Evaluation of Current Symptoms in Postoperative Achilles Tendons: A Multimodal Ultrasound Study
Abstract
1. Introduction
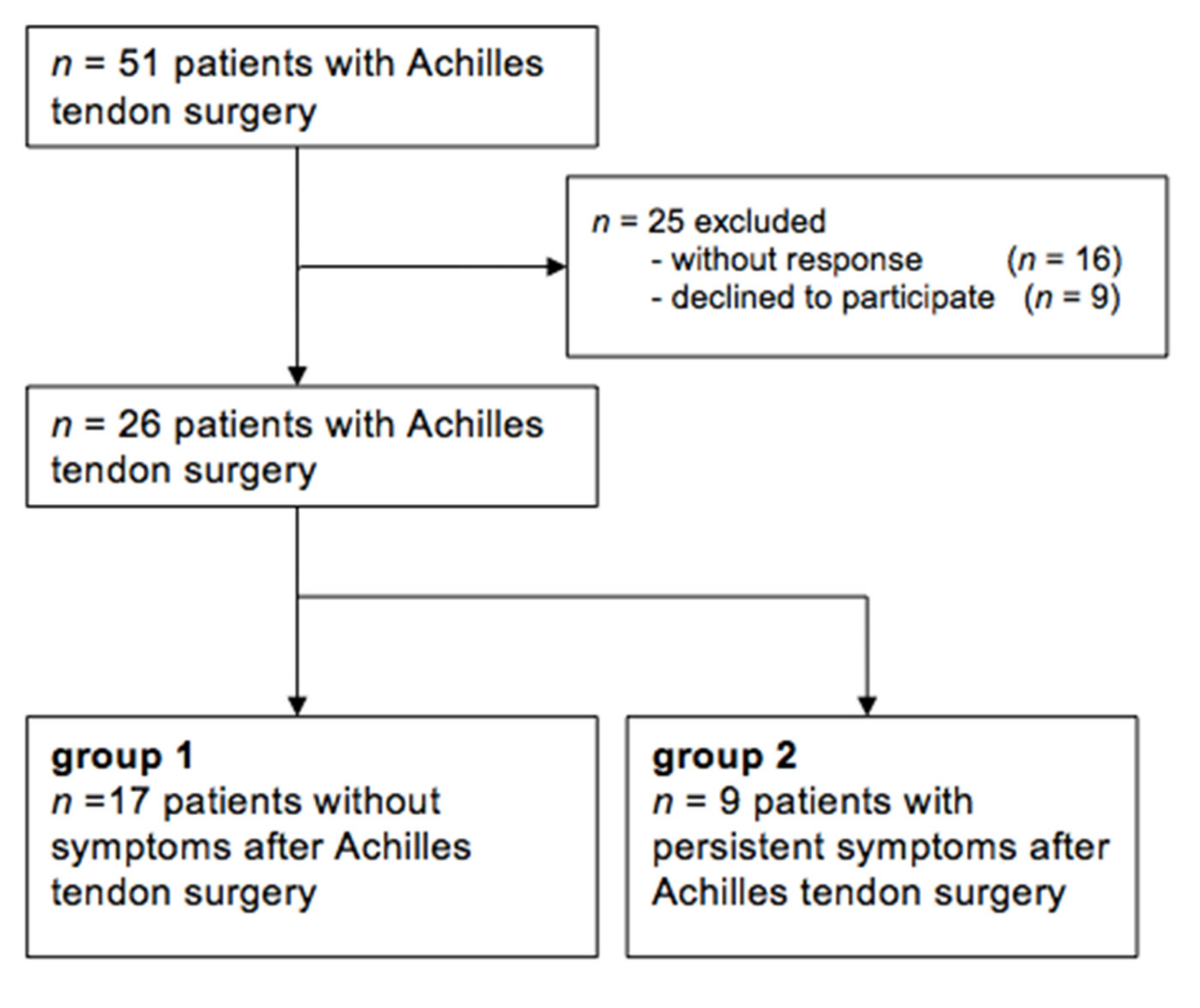
2. Materials and Methods
2.1. Patients
- Group 1: VISA-A score > = 90 representing asymptomatic Achilles tendons;
- Group 2: VISA-A score < 90 representing symptomatic Achilles tendons.
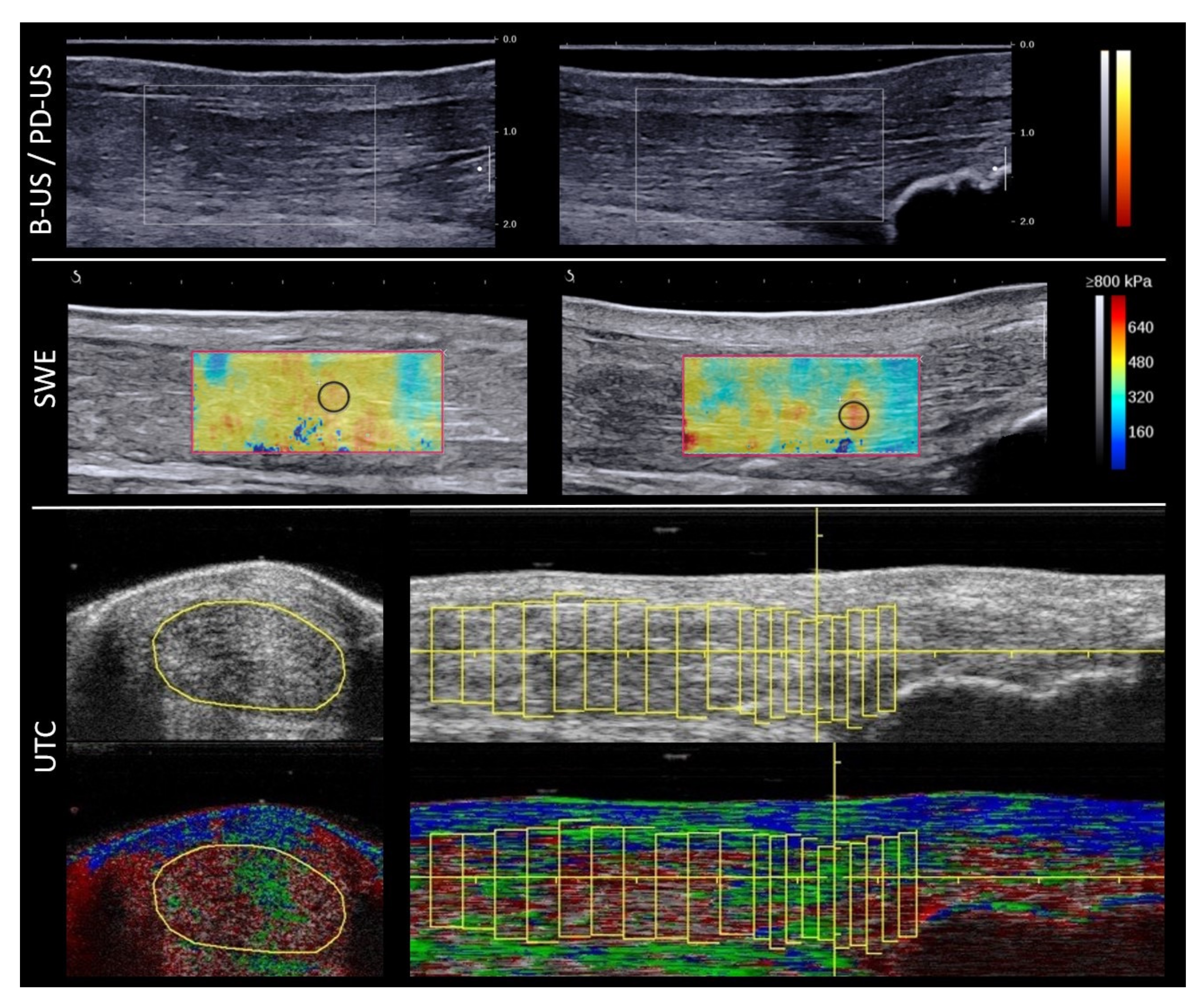
2.2. Data Acquisition with B-Mode, PD-US and SWE
2.3. UTC Data Acquisation
- Echo type I—intact and aligned tendon bundles (green);
- Echo type II—discontinuous wavy tendon bundles (blue);
- Echo type III—mainly fibrillar matrix (red);
- Echo type IV—mainly amorphous matrix (black).
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Zhuang, H.; Shao, D.; Wang, L.; Shi, M. High-Frequency Color Doppler Ultrasound in Diagnosis, Treatment, and Rehabilitation of Achilles Tendon Injury. Med Sci. Monit. 2017, 23, 5752–5759. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, A.; Massari, L.; Traina, G.C.; Mannella, P. The echographic and clinical follow-up of patients operated on for subcutaneous rupture of the Achilles tendon. La Radiol. Med. 1996, 91, 28–32. [Google Scholar]
- Bleakney, R.R.; Tallon, C.; Wong, J.K.; Lim, K.P.; Maffulli, N. Long-term Ultrasonographic Features of the Achilles Tendon After Rupture. Clin. J. Sport Med. 2002, 12, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Ooi, C.C.; Connell, D. Tendinopathy: Is Imaging Telling Us the Entire Story? J. Orthop. Sports Phys. Ther. 2015, 45, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Kälebo, P.; Tidebrant, G.; Movin, T.; Karlsson, J. The ultrasonographic appearance of the ruptured Achilles tendon during healing: A longitudinal evaluation of surgical and nonsurgical treatment, with comparisons to MRI appearance. Knee Surg. Sports Traumatol. Arthrosc. 2001, 10, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rupp, S.; Tempelhof, S.; Fritsch, E. Ultrasound of the Achilles tendon after surgical repair: Morphology and function. Br. J. Radiol. 1995, 68, 454–458. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Rath, B.; Betsch, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies: A Double-blinded, Longitudinal Clinical Study. Acad. Radiol. 2018, 25, 265–272. [Google Scholar] [CrossRef]
- Washburn, N.; Onishi, K.; Wang, J.H.-C. Ultrasound elastography and ultrasound tissue characterisation for tendon evaluation. J. Orthop. Transl. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Wan, W.-B.; Wang, Y.-X.; Jiao, Z.-Y.; Zhang, L.-H.; Luo, Y.-K.; Tang, P.-F. Evaluation of Elastic Stiffness in Healing Achilles Tendon After Surgical Repair of a Tendon Rupture Using In Vivo Ultrasound Shear Wave Elastography. Med Sci. Monit. 2016, 22, 1186–1191. [Google Scholar] [CrossRef]
- Frankewycz, B.; Penz, A.; Weber, J.; Da Silva, N.P.; Freimoser, F.; Bell, R.; Nerlich, M.; Jung, E.M.; Docheva, D.; Pfeifer, C.G. Achilles tendon elastic properties remain decreased in long term after rupture. Knee Surg. Sports Traumatol. Arthrosc. 2017, 26, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Kudaş, S.; Özcan, A.Ş.; Ipek, A.; Karaoğlanoğlu, M.; Arslan, H.; Bozkurt, M. Real-time sonoelastography of the Achilles tendon: Pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skelet. Radiol. 2012, 41, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.V.; Bartels, E.M.; Langberg, H. The Victorian Institute of Sports Assessment—Achilles Questionnaire (VISA-A)—A Reliable Tool for Measuring Achilles Tendinopathy. Int. J. Sports Phys. Ther. 2012, 7, 76–84. [Google Scholar]
- Lohrer, H.; Nauck, T. Cross-cultural adaptation and validation of the VISA-A questionnaire for German-speaking Achilles tendinopathy patients. BMC Musculoskelet. Disord. 2009, 10, 134. [Google Scholar] [CrossRef]
- Kostuj, T.S.; Baums, M.H.; Lieske, S. German Validation of the AOFAS ankle hindfoot scale. Fuß Sprunggelenk. 2014, 12, 100–106. [Google Scholar] [CrossRef]
- Ohberg, L.; Alfredson, H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: Pilot study of a new treatment. Br. J. Sports Med. 2002, 36, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Chu, K.; Lai, C.W.K. Sonographic evaluation of the immediate effects of eccentric heel drop exercise on Achilles tendon and gastrocnemius muscle stiffness using shear wave elastography. PeerJ 2017, 5, e3592. [Google Scholar] [CrossRef]
- Van Schie, H.T.; de Vos, R.J.; de Jonge, S.; Bakker, E.M.; Heijboer, M.P.; Verhaar, J.A.; Tol, J.L.; Weinans, H. Ultrasonographic tissue characterisation of human Achilles tendons: Quantification of tendon structure through a novel non-invasive approach. Br. J. Sports Med. 2010, 44, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Rabello, L.M.; Akker-Scheek, I.V.D.; Kuipers, I.F.; Diercks, R.L.; Brink, M.S.; Zwerver, J. Bilateral changes in tendon structure of patients diagnosed with unilateral insertional or midportion achilles tendinopathy or patellar tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1631–1638. [Google Scholar] [CrossRef]
- Docking, S.I.; Rosengarten, S.D.; Daffy, J.; Cook, J. Structural integrity is decreased in both Achilles tendons in people with unilateral Achilles tendinopathy. J. Sci. Med. Sport 2015, 18, 383–387. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, S.; Rozenberg, R.; Vieyra, B.; Stam, H.J.; Aanstoot, H.J.; Weinans, H.; van Schie, H.T.; Praet, S.F. Achilles tendons in people with type 2 diabetes show mildly compromised structure: An ultrasound tissue characterisation study. Br. J. Sports Med. 2015, 49, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Betsch, M.; Quack, V.; Bejder, L.; Schrading, S.; Tingart, M.; Dirrichs, T. Shear wave elastography for treatment monitoring of plantar fasciitis. J. Sports Med. Phys. Fit. 2020, 60, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Scott, A.; Carpentier, G.; Lian, Ø.; Van Kuppevelt, T.; Gossard, C.; Papy-Garcia, D.; Tassoni, M.-C.; Martelly, I. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. Br. J. Sports Med. 2014, 48, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef]
- Drew, B.T.; Smith, T.O.; Littlewood, C.; Sturrock, B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: A systematic review. Br. J. Sports Med. 2014, 48, 966–972. [Google Scholar] [CrossRef]
- De Vos, R.J.; Heijboer, M.P.; Weinans, H.; Verhaar, J.A.; van Schie, J.T. Tendon structure’s lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J. Sport Rehabil. 2012, 21, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Draghi, A.G.; Bortolotto, C.; Draghi, F. Sonography of the Achilles Tendon After Complete Rupture Repair: What the Radiologist Should Know. J. Ultrasound Med. 2016, 35, 2529–2536. [Google Scholar] [CrossRef]
- Barile, A.; Bruno, F.; Mariani, S.; Arrigoni, F.; Brunese, L.; Zappia, M.; Splendiani, A.; Di Cesare, E.; Masciocchi, C. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: A brief diagnostic imaging review. Musculoskelet. Surg. 2017, 101, 51–61. [Google Scholar] [CrossRef]
- Chianca, V.; Zappia, M.; Oliva, F.; Luca, B.; Maffulli, N. Post-operative MRI and US appearance of the Achilles tendons. J. Ultrasound 2020, 23, 387–395. [Google Scholar] [CrossRef]
- Maranho, D.A.C.; Nogueira-Barbosa, M.H.; Simão, M.N.; Volpon, J.B. Ultrasonographic Evaluation of Achilles Tendon Repair After Percutaneous Sectioning for the Correction of Congenital Clubfoot Residual Equinus. J. Pediatr. Orthop. 2009, 29, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.A.; Cho, K.H. Postoperative ultrasonography of the musculoskeletal system. Ultrasonography 2015, 34, 195–205. [Google Scholar] [CrossRef]
- Cohen, M. US imaging in operated tendons. J. Ultrasound 2012, 15, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.S.; Huang, Y.P.; Wang, T.G.; Wu, C.S.; Jiang, C.C. Sonographic Appearance of Surgically Repaired Achilles Tendons. Taiwan J. Phys. Med. Rehabil. 2008, 36, 23–30. [Google Scholar]
- Miquel, A.; Molina, V.; Phan, C.; Lesavre, A.; Menu, Y. Ultrasound of the Achilles tendon after percutaneous repair. J. Radiol. 2009, 90, 305–309. [Google Scholar] [CrossRef]
- Hirschmuller, A.; Frey, V.; Deibert, P.; Konstantinidis, L.; Mayer, F.; Sudkamp, N.; Helwig, P. Achilles tendon power Doppler sonography in 953 long distance runners—A cross sectional study. Ultraschall Med. 2010, 31, 387–393. [Google Scholar] [PubMed]
| Parameter | Asymptomatic: n = 17 Participants n = 18 Tendons | Symptomatic: n = 9 Participants n = 10 Tendons | Significance |
|---|---|---|---|
| Insertional AT (tendons) | 5 | 6 | |
| Insertional rupture (tendons) | 1 | 0 | |
| Mid-portion AT (tendons) | 4 | 2 | |
| Mid-portion rupture (tendons) | 8 | 2 | |
| Male (%) | 83.3 | 44.4 | |
| Age (years) | 55 (29–79; SD 14.5) | 53 (44–61; SD 5.9) | t(24) = 0.258, p = 0.799 |
| Sport (hours/week) | 3.0 (2–4; SD 0.9) n = 6 | 0 | |
| Body mass index (kg/m2) | 26.3 (23–30; SD 2.3) | 29.4 (23–40; SD 7.4) | t(24) = −1.629, p = 0.116 |
| Postoperative time (months) | 25 (6–60; SD 13) | 21 (3–60; SD 17) | t(24) = 0.607, p = 0.550 |
| Months until symptom relief | 7.6 (3–14; SD 4) | ||
| VISA-A score | 98 (90–100; SD 3) | 48 (22–85; SD 25) | t(24) = 8.267, p < 0.001 |
| AOFAS score | 97 (90–100; SD 5) | 76 (69–85; SD 5) | t(24) = 10.642, p < 0.001 |
| Roles and Maudesly score (n)—excellent/good/acceptable/poor | 7/10/0/0 | 0/0/3/6 | |
| Likert scale—completely recovered/much improved/somewhat improved/hardly improved/not improved/worse | 11/6/0/0/ 0/0 | 0/2/4/3/ 0/0 |
| Modality | Parameter | Asymptomatic Postoperative | Symptomatic Postoperative | Significance |
|---|---|---|---|---|
| B-US | thickness longitudinal (cm) thickness transverse (cm) cross-sectional area (cm2) hypoechogenicity fascicle irregularity peritendinous fluid calcification bursitis | 1.1; SD 0.25 | 1.0; SD 0.38 | t(26) = 0.215, p = 0.831 |
| 1.1; SD 0.21 | 1.1; SD 0.42 | t(26) = −0.232, p = 0.818 | ||
| 1.8; SD 0.84 n = 16 n = 18 n = 0 n = 2 n = 3 | 2.0; SD 0.9 n = 10 n = 10 n = 2 n = 4 n = 2 | t(26) = −0.46, p = 0.646 U = 80, p = 0.654 U = 90, p = 1.000 U = 72, p = 0.408 U = 64, p = 0.226 U = 87, p = 0.906 | ||
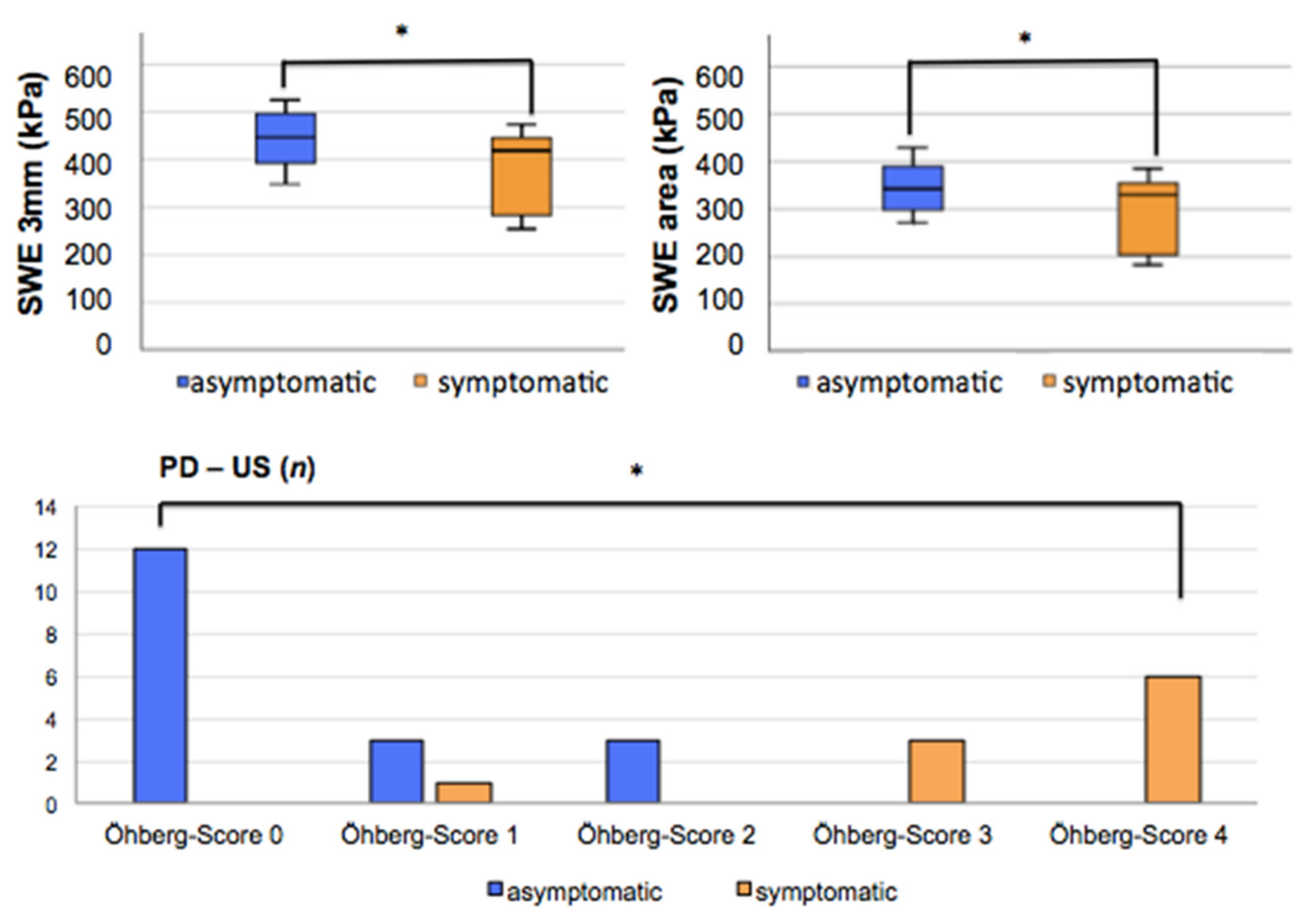
| PD-US | Öhberg score 0 Öhberg score 1 Öhberg score 2 Öhberg score 3 Öhberg score 4 | n = 12 n = 3 n = 3 n = 0 n = 0 | n = 0 n = 1 n = 0 n = 3 n = 6 | t(26) = −8.624, p < 0.001 * |
| SWE | SWE 3 mm (kPa) (m/s) SWE area (kPa) (m/s) | 443.7 (357–52; SD 55) 12.1; SD 0.8 | 384.6 (253–478; SD 83) 11.3; SD 1.2 | t(26) = 2.277, p = 0.031 * |
| 347.4 (281–428; SD 49) 10.8; SD 0.8 | 298.6 (194–383; SD 75) 9.97; SD 1.2 | t(26 = 2.098, p = 0.046 * | ||
| UTC | echo type 1 echo type 2 echo type 3 echo type 4 | 27.4 (14–47; SD 10) | 29.4 (11–59; SD 18) | t(24) = −0.383, p = 0.705 |
| 19.9 (13–26; SD 4) | 19.9 (10-32; SD 8) | t(24) = 0.008, p = 0.993 | ||
| 32.3 (17–48; SD 8) | 33.4 (9–53; SD 16) | t(24) = −0.222, p = 0.826 | ||
| 20.4 (12–27; SD 5) | 17.3 (4–28; SD 9) | t(24) = 1.142, p = 0.265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, P.; Betsch, M.; Fuss, B.; Dirrichs, T.; Tingart, M.; Quack, V.; Gatz, M. Evaluation of Current Symptoms in Postoperative Achilles Tendons: A Multimodal Ultrasound Study. Healthcare 2021, 9, 288. https://doi.org/10.3390/healthcare9030288
Nunes P, Betsch M, Fuss B, Dirrichs T, Tingart M, Quack V, Gatz M. Evaluation of Current Symptoms in Postoperative Achilles Tendons: A Multimodal Ultrasound Study. Healthcare. 2021; 9(3):288. https://doi.org/10.3390/healthcare9030288
Chicago/Turabian StyleNunes, Priscila, Marcel Betsch, Bernhard Fuss, Timm Dirrichs, Markus Tingart, Valentin Quack, and Matthias Gatz. 2021. "Evaluation of Current Symptoms in Postoperative Achilles Tendons: A Multimodal Ultrasound Study" Healthcare 9, no. 3: 288. https://doi.org/10.3390/healthcare9030288
APA StyleNunes, P., Betsch, M., Fuss, B., Dirrichs, T., Tingart, M., Quack, V., & Gatz, M. (2021). Evaluation of Current Symptoms in Postoperative Achilles Tendons: A Multimodal Ultrasound Study. Healthcare, 9(3), 288. https://doi.org/10.3390/healthcare9030288





