.; Drew, D. American Society for Pain Management Nursing Position Statement: Prescribing and Administering Opioid Doses Based Solely on Pain Intensity. Pain Manag. Nurs. 2016, 17, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C. One Size Does Not Fit All: Opioid Dose Range Orders. J. PeriAnesth. Nurs. 2014, 29, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.J.; Gordon, D.B.; Morgan, B.; Manworren, R.C. ’’As-Needed’’ Range Orders for Opioid Analgesics in the Management of Pain: A Consensus Statement of the American Society for Pain Management Nursing and the American Pain Society. Pain Manag. Nurs. 2018, 19, 207–210. [Google Scholar] [CrossRef]
- Smetzer, J.L.; Cohen, M.R. Pain Scales Don’t Weigh Every Risk. J. Pain Palliat. Care Pharmacother. 2003, 17, 67–70. [Google Scholar] [CrossRef]
- Van Boekel, R.L.M.; Vissers, K.C.P.; Van Der Sande, R.; Bronkhorst, E.; Lerou, J.G.C.; Steegers, M.A.H. Moving beyond pain scores: Multidimensional pain assessment is essential for adequate pain management after surgery. PLoS ONE 2017, 12, e0177345. [Google Scholar] [CrossRef]
- Scher, C.; Petti, E.; Meador, L.; Van Cleave, J.H.; Liang, E.; Reid, M.C. Multidimensional Pain Assessment Tools for Ambulatory and Inpatient Nursing Practice. Pain Manag. Nurs. 2020, 21, 416–422. [Google Scholar] [CrossRef]
- Hirsh, A.T.; Anastas, T.M.; Miller, M.M.; Quinn, P.D.; Kroenke, K. Patient race and opioid misuse history influence provider risk perceptions for future opioid-related problems. Am. Psychol. 2020, 75, 784–795. [Google Scholar] [CrossRef]
- Anderson, K.O.; Green, C.R.; Payne, R. Racial and Ethnic Disparities in Pain: Causes and Consequences of Unequal Care. J. Pain 2009, 10, 1187–1204. [Google Scholar] [CrossRef]
- Green, C.R.; Anderson, K.O.; Baker, T.A.; Campbell, L.C.; Decker, S.; Fillingim, R.B.; Kaloukalani, D.A.; Lasch, K.E.; Myers, C.; Tait, R.C.; et al. The Unequal Burden of Pain: Confronting Racial and Ethnic Disparities in Pain. Pain Med. 2003, 4, 277–294. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Johns, M. Review of nonopioid multimodal analgesia for surgical and trauma patients. Am. J. Health Pharm. 2020, 77, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.-H.; Ho, A.K.; Mizubuti, G.B.; Klar, G.; Karmakar, M.K. Regional analgesia for patients with traumatic rib fractures: A narrative review. J. Trauma Acute Care Surg. 2020, 88, e22–e30. [Google Scholar] [CrossRef]
- Saranteas, T.; Koliantzaki, I.; Savvidou, O.; Tsoumpa, M.; Eustathiou, G.; Kontogeorgakos, V.; Souvatzoglou, R. Acute pain management in trauma: Anatomy, ultrasound-guided peripheral nerve blocks and special considerations. Minerva Anestesiol. 2019, 85, 763–773. [Google Scholar] [CrossRef]
- Hamrick, K.L.; Beyer, C.A.; Lee, J.A.; Cocanour, C.S.; Duby, J.J. Multimodal Analgesia and Opioid Use in Critically Ill Trauma Patients. J. Am. Coll. Surg. 2019, 228, 769–775.e1. [Google Scholar] [CrossRef]
- Czernicki, M.; Kunnumpurath, S.; Park, W.; Kunnumpurath, A.; Kodumudi, G.; Tao, J.; Kodumudi, V.; Vadivelu, N.; Urman, R.D. Perioperative Pain Management in the Critically Ill Patient. Curr. Pain Headache Rep. 2019, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Haro, R.; Morales-Sarabia, J.; Ferrer-Gomez, C.; De Andrés, J. Regional analgesia techniques for pain management in patients admitted to the intensive care unit. Minerva Anestesiol. 2019, 85, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, E.; Kinirons, B. Regional anaesthesia in the elderly patient a current perspective. Curr. Opin. Anaesthesiol. 2021, 34, 48–53. [Google Scholar] [CrossRef]
- Villatte, G.; Mathonnet, M.; Villeminot, J.; Savary, M.; Theissen, A.; Ostermann, S.; Erivan, R.; Raynaud-Simon, A.; Slim, K. Interest of enhanced recovery programs in the elderly during total hip arthroplasty A systematic review. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 234–242. [Google Scholar]
- Belcaid, I.; Eipe, N. Perioperative Pain Management in Morbid Obesity. Drugs 2019, 79, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L. Opioid Management in Pregnancy and Postpartum. Obstet. Gynecol. Clin. North Am. 2020, 47, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Peahl, A.F.; Smith, R.; Johnson, T.R.; Morgan, D.M.; Pearlman, M.D. Better late than never: Why obstetricians must implement enhanced recovery after cesarean. Am. J. Obstet. Gynecol. 2019, 221, 117-e1–117-e7. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee. Opinion No. 742 Summary: Postpartum Pain Management. Obstet. Gynecol. 2018, 132, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.D.; Carvalho, B. Optimal Pain Management After Cesarean Delivery. Anesthesiol. Clin. 2017, 35, 107–124. [Google Scholar] [CrossRef]
- Wu, M.-S.; Chen, K.-H.; Chen, I.-F.; Huang, S.K.; Tzeng, P.-C.; Yeh, M.-L.; Lee, F.-P.; Lin, J.-G.; Chen, C. The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150367. [Google Scholar] [CrossRef]
- Sun, J.-N.; Chen, W.; Zhang, Y.; Zhang, Y.; Feng, S.; Chen, X.-Y. Does cognitive behavioral education reduce pain and improve joint function in patients after total knee arthroplasty? A randomized controlled trial. Int. Orthop. 2020, 44, 2027–2035. [Google Scholar] [CrossRef]
- Nicholls, J.L.; Azam, M.A.; Burns, L.C.; Englesakis, M.; Sutherland, A.M.; Weinrib, A.Z.; Katz, J.; Clarke, H. Psychological treatments for the management of postsurgical pain: A systematic review of randomized controlled trials. Patient Relat. Outcome Meas. 2018, 9, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhuo, Y.; Feng, E.; Wang, W.; Lin, W.; Lin, F.; Li, Z.; Lin, L.; Xiao, L.; Wang, H.; et al. The effect of musical interventions in improving short-term pain outcomes following total knee replacement: A meta-analysis and systematic review. J. Orthop. Surg. Res. 2020, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thybo, K.H.; Hägi-Pedersen, D.; Dahl, J.B.; Wetterslev, J.; Nersesjan, M.; Jakobsen, J.C.; Pedersen, N.A.; Overgaard, S.; Schrøder, H.M.; Schmidt, H.; et al. Effect of Combination of Paracetamol (Acetaminophen) and Ibuprofen vs Either Alone on Patient-Controlled Morphine Consumption in the First 24 Hours After Total Hip Arthroplasty. JAMA 2019, 321, 562–571. [Google Scholar] [CrossRef]
- Derry, C.J.; Derry, S.; Moore, R.A. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst. Rev. 2013, 2013, CD010210. [Google Scholar] [CrossRef]
- Ong, C.K.S.; Seymour, R.A.; Lirk, P.; Merry, A.F. Combining Paracetamol (Acetaminophen) with Nonsteroidal Antiinflammatory Drugs: A Qualitative Systematic Review of Analgesic Efficacy for Acute Postoperative Pain. Anesth. Analg. 2010, 110, 1170–1179. [Google Scholar] [CrossRef]
- Prescott, L.F. Paracetamol: Past, present, and future. Am. J. Ther. 2000, 7, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.A.; Hogg, M.; Bjorksten, A.R.; Williams, D.L.; Leslie, K.; Jamsen, K.; Kirkpatrick, C. Comparative Plasma and Cerebrospinal Fluid Pharmacokinetics of Paracetamol After Intravenous and Oral Administration. Anesth. Analg. 2016, 123, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Stundner, O.; Poeran, J.; Ladenhauf, H.N.; Berger, M.M.; Levy, S.B.; Zubizarreta, N.; Mazumdar, M.; Bekeris, J.; Liu, J.; Galatz, L.M.; et al. Effectiveness of intravenous acetaminophen for postoperative pain management in hip and knee arthroplasties: A population-based study. Reg. Anesth. Pain Med. 2019, 44, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.C.; Nadpara, P.A.; Taylor, P.D.; Brophy, G.M. Intravenous Versus Oral Acetaminophen for Pain Control in Neurocritical Care Patients. Neurocrit. Care 2016, 25, 400–406. [Google Scholar] [CrossRef]
- White, P.F. Cost-effective multimodal analgesia in the perioperative period: Use of intravenous vs. oral acetaminophen. J. Clin. Anesth. 2020, 61, 109625. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.R.; Huiras, P.; Empfield, J.; Horbowicz, K.J.; Lewis, K.; McAneny, D.; Twitchell, D. Controlling postoperative use of i.v. acetaminophen at an academic medical center. Am. J. Health Pharm. 2018, 75, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.Y. The Growing Case Against IV Tylenol, Once Seen as a Solution to the OPIOID crisis. The Washington Post, 19 June 2018. Available online: https://www.washingtonpost.com/news/wonk/wp/2018/06/19/the-growing-case-against-iv-tylenol-once-seen-as-a-solution-to-the-opioid-crisis/ (accessed on 2 January 2021).
- Foley, M.K.H.; Anderson, J.; Mallea, L.; Morrison, K.; Downey, M. Effects of Healing Touch on Postsurgical Adult Outpatients. J. Holist. Nurs. 2016, 34, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.-I.; Aller, M.-A.; Arias, J. Surgical inflammation: A pathophysiological rainbow. J. Transl. Med. 2009, 7, 19. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Fujie, K.; Taguchi, N.; Ma, E.; Shimizu, T.; Hashimoto, K. Short-Term Effects of Early Postoperative Celecoxib Administration for Pain, Sleep Quality, and Range of Motion After Total Knee Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2021, 36, 526–531. [Google Scholar] [CrossRef]
- Oxford League Table of Analgesic Efficacy. Available online: http://www.bandolier.org.uk/booth/painpag/Acutrev/Analgesics/lftab.html (accessed on 28 February 2021).
- Yurashevich, M.; Pedro, C.; Fuller, M.; Habib, A. Intra-operative ketorolac 15 mg versus 30 mg for analgesia following cesarean delivery: A retrospective study. Int. J. Obstet. Anesth. 2020, 44, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Motov, S.; Masoudi, A.; Drapkin, J.; Sotomayor, C.; Kim, S.; Butt, M.; Likourezos, A.; Fassassi, C.; Hossain, R.; Brady, J.; et al. Randomized Trial Comparing 3 Doses of Oral Ibuprofen for Management of Pain in Adult EM Patients. J. Emerg. Med. 2020, 59, 759–760. [Google Scholar] [CrossRef]
- Motov, S.; Yasavolian, M.; Likourezos, A.; Pushkar, I.; Hossain, R.; Drapkin, J.; Cohen, V.; Filk, N.; Smith, A.; Huang, F.; et al. Comparison of Intravenous Ketorolac at Three Single-Dose Regimens for Treating Acute Pain in the Emergency Department: A Randomized Controlled Trial. Ann. Emerg. Med. 2017, 70, 177–184. [Google Scholar] [CrossRef]
- Zhou, T.J.; Tang, J.; White, P.F. Propacetamol Versus Ketorolac for Treatment of Acute Postoperative Pain After Total Hip or Knee Replacement. Anesth. Analg. 2001, 92, 1569–1575. [Google Scholar] [CrossRef]
- CPIC. Clinical Pharmacogenetics Implementation Consortium Guidelines. 11 August 2020. Available online: https://cpicpgx.org/guidelines/ (accessed on 28 February 2021).
- Abdallah, F.W.; Hussain, N.; Weaver, T.; Brull, R. Analgesic efficacy of cannabinoids for acute pain management after surgery: A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2020, 45, 509–519. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Selph, S.S.; Buckley, D.I.; Holmes, R.S.; Mauer, K.; Ramirez, S.; Hsu, F.C.; Dana, T.; Fu, R.; Chou, R. Nonopioid Pharmacologic Treatments for Chronic Pain. In Nonopioid Pharmacologic Treatments for Chronic Pain; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020. [Google Scholar]
- Mun, C.J.; Letzen, J.E.; Peters, E.N.; Campbell, C.M.; Vandrey, R.; Gajewski-Nemes, J.; DiRenzo, D.; Caufield-Noll, C.; Finan, P.H. Cannabinoid effects on responses to quantitative sensory testing among individuals with and without clinical pain: A systematic review. Pain 2020, 161, 244–260. [Google Scholar] [CrossRef]
- Moore, A.B.; Navarrett, S.; Herzig, S.J. Potentially Inappropriate Use of Intravenous Opioids in Hospitalized Patients. J. Hosp. Med. 2019, 14, 678–680. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Park, J.S.; Park, S.Y.; Kim, H.J.; Yeo, J.; Kim, J.-C.; Park, S.; Choi, G.-S. Can intravenous patient-controlled analgesia be omitted in patients undergoing laparoscopic surgery for colorectal cancer? Ann. Surg. Treat. Res. 2015, 88, 86–91. [Google Scholar] [CrossRef]
- McEvoy, M.D.; Wanderer, J.P.; King, A.B.; Geiger, T.M.; Tiwari, V.; Terekhov, M.; Ehrenfeld, J.M.; Furman, W.R.; Lee, L.A.; Sandberg, W.S. A perioperative consult service results in reduction in cost and length of stay for colorectal surgical patients: Evidence from a healthcare redesign project. Perioper. Med. 2016, 5, 3. [Google Scholar] [CrossRef]
- Institute for Safe Medication Practices (ISMP). Safety Issues with PCA Part I—How Errors Occur. 10 July 2003. Available online: https://www.ismp.org/resources/safety-issues-pca-part-i-how-errors-occur (accessed on 17 January 2021).
- Institute for Safe Medication Practices (ISMP). Safety Issues with PCA Part II—How to Prevent Errors. 24 July 2003. Available online: https://www.ismp.org/resources/safety-issues-pca-part-ii-how-prevent-errors (accessed on 17 January 2021).
- Smith, H.S. Opioid Metabolism. Mayo Clin. Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef]
- Coller, J.K.; Christrup, L.L.; Somogyi, A.A. Role of active metabolites in the use of opioids. Eur. J. Clin. Pharmacol. 2008, 65, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. The Metabolism of Opioid Agents and the Clinical Impact of Their Active Metabolites. Clin. J. Pain 2011, 27, 824–838. [Google Scholar] [CrossRef]
- Overholser, B.R.; Foster, D.R. Opioid pharmacokinetic drug-drug interactions. Am. J. Manag. Care 2011, 17, 276–287. [Google Scholar]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021. [Google Scholar] [CrossRef]
- Davison, S.N. Clinical Pharmacology Considerations in Pain Management in Patients with Advanced Kidney Failure. Clin. J. Am. Soc. Nephrol. 2019, 14, 917–931. [Google Scholar] [CrossRef]
- Crews, K.R.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Klein, T.E.; Caudle, K.E.; Haidar, C.E.; Shen, D.D.; Callaghan, J.T.; Sadhasivam, S.; et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for Cytochrome P450 2D6 Genotype and Codeine Therapy: 2014 Update. Clin. Pharmacol. Ther. 2014, 95, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Miotto, K.; Cho, A.K.; Khalil, M.A.; Blanco, K.; Sasaki, J.D.; Rawson, R. Trends in Tramadol. Anesth. Analg. 2017, 124, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-analysis of Population-scale Sequencing Projects. Clin. Pharmacol. Ther. 2017, 102, 688–700. [Google Scholar] [CrossRef]
- Ren, Z.-Y.; Xu, X.-Q.; Bao, Y.-P.; He, J.; Shi, L.; Deng, J.-H.; Gao, X.-J.; Tang, H.-L.; Wang, Y.-M.; Lu, L. The impact of genetic variation on sensitivity to opioid analgesics in patients with postoperative pain: A systematic review and meta-analysis. Pain Physician 2015, 18, 131–152. [Google Scholar] [PubMed]
- Comelon, M.; Raeder, J.; Drægni, T.; Lieng, M.; Lenz, H. Tapentadol versus oxycodone analgesia and side effects after laparoscopic hysterectomy. Eur. J. Anaesthesiol. 2021. [Google Scholar] [CrossRef]
- Wang, X.; Narayan, S.W.; Penm, J.; Patanwala, A.E. Efficacy and Safety of Tapentadol Immediate Release for Acute Pain. Clin. J. Pain 2020, 36, 399–409. [Google Scholar] [CrossRef]
- Wei, J.; Lane, N.E.; Bolster, M.B.; Dubreuil, M.; Zeng, C.; Misra, D.; Lu, N.; Choi, H.K.; Lei, G.; Zhang, Y. Association of Tramadol Use With Risk of Hip Fracture. J. Bone Miner. Res. 2020, 35, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, P.; Grilli, M.; Canonico, P.L.; Collino, M.; Dickenson, A.H. Pharmacological rationale for tapentadol therapy: A review of new evidence. J. Pain Res. 2019, 12, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Dubreuil, M.; LaRochelle, M.R.; Lu, N.; Wei, J.; Choi, H.K.; Lei, G.; Zhang, Y. Association of Tramadol With All-Cause Mortality Among Patients With Osteoarthritis. JAMA 2019, 321, 969–982. [Google Scholar] [CrossRef]
- Faria, J.; Barbosa, J.; Moreira, R.; Queirós, O.; Carvalho, F.; Dinis-Oliveira, R. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur. J. Pain 2018, 22, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, N.; Chang, D.; Helander, E.M.; Bordelon, G.J.; Kai, A.; Kaye, A.D.; Hsu, D.; Bang, D.; Julka, I. Ketorolac, Oxymorphone, Tapentadol, and Tramadol. Anesthesiol. Clin. 2017, 35, e1–e20. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Queirós, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative metabolism of tramadol and tapentadol: A toxicological perspective. Drug Metab. Rev. 2016, 48, 577–592. [Google Scholar] [CrossRef]
- “Weak” Opioid Analgesics. Codeine, Dihydrocodeine and Tramadol: No Less Risky Than Morphine. Prescrire Int. 2016, 25, 45–50. Available online: https://www.ncbi.nlm.nih.gov/pubmed/27042732 (accessed on 14 September 2020).
- Brennan, M.J. The Clinical Implications of Cytochrome P450 Interactions with Opioids and Strategies for Pain Management. J. Pain Symptom Manag. 2012, 44, S15–S22. [Google Scholar] [CrossRef]
- Pergolizzi, J.V. Quantifying the impact of drug-drug interactions associated with opioids. Am. J. Manag. Care 2011, 17, 288–292. [Google Scholar]
- Li, P.H.; Ue, K.L.; Wagner, A.; Rutkowski, R.; Rutkowski, K. Opioid Hypersensitivity: Predictors of Allergy and Role of Drug Provocation Testing. J. Allergy Clin. Immunol. Pract. 2017, 5, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Kalangara, J.; Vanijcharoenkarn, K.; Lynde, G.C.; McIntosh, N.; Kuruvilla, M. Approach to Perioperative Anaphylaxis in 2020: Updates in Diagnosis and Management. Curr. Allergy Asthma Rep. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Histamine-Releasing and Allergenic Properties of Opioid Analgesic Drugs: Resolving the Two. Anaesth. Intensiv. Care 2012, 40, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.Z.; Mueller, S.W.; Reynolds, P.M. Assessment of Opioid Cross-reactivity and Provider Perceptions in Hospitalized Patients with Reported Opioid Allergies. Ann. Pharmacother. 2019, 53, 1117–1123. [Google Scholar] [CrossRef]
- Swarm, R.A.; Paice, J.A.; Anghelescu, D.L.; Are, M.; Bruce, J.Y.; Buga, S.; Chwistek, M.; Cleeland, C.; Craig, D.; Gafford, E.; et al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 977–1007. [Google Scholar] [CrossRef]
- Said, E.T.; Drueding, R.E.; Martin, E.I.; Furnish, T.J.; Meineke, M.N.; Sztain, J.F.; Abramson, W.B.; Swisher, M.W.; Jacobsen, G.R.; Gosman, A.A.; et al. The Implementation of an Acute Pain Service for Patients Undergoing Open Ventral Hernia Repair with Mesh and Abdominal Wall Reconstruction. World J. Surg. 2021, 45, 1102–1108. [Google Scholar] [CrossRef]
- Bui, B.T.; Grygiel, M.R.; Konstantatos, M.B.A.; Christelis, N.; Liew, M.B.S.; Hopkins, B.R.; Dooley, B.M. The impact of an innovative pharmacist-led inpatient opioid de-escalation intervention in post-operative orthopedic patients. J. Opioid Manag. 2020, 16, 167–176. [Google Scholar] [CrossRef]
- Lovasi, O.; Lám, J.; Kósik, N. Az akutfájdalom-kezelő szolgálat szerepe a műtét utáni fájdalomcsillapításban. Orvosi Hetil. 2020, 161, 575–581. [Google Scholar] [CrossRef]
- Mitra, S.; Jain, K.; Singh, J.; Jindal, S.; Saxena, P.; Singh, M.; Saroa, R.; Ahuja, V.; Kang, J.; Garg, S. Does an acute pain service improve the perception of postoperative pain management in patients undergoing lower limb surgery? A prospective controlled non-randomized study. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, 187–194. [Google Scholar] [CrossRef]
- Said, E.T.; Sztain, J.F.; Abramson, W.B.; Meineke, M.N.; Furnish, T.J.; Schmidt, U.H.; Manecke, G.R.; Gabriel, R.A. A Dedicated Acute Pain Service Is Associated with Reduced Postoperative Opioid Requirements in Patients Undergoing Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. Anesth. Analg. 2018, 127, 1044–1050. [Google Scholar] [CrossRef]
- Zaccagnino, M.P.; Bader, A.M.; Sang, C.N.; Correll, D.J. The Perioperative Surgical Home. Anesth. Analg. 2017, 125, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.R.; Stanley, A.Y. Literature Review: Assessment of Opioid-related Sedation and the Pasero Opioid Sedation Scale. J. PeriAnesth. Nurs. 2019, 34, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Jungquist, C.R.; Quinlan-Colwell, A.; Vallerand, A.; Carlisle, H.L.; Cooney, M.; Dempsey, S.J.; Dunwoody, D.; Maly, A.; Meloche, K.; Meyers, A.; et al. American Society for Pain Management Nursing Guidelines on Monitoring for Opioid-Induced Advancing Sedation and Respiratory Depression: Revisions. Pain Manag. Nurs. 2020, 21, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Jungquist, C.R.; Smith, K.; Nicely, K.L.W.; Polomano, R.C. Monitoring Hospitalized Adult Patients for Opioid-Induced Sedation and Respiratory Depression. AJN Am. J. Nurs. 2017, 117, S27–S35. [Google Scholar] [CrossRef]
- Lam, T.; Nagappa, M.; Wong, J.; Singh, M.; Wong, D.; Chung, F. Continuous Pulse Oximetry and Capnography Monitoring for Postoperative Respiratory Depression and Adverse Events. Anesth. Analg. 2017, 125, 2019–2029. [Google Scholar] [CrossRef]
- Steele, T.; Eidem, L.; Bond, J. Impact of Adoption of Smart Pump System with Continuous Capnography Monitoring on Opioid-Related Adverse Event Rates: Experience From a Tertiary Care Hospital. J. Patient Saf. 2019, 16, e194–e198. [Google Scholar] [CrossRef]
- Kim, B.; Nolan, S.; Beaulieu, T.; Shalansky, S.; Ti, L. Inappropriate opioid prescribing practices: A narrative review. Am. J. Health Pharm. 2019, 76, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Parhami, I.; Massey, J.; Trimzi, I.; Huckshorn, K.; Gallucci, G. Risks Associated with Co-Prescribing Opioids and Benzodiazepines and Delaware’s Prescription Drug Monitoring Program. Del. Med. J. 2015, 87, 270–274. [Google Scholar]
- Weinstein, S.; Poultsides, L.; Baaklini, L.; Mörwald, E.; Cozowicz, C.; Saleh, J.; Arrington, M.; Poeran, J.; Zubizarreta, N.; Memtsoudis, S. Postoperative delirium in total knee and hip arthroplasty patients: A study of perioperative modifiable risk factors. Br. J. Anaesth. 2018, 120, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Rade, M.C.; YaDeau, J.T.; Ford, C.; Reid, M.C. Postoperative Delirium in Elderly Patients After Elective Hip or Knee Arthroplasty Performed Under Regional Anesthesia. HSS J. 2011, 7, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Rizk, P.; Morris, W.; Oladeji, P.; Huo, M. Review of Postoperative Delirium in Geriatric Patients Undergoing Hip Surgery. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 100–105. [Google Scholar] [CrossRef]
- Rogers, E.; Mehta, S.; Shengelia, R.; Reid, M.C. Four Strategies for Managing Opioid-Induced Side Effects in Older Adults. Clin. Geriatr. 2013, 21. [Google Scholar]
- American Society of Anesthesiologists Task Force on Neuraxial Opioids. Practice Guidelines for the Prevention, Detection, and Management of Respiratory Depression Associated with Neuraxial Opioid Administration. Anesthesiology 2016, 124, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morlion, B. Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med. 2016, 18, 1837–1863. [Google Scholar] [CrossRef]
- Drewes, A.M.; Munkholm, P.; Simrén, M.; Breivik, H.; Kongsgaard, U.E.; Hatlebakk, J.G.; Agreus, L.; Friedrichsen, M.; Christrup, L.L. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction–Recommendations of the Nordic Working Group. Scand. J. Pain 2016, 11, 111–122. [Google Scholar] [CrossRef]
- Alvaro, D.; Caraceni, A.T.; Coluzzi, F.; Gianni, W.; Lugoboni, F.; Marinangeli, F.; Massazza, G.; Pinto, C.; Varrassi, G. What to Do and What Not to Do in the Management of Opioid-Induced Constipation: A Choosing Wisely Report. Pain Ther. 2020, 9, 657–667. [Google Scholar] [CrossRef]
- Yue, C.; Liu, Y.; Zhang, X.; Xu, B.; Sheng, H. Randomised controlled trial of a comprehensive protocol for preventing constipation following total hip arthroplasty. J. Clin. Nurs. 2020, 29, 2863–2871. [Google Scholar] [CrossRef]
- Woelk, C.J. The hand that writes the opioid. Can. Fam. Physician Med. Fam. Can. 2007, 53, 1015–1017. [Google Scholar]
- Schwenk, E.S.; Grant, A.E.; Torjman, M.C.; McNulty, S.E.; Baratta, J.L.; Viscusi, E.R. The Efficacy of Peripheral Opioid Antagonists in Opioid-Induced Constipation and Postoperative Ileus. Reg. Anesth. Pain Med. 2017, 42, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Nishie, K.; Yamamoto, S.; Yamaga, T.; Horigome, N.; Hanaoka, M. Peripherally acting μ-opioid antagonist for the treatment of opioid-induced constipation: Systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2018, 34, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.H.; Bogdanovski, D.A.; Paglinco, S.R.; Barratt-Stopper, P.; Rolandelli, R.H. Cost and efficacy examination of alvimopan for the prevention of postoperative ileus. J. Investig. Med. 2017, 65, 949–952. [Google Scholar] [CrossRef]
- Kelley, S.R.; Wolff, B.G.; Lovely, J.K.; Larson, D.W. Fast-track pathway for minimally invasive colorectal surgery with and without alvimopan (Entereg)TM: Which is more cost-effective? Am. Surg. 2013, 79, 630–633. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23711275 (accessed on 14 September 2020). [CrossRef] [PubMed]
- Goodstein, T.; Launer, B.; White, S.; Lyon, M.; George, N.; Deronde, K.; Burke, M.; O’Donnell, C.; Lyda, C.; Kiser, T.H.; et al. A Retrospective Study of Patients Undergoing Radical Cystectomy and Receiving Peri-Operative Naloxegol or Alvimopan: Comparison of Length of Stay. J. Surg. 2018, 6, 129–134. [Google Scholar] [CrossRef]
- Marciniak, C.M.; Toledo, S.; Lee, J.; Jesselson, M.; Bateman, J.; Grover, B.; Tierny, J. Lubiprostonevs Sennain postoperative orthopedic surgery patients with opioid-induced constipation: A double-blind, active-comparator trial. World J. Gastroenterol. 2014, 20, 16323–16333. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.D.; Detriche, O.; Forget, P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Tubog, T.D.; Harenberg, J.L.; Buszta, K.; Hestand, J.D. Prophylactic Nalbuphine to Prevent Neuraxial Opioid-Induced Pruritus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. PeriAnesth. Nurs. 2019, 34, 491–501.e8. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, R.G. Nalbuphine for Treatment of Opioid-induced Pruritus. Clin. J. Pain 2016, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
- McNicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015, 2015, CD003348. [Google Scholar] [CrossRef]
- Mancini, R.; Filicetti, M. Pain management of opioid-tolerant patients undergoing surgery. Am. J. Health Pharm. 2010, 67, 872–875. [Google Scholar] [CrossRef]
- Oliver, J.; Coggins, C.; Compton, P.; Hagan, S.; Matteliano, D.; Stanton, M.; Marie, B.S.; Strobbe, S.; Turner, H.N. American Society for Pain Management Nursing Position Statement: Pain Management in Patients with Substance Use Disorders. Pain Manag. Nurs. 2012, 13, 169–183. [Google Scholar] [CrossRef]
- Li, W.T.; Bell, K.L.; Yayac, M.; Barmann, J.A.; Star, A.M.; Austin, M.S. A Postdischarge Multimodal Pain Management Cocktail Following Total Knee Arthroplasty Reduces Opioid Consumption in the 30-Day Postoperative Period: A Group-Randomized Trial. J. Arthroplast. 2021, 36, 164–172.e2. [Google Scholar] [CrossRef]
- MacPherson, R.; Pattullo, G. Management of postsurgical pain in the community. Aust. Prescr. 2020, 43, 191–194. [Google Scholar] [CrossRef]
- Hill, M.V.; McMahon, M.L.; Stucke, R.S.; Barth, R.J. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann. Surg. 2017, 265, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.V.; Howard, R.A.; Gunaseelan, V.; Brummett, C.M.; Waljee, J.F.; Englesbe, M.J. Statewide Implementation of Postoperative Opioid Prescribing Guidelines. N. Engl. J. Med. 2019, 381, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.V.; Stucke, R.S.; Billmeier, S.E.; Kelly, J.L.; Barth, R.J. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J. Am. Coll. Surg. 2018, 226, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Thiels, C.A.; Ubl, D.S.; Yost, K.J.; Dowdy, S.C.; Mabry, T.M.; Gazelka, H.M.; Cima, R.R.; Habermann, E.B. Results of a Prospective, Multicenter Initiative Aimed at Developing Opioid-prescribing Guidelines After Surgery. Ann. Surg. 2018, 268, 457–468. [Google Scholar] [CrossRef]
- Kelley-Quon, L.I.; Kirkpatrick, M.G.; Ricca, R.L.; Baird, R.; Harbaugh, C.M.; Brady, A.; Garrett, P.; Wills, H.; Argo, J.; Diefenbach, K.A.; et al. Guidelines for Opioid Prescribing in Children and Adolescents After Surgery. JAMA Surg. 2021, 156, 76. [Google Scholar] [CrossRef]
- Genord, C.; Frost, T.; Eid, D. Opioid exit plan: A pharmacist’s role in managing acute postoperative pain. J. Am. Pharm. Assoc. 2017, 57, S92–S98. [Google Scholar] [CrossRef]
- Brandal, D.; Keller, M.S.; Lee, C.; Grogan, T.; Fujimoto, Y.; Gricourt, Y.; Yamada, T.; Rahman, S.; Hofer, I.; Kazanjian, K.; et al. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital. Anesth. Analg. 2017, 125, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Loomis, E.; McNaughton, D.B.; Genord, C.K. A Quality Improvement Initiative Addressing Safe Opioid Prescribing and Disposal Post-Cesarean Delivery. Pain Manag. Nurs. in press.
- Kumar, K.; Gulotta, L.V.; Dines, J.S.; Allen, A.A.; Cheng, J.; Fields, K.G.; YaDeau, J.T.; Wu, C.L. Unused Opioid Pills After Outpatient Shoulder Surgeries Given Current Perioperative Prescribing Habits. Am. J. Sports Med. 2017, 45, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Mayman, D.J.; Jerabek, S.A.; Sculco, P.K.; Haas, S.B.; Ast, M.P. Reduction of Opioids Prescribed Upon Discharge After Total Knee Arthroplasty Significantly Reduces Consumption: A Prospective Study Comparing Two States. J. Arthroplast. 2021, 36, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Hevesi, M.; Ubl, D.S.; Habermann, E.B.; Gazelka, H.M.; Trousdale, R.T.; Turner, N.S.; Pagnano, M.W.; Mabry, T.M. Implementation of Procedure-Specific Opioid Guidelines. JBJS Open Access 2020, 5, e0050. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.J.; Chen, C.J.; Clifford, H.; Xue, Z.; Wang, S.; Argenziano, M.; Landau, R.; Meng, M.-L. Introduction of an Analgesia Prescription Guideline Can Reduce Unused Opioids After Cardiac Surgery: A Before and After Cohort Study. J. Cardiothorac. Vasc. Anesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Solouki, S.; Vega, M.; Agalliu, I.; Abraham, N.E. Patient Satisfaction and Refill Rates After Decreasing Opioids Prescribed for Urogynecologic Surgery. Female Pelvic Med. Reconstr. Surg. 2020, 26, e78–e82. [Google Scholar] [CrossRef]
- Fleischman, A.N.; Tarabichi, M.; Foltz, C.; Makar, G.; Hozack, W.J.; Austin, M.S.; Chen, A.F.; Star, A.M.; Greenky, M.; Henstenburg, B.; et al. Cluster-Randomized Trial of Opiate-Sparing Analgesia after Discharge from Elective Hip Surgery. J. Am. Coll. Surg. 2019, 229, 335–345.e5. [Google Scholar] [CrossRef]
- Hartford, L.B.; Van Koughnett, J.A.M.; Murphy, P.B.; Knowles, S.A.; Wigen, R.B.; Allen, L.J.; Clarke, C.F.M.; Brackstone, M.; Gray, D.K.; Maciver, A.H. The Standardization of Outpatient Procedure (STOP) Narcotics: A Prospective Health Systems Intervention to Reduce Opioid Use in Ambulatory Breast Surgery. Ann. Surg. Oncol. 2019, 26, 3295–3304. [Google Scholar] [CrossRef]
- Holte, A.J.; Carender, C.N.; Noiseux, N.O.; Otero, J.E.; Brown, T.S. Restrictive Opioid Prescribing Protocols Following Total Hip Arthroplasty and Total Knee Arthroplasty Are Safe and Effective. J. Arthroplast. 2019, 34, S135–S139. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.J.; Grace, T.R.; Khanna, K.; Barry, J.; Hansen, E.N. A Goal-directed Quality Improvement Initiative to Reduce Opioid Prescriptions After Orthopaedic Procedures. JAAOS Glob. Res. Rev. 2019, 3, e109. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Hevesi, M.; Trousdale, E.R.; Ubl, D.S.; Gazelka, H.M.; Habermann, E.B.; Trousdale, R.T.; Pagnano, M.W.; Mabry, T.M. The 2018 Chitranjan S. Ranawat, MD Award: Developing and Implementing a Novel Institutional Guideline Strategy Reduced Postoperative Opioid Prescribing After TKA and THA. Clin. Orthop. Relat. Res. 2019, 477, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.J.; Ashraf, S. Intestinal Anastomosis (Chapter 29). 2008. Available online: http://www.acssurgery.com/acs/Chapters/CH0529.htm (accessed on 13 December 2020).
- Lawrence, A.E.; Carsel, A.J.; Leonhart, K.L.; Richards, H.W.; Harbaugh, C.M.; Waljee, J.F.; McLeod, D.J.; Walz, P.C.; Minneci, P.C.; Deans, K.J.; et al. Effect of Drug Disposal Bag Provision on Proper Disposal of Unused Opioids by Families of Pediatric Surgical Patients. JAMA Pediatr. 2019, 173, e191695. [Google Scholar] [CrossRef]
- Hite, M.; Dippre, A.; Heldreth, A.; Cole, D.; Lockett, M.; Klauber-Demore, N.; Abbott, A.M. A Multifaceted Approach to Opioid Education, Prescribing, and Disposal for Patients with Breast Cancer Undergoing Surgery. J. Surg. Res. 2021, 257, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Ramel, C.L.; Habermann, E.B.; Thiels, C.A.; Dierkhising, R.A.; Cunningham, J.L. Provision of a Drug Deactivation System for Unused Opioid Disposal at Surgical Dismissal. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 357–361. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Where and How to Dispose of Unused Medicines. 10 September 2020. Available online: https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines (accessed on 8 January 2021).
- US Environmental Protection Agency. How to Dispose of Medicines Properly. In April 2011. Available online: https://archive.epa.gov/region02/capp/web/pdf/ppcpflyer.pdf (accessed on 8 January 2021).
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.; Davis, N.L. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef]
- Mudumbai, S.C.; Lewis, E.T.; Oliva, E.M.; Chung, P.D.; Harris, B.; Trafton, J.; Mariano, E.R.; Wagner, T.; Clark, J.D.; Stafford, R.S. Overdose Risk Associated with Opioid Use upon Hospital Discharge in Veterans Health Administration Surgical Patients. Pain Med. 2018, 20, 1020–1031. [Google Scholar] [CrossRef]
- Han, J.K.; Hill, L.G.; Koenig, M.E.; Das, N. Naloxone Counseling for Harm Reduction and Patient Engagement. Fam. Med. 2017, 49, 730–733. [Google Scholar]
- Punzal, M.; Santos, P.; Li, X.; Oyler, D.R.; Hall, A.M. Current practices in naloxone prescribing upon hospital discharge. J. Opioid Manag. 2019, 15, 357–361. [Google Scholar] [CrossRef]
- Shah, A.; Hayes, C.J.; Martin, B.C. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use—United States, 2006–2015. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 265–269. [Google Scholar] [CrossRef]
- Stapler, S.J.; Brockhaus, K.K.; Battaglia, M.A.; Mahoney, S.T.; McClure, A.M.; Cleary, R.K. A single institution analysis of targeted colorectal surgery enhanced recovery pathway strategies that decrease readmissions. Dis. Colon Rectum. in press.
- L’Hermite, J.; Pagé, M.G.; Chevallier, T.; Occean, B.; Viel, E.; Bredeau, O.; Lefrant, J.-Y.; Cuvillon, P. Characterisation of pragmatic postoperative PAin trajectories over seven days and their association with CHronicity after 3 months: A prospective, pilot cohort study (PATCH study). Anaesth. Crit. Care Pain Med. 2020, 40, 100793. [Google Scholar] [CrossRef]
- Ellis, J.L.; Ghiraldi, E.M.; Cohn, J.A.; Nitti, M.; Friedlander, J.I.; Ginzburg, S.; Sterious, S.N.; Abbosh, P.; Ohmann, E.; Uzzo, R.G.; et al. Prescribing Trends in Post-operative Pain Management After Urologic Surgery: A Quality Care Investigation for Healthcare Providers. Urology 2021. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.M. JCAHO Pain Management Standards Are Unveiled. JAMA 2000, 284, 428–429. [Google Scholar] [CrossRef]
- Porter, J.; Jick, H. Addiction Rare in Patients Treated with Narcotics. N. Engl. J. Med. 1980, 302, 123. [Google Scholar] [CrossRef] [PubMed]
- Society, A.P. Principles of analgesic use in the treatment of acute pain and cancer pain. Am. Pain Soc. 1999, 9, 601–612. [Google Scholar]
- Scher, C.; Meador, L.; Van Cleave, J.H.; Reid, M.C. Moving Beyond Pain as the Fifth Vital Sign and Patient Satisfaction Scores to Improve Pain Care in the 21st Century. Pain Manag. Nurs. 2018, 19, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.E.; Vlahos, A.L.; Ledgerwood, A.M. Kindness Kills: The Negative Impact of Pain as the Fifth Vital Sign. J. Am. Coll. Surg. 2007, 205, 101–107. [Google Scholar] [CrossRef]
- Chee, T.T.; Ryan, A.M.; Wasfy, J.H.; Borden, W.B. Current State of Value-Based Purchasing Programs. Circulation 2016, 133, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Bledsoe, G.H.; Armstrong, J.H. Are Pain Management Questions in Patient Satisfaction Surveys Driving the Opioid Epidemic? Am. J. Public Health 2016, 106, 985–986. [Google Scholar] [CrossRef]
- Anson, P. AMA Drops Pain as Vital Sign—Pain News Network. Pain News Network; 16 Jun 2016. Available online: https://www.painnewsnetwork.org/stories/2016/6/16/ama-drops-pain-as-vital-sign (accessed on 1 January 2021).
- Thompson, C.A. HCAHPS survey to measure pain communication, not management. Am. J. Health Pharm. 2017, 74, 1924–1926. [Google Scholar] [CrossRef]
- Wen, H.; Hockenberry, J.M.; Jeng, P.J.; Bao, Y. Prescription Drug Monitoring Program Mandates: Impact on Opioid Prescribing and Related Hospital Use. Health Aff. 2019, 38, 1550–1556. [Google Scholar] [CrossRef]
- Hospital Compare. In the Center for Medicare and Medicaid Services. Available online: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalCompare (accessed on 2 January 2021).
- Leapfrog Hospital Safety Grade. In Leapfrod Group. 25 March 2016. Available online: https://www.leapfroggroup.org/data-users/leapfrog-hospital-safety-grade (accessed on 2 January 2021).
- Huesch, M.D.; Currid-Halkett, E.; Doctor, J.N. Public hospital quality report awareness: Evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open 2014, 4, e004417. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.M.; Caturegli, I.; O’Hara, N.N.; O’Toole, R.V.; Dalury, D.F.; Harris, A.D.; Manson, T.T. What publicly available quality metrics do hip and knee arthroplasty patients care about most when selecting a hospital in Maryland: A discrete choice experiment. BMJ Open 2019, 9, e028202. [Google Scholar] [CrossRef] [PubMed]
- National Quality Partners PlaybookTM: Opioid Stewardship. Available online: https://store.qualityforum.org/products/national-quality-partners-playbook%E2%84%A2-opioid-stewardship (accessed on 1 January 2021).
- Ghafoor, V.L.; Phelps, P.K.; Pastor, I.J.; Meisel, S. Transformation of Hospital Pharmacist Opioid Stewardship. Hosp. Pharm. 2019, 54, 266–273. [Google Scholar] [CrossRef]
- Quinlan, J.; Rann, S.; Bastable, R.; Levy, N. Perioperative opioid use and misuse. Clin. Med. 2019, 19, 441–445. [Google Scholar] [CrossRef]
- Phelps, P.; Achey, T.S.; Mieure, K.D.; Cuellar, L.; MacMaster, H.; Pecho, R.; Ghafoor, V. A Survey of Opioid Medication Stewardship Practices at Academic Medical Centers. Hosp. Pharm. 2018, 54, 57–62. [Google Scholar] [CrossRef]
- Rizk, E.; Swan, J.T.; Fink, E. Prioritization of quality indicators for opioid stewardship. Am. J. Health Pharm. 2019, 76, 1458–1459. [Google Scholar] [CrossRef]
- Tichy, E.M.; Schumock, G.T.; Hoffman, J.M.; Suda, K.J.; Rim, M.H.; Tadrous, M.; Stubbings, J.; Cuellar, S.; Clark, J.S.; Wiest, M.D.; et al. National trends in prescription drug expenditures and projections for 2020. Am. J. Health Pharm. 2020, 77, 1213–1230. [Google Scholar] [CrossRef]
- Hyland, S.J.; Kramer, B.J. Curbing the enthusiasm: Stewardship of high risk, high-cost drugs in perioperative settings. In Proceedings of the American College of Clinical Pharmacy Annual Meeting, Seattle, WA, USA, 22 October 2018. [Google Scholar]
- Patel, G.P.; Hyland, S.J.; Birrer, K.L.; Wolfe, R.C.; Lovely, J.K.; Smith, A.N.; Dixon, R.L.; Johnson, E.G.; Gaviola, M.L.; Giancarelli, A.; et al. Perioperative clinical pharmacy practice: Responsibilities and scope within the surgical care continuum. J. Am. Coll. Clin. Pharm. 2019, 3, 501–519. [Google Scholar] [CrossRef]
- Oddis, J.A. Report of the ASHP Opioid Task Force. Am. J. Health Pharm. 2020, 77, 1158–1165. [Google Scholar] [CrossRef]
- Coulson, E.E.; Kral, L.A. The Clinical Pharmacist’s Role in Perioperative Surgical Pain Management. J. Pain Palliat. Care Pharmacother. 2020, 34, 120–126. [Google Scholar] [CrossRef]
- Hyland, S.J.; Kramer, B.J.; Fada, R.A.; Lucki, M.M. Clinical Pharmacist Service Associated With Improved Outcomes and Cost Savings in Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 2307.e1–2317.e1. [Google Scholar] [CrossRef]
- Poirier, R.H.; Brown, C.S.; Baggenstos, Y.T.; Walden, S.G.; Gann, N.Y.; Patty, C.M.; Sandoval, R.A.; McNulty, J.R. Impact of a pharmacist-directed pain management service on inpatient opioid use, pain control, and patient safety. Am. J. Health Pharm. 2018, 76, 17–25. [Google Scholar] [CrossRef]
- Brown, R.F.; Brockhaus, K.; Rajkumar, D.; Battaglia, M.; Cleary, R.K. Postoperative Pain After Enhanced Recovery Pathway Robotic Colon and Rectal Surgery, Diseases of the Colon & Rectum: 26 January 2021–Volume Publish Ahead of Print–Issue. Available online: https://journals.lww.com/dcrjournal/Abstract/9000/Postoperative_Pain_After_Enhanced_Recovery_Pathway.99586.aspx (accessed on 13 January 2021). [CrossRef]
- Hefti, E.; Remington, M.; Lavallee, C. Hospital consumer assessment of healthcare providers and systems scores relating to pain following the incorporation of clinical pharmacists into patient education prior to joint replacement surgery. Pharm. Pract. 2017, 15, 1071. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Yang, J. Oliceridine for the Management of Acute Postoperative Pain. Ann. Pharmacother. 2021. [Google Scholar] [CrossRef]
- Bergese, S.D.; Brzezinski, M.; Hammer, G.B.; Beard, T.L.; Pan, P.H.; Mace, S.E.; Berkowitz, R.D.; Cochrane, K.; Wase, L.; Minkowitz, H.S.; et al. ATHENA: A Phase 3, Open-Label Study Of The Safety And Effectiveness Of Oliceridine (TRV130), A G-Protein Selective Agonist At The μ-Opioid Receptor, In Patients With Moderate To Severe Acute Pain Requiring Parenteral Opioid Therapy. J. Pain Res. 2019, 12, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.; Nahar, N.; Saha, M.; Islam, M.S.; Reza, H.M.; Sharker, S.M. Local drug delivery from surgical thread for area-specific anesthesia. Biomed. Phys. Eng. Express 2020, 6, 015028. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.A.; Ilfeld, B.M. Acute postoperative pain management with percutaneous peripheral nerve stimulation: The SPRINT neuromodulation system. Expert Rev. Med. Devices 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Nishizawa, D.; Hasegawa, J.; Nakayama, K.; Fukuda, K.; Ichinohe, T.; Mieda, T.; Tsujita, M.; Nakagawa, H.; Kitamura, A.; et al. Effects of rs958804 and rs7858836 single-nucleotide polymorphisms of the ASTN2 gene on pain-related phenotypes in patients who underwent laparoscopic colectomy and mandibular sagittal split ramus osteotomy. Neuropsychopharmacol. Rep. 2021. [Google Scholar] [CrossRef]
- Kost, J.A.; Ruaño, G. Pharmacogenetics and the Personalization of Pain Management: A Potential Role in Precision Opioid Treatment. Conn Med. 2020, 84, 13–18. Available online: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=00106178&AN=141394148&h=WC%2F%2Fj3BfxC2wXRUHETAEgOc8WWLzGjPsSIu5o%2B5CYrADbW1dD9RcXpItvR%2FougtY3a4MTecX%2FdaoMpY7Chi4Zg%3D%3D&crl=c (accessed on 1 January 2021).
- Chen, Y.K.; Boden, K.A.; Schreiber, K.L. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: A narrative review. Anaesthesia 2021, 76, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ardeljan, L.D.; Waldfogel, J.M.; Bicket, M.C.; Hunsberger, J.B.; Vecchione, T.M.; Arwood, N.; Eid, A.; Hatfield, L.A.; McNamara, L.; Duncan, R.; et al. Current state of opioid stewardship. Am. J. Health Pharm. 2020, 77, 636–643. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).