Primary Extra-Nodal DLBCL of Glands: Our Experiences outside Guidelines of Treatment
Abstract
1. Introduction
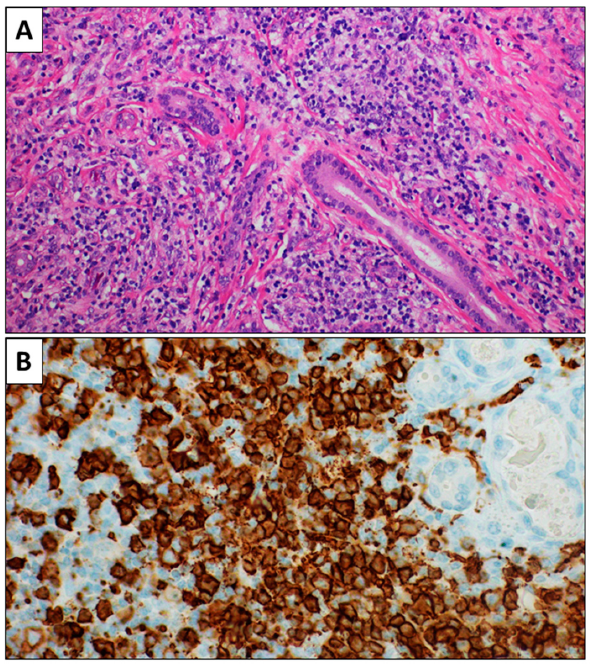
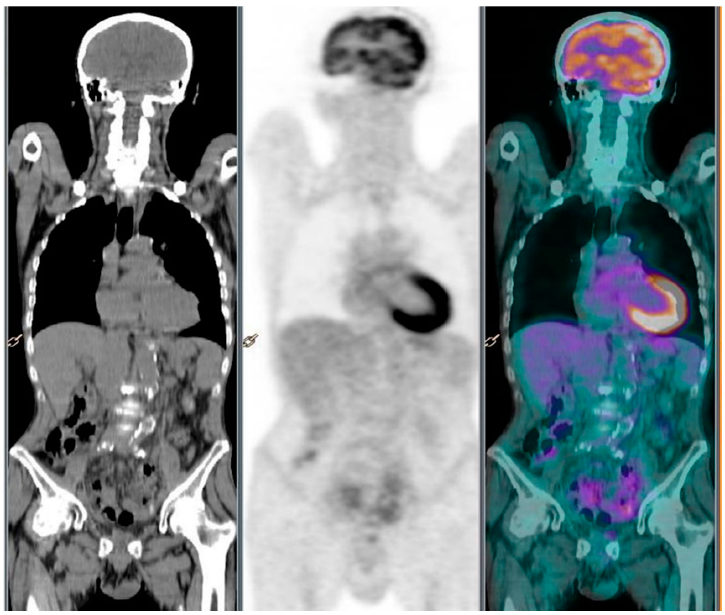
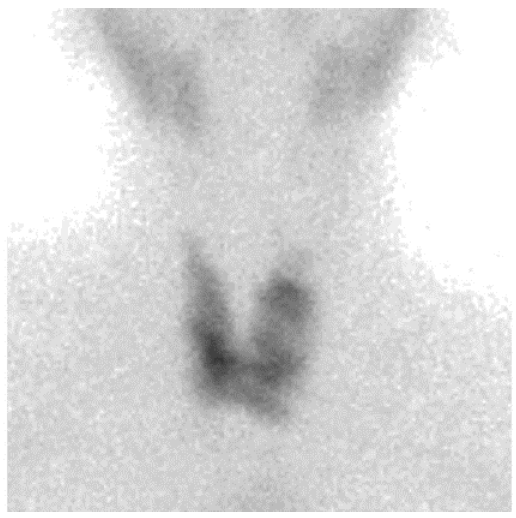
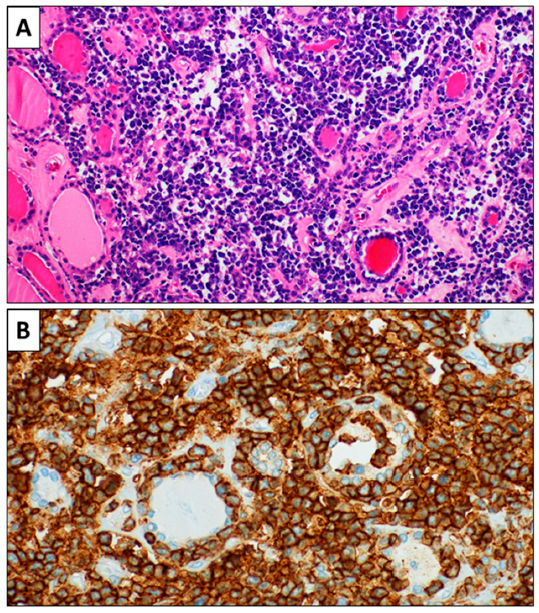
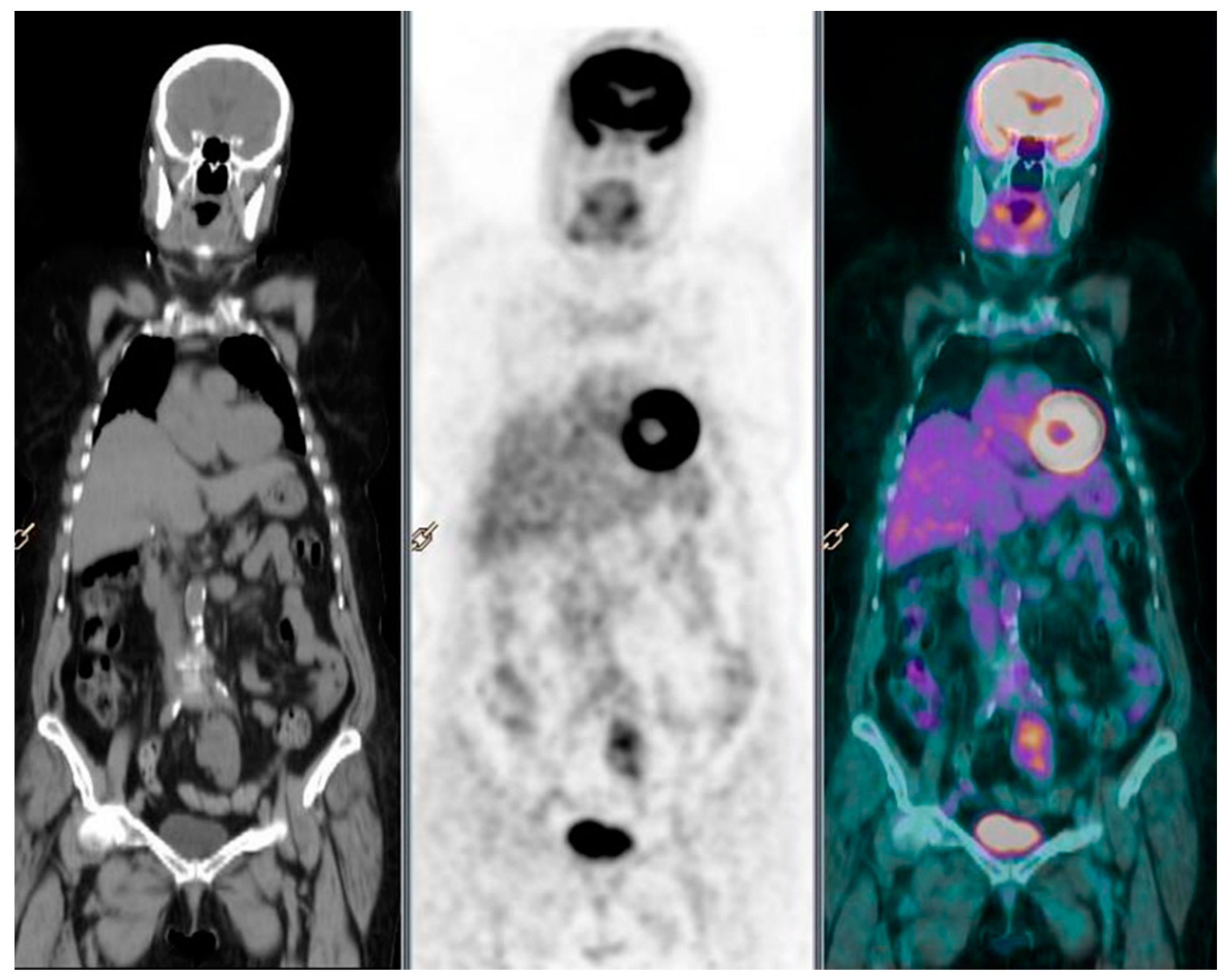
2. Case Report 1
3. Case Report 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Roggero, E.; Bertoni, F.; Cavalli, F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann. Oncol. 1997, 8, 727–737. [Google Scholar] [CrossRef]
- Fiorelli, A.; D’Andrilli, A.; Carlucci, A.; Vicidomini, G.; Loizzi, D.; Ardò, N.P.; Marasco, R.D.; Ventura, L.; Ampollini, L.; Carbognani, P.; et al. Prognostic factors of lung cancer in lymphoma survivors (the LuCiLyS study). Transl. Lung Cancer Res. 2020, 9, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.; Sica, A.; Ronchi, A.; Caccavale, S.; Franco, R.; Argenziano, G. Primary Cutaneous B-Cell Lymphomas: An Update. Front. Oncol. 2020, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Di Rocco, A.; Evangelista, A.; Quaglia, F.M.; Tisi, M.C.; Morello, L.; Zilioli, V.R.; Rusconi, C.; Hohaus, S.; Sciarra, R.; et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: Results from the MANTLE-FIRST study. Leukemia 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H. World Health Organization, International Agency for Research on Cancer WHO classification of tumours of haematopoietic and lymphoid tissues. In World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Castillo, J.J.; Winer, E.S.; Olszewski, A.J. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: An analysis of the Surveillance, Epidemiology and End Results database. Am. J. Hematol. 2014, 89, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Urraro, F.; Sangiovanni, A.; Russo, G.M.; Russo, C.; Grassi, R.; Agostini, A.; Belfiore, M.P.; Cellina, M.; Floridi, C.; et al. Extranodal Lymphomas: A pictorial review for CT and MRI classification. Acta Biomed. 2020, 91, 34–42. [Google Scholar] [CrossRef] [PubMed]
- El-Galaly, T.C.; Villa, D.; Alzahrani, M.; Hansen, J.W.; Sehn, L.H.; Wilson, D.; Brown, P.D.N.; Loft, A.; Iyer, V.; Johnsen, H.E.; et al. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: A Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am. J. Hematol. 2015, 90, 1041–1046. [Google Scholar] [CrossRef]
- Hui, D.; Proctor, B.; Donaldson, J.; Shenkier, T.; Hoskins, P.; Klasa, R.; Savage, K.; Chhanabhai, M.; Gascoyne, R.D.; Connors, J.M.; et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk. Lymphoma 2010, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Sagnelli, C.; Papa, A.; Ciccozzi, M.; Sagnelli, E.; Calogero, A.; Martinelli, E.; Casale, B. An Anecdotal Case Report of Chronic Lymphatic Leukemia with del(11q) Treated with Ibrutinib: Artificial Nourishment and Physical Activity Program. Int. J. Environ. Res. Public Health 2020, 17, 1929. [Google Scholar] [CrossRef]
- Feugier, P.; van Hoof, A.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Fermé, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients with Diffuse Large B-Cell Lymphoma: A Study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef]
- Sica, A.; Vitiello, P.; Caccavale, S.; Sagnelli, C.; Calogero, A.; Dodaro, C.A.; Pastore, F.; Ciardiello, F.; Argenziano, G.; Reginelli, A.; et al. Primary cutaneous DLBCL non-GCB type: Challenges of a rare case. Open Med. 2020, 15, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Marino, F.Z.; Vitiello, P.; Caccavale, S.; Argenziano, G.; Crisci, S.; Franco, R.; Sica, A. A case of primary cutaneous B-cell lymphoma with immature features in an old man. Diffuse Large B-cell Lymhpoma with immature features or B-cell Lymhpoblastic Lymphoma? J. Cutan. Pathol. 2020. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Schubert, J.; Ziepert, M.; Schmits, R.; Mohren, M.; Lengfelder, E.; Reiser, M.; Nickenig, C.; Clemens, M.; Peter, N.; et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol. 2008, 9, 105–116. [Google Scholar] [CrossRef]
- Vitolo, U.; Seymour, J.F.; Martelli, M.; Illerhaus, G.; Illidge, T.; Zucca, E.; Campo, E.; Ladetto, M. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v91–v102. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Hutchings, M.; Ladetto, M.; Goede, V.; Mey, U.; Soubeyran, P.; Spina, M.; Stauder, R.; Trněný, M.; Wedding, U.; et al. ESMO Consensus Conference on malignant lymphoma: General perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann. Oncol. 2018, 29, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Zelenetz, A.D.; Gordon, L.I.; Abramson, J.S.; Advani, R.H.; Bartlett, N.L.; Caimi, P.F.; Chang, J.E.; Chavez, J.C.; Christian, B.; Fayad, L.E.; et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3. J. Natl. Compr. Cancer Netw. 2019, 17, 650–661. [Google Scholar] [CrossRef]
- Cunningham, D.; Hawkes, E.A.; Jack, A.; Qian, W.; Smith, P.; Mouncey, P.; Pocock, C.; Ardeshna, K.M.; Radford, J.A.; McMillan, A.; et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: A phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013, 381, 1817–1826. [Google Scholar] [CrossRef]
- Sica, A.; Vitiello, P.; Papa, A.; Calogero, A.; Sagnelli, C.; Casale, D.; Mottola, M.; Svanera, G.; Dodaro, C.A.; Martinelli, E.; et al. Use of rituximab in NHL malt type pregnant in I° trimester for two times. Open Med. 2019, 14, 757–760. [Google Scholar] [CrossRef]
- Lamy, T.; Damaj, G.; Soubeyran, P.; Gyan, E.; Cartron, G.; Bouabdallah, K.; Gressin, R.; Cornillon, J.; Banos, A.; Le Du, K.; et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018, 131, 174–181. [Google Scholar] [CrossRef]
- Cascone, R.; Sica, A.; Sagnelli, C.; Carlucci, A.; Calogero, A.; Santini, M.; Fiorelli, A. Endoscopic Treatment and Pulmonary Rehabilitation for Management of Lung Abscess in Elderly Lymphoma Patients. Int. J. Environ. Res. Public Health 2020, 17, 997. [Google Scholar] [CrossRef]
- Sica, A.; Casale, D.; Rossi, G.; Casale, B.; Ciccozzi, M.; Fasano, M.; Ciotti, M.; Sagnelli, E.; Papa, A.; Sagnelli, C. The impact of the SARS-CoV-2 infection, with special reference to the hematological setting. J. Med. Virol. 2021, 93, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, C.; Díaz-López, A.; Dlouhy, I.; Rovira, J.; Lopez-Guillermo, A.; Alonso, S.; Martín, A.; Sancho, J.M.; García, O.; Sánchez, J.M.; et al. Validation of the NCCN-IPI for diffuse large B-cell lymphoma (DLBCL): The addition of β2-microglobulin yields a more accurate GELTAMO-IPI. Br. J. Haematol. 2017, 176, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sehn, L.H.; Rademaker, A.W.; Gordon, L.I.; LaCasce, A.S.; Crosby-Thompson, A.; Vanderplas, A.; Zelenetz, A.D.; Abel, G.A.; Rodriguez, M.A.; et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014, 123, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C. Confirmation of the molecular classification of diffuse large B-cell lym-phoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Bagaglio, S.; Uberti-Foppa, C.; Sagnelli, C.; Lai, A.; Hasson, H.; Salpietro, S.; Messina, E.; Morsica, G.; Zaffina, C.; Sica, A.; et al. HIV-1 recombinant forms in immigrants regularly residing in Milan, northern Italy. Infection 2020, 48, 553–558. [Google Scholar] [CrossRef]
- Takahashi, H.; Tomita, N.; Yokoyama, M.; Tsunoda, S.; Yano, T.; Murayama, K.; Hashimoto, C.; Tamura, K.; Sato, K.; Ishigatsubo, Y. Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era. Cancer 2012, 118, 4166–4172. [Google Scholar] [CrossRef]
- Merli, M.; Frigeni, M.; Alric, L.; Visco, C.; Besson, C.; Mannelli, L.; Di Rocco, A.; Ferrari, A.; Farina, L.; Pirisi, M.; et al. Direct-Acting Antivirals in Hepatitis C Virus-Associated Diffuse Large B-cell Lymphomas. Oncologist 2019, 24, e720–e729. [Google Scholar] [CrossRef]
- Merli, M.; DeFrancesco, I.; Visco, C.; Besson, C.; Di Rocco, A.; Arcari, A.; Sica, A.; Cencini, E.; Tisi, M.C.; Frigeni, M.; et al. Direct-acting antivirals in relapsed or refractory hepatitis C virus-associated diffuse large B-cell lymphoma. Leuk. Lymphoma 2020, 61, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-S.; Chen, J.-H.; Huang, T.-C.; Wu, Y.-Y.; Chang, P.-Y.; Dai, M.-S.; Chen, Y.-C.; Ho, C.-L.; Ho, C.-L. Diffuse large B-cell lymphoma: Sites of extranodal involvement are a stronger prognostic indicator than number of extranodal sites in the rituximab era. Leuk. Lymphoma 2015, 56, 2047–2055. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nat. Cell Biol. 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The Use of Molecular Profiling to Predict Survival after Chemotherapy for Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Winer, E.S.; Castillo, J.J. Validation of clinical prognostic indices for diffuse large B-cell lymphoma in the National Cancer Data Base. Cancer Causes Control. 2015, 26, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Vitiello, P.; Ronchi, A.; Casale, B.; Calogero, A.; Sagnelli, E.; Nachtigal, G.C.; Troiani, T.; Franco, R.; Argenziano, G.; et al. Primary Cutaneous Anaplastic Large Cell Lymphoma (pcALCL) in the Elderly and the Importance of Sport Activity Training. Int. J. Environ. Res. Public Health 2020, 17, 839. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Johnson, N.A.; Slack, G.W.; Savage, K.J.; Connors, J.M.; Ben-Neriah, S.; Rogic, S.; Scott, D.W.; Tan, K.L.; Steidl, C.; Sehn, L.H.; et al. Concurrent Expression of MYC and BCL2 in Diffuse Large B-Cell Lymphoma Treated with Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J. Clin. Oncol. 2012, 30, 3452–3459. [Google Scholar] [CrossRef]
- Sica, A.; Casale, B.; Sagnelli, C.; di Dato, M.T.; Buonavolontà, P.; Salzano, A.M.; Sagnelli, E.; Famiglietti, V.; Saracco, E.; Tammaro, D.; et al. All-in-One Spinal Cord Stimulation in Lymphoproliferative Diseases. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Shao, H.; Sokol, L.; Sotomayor, E.; Letson, D.; Bui, M.M. Prognostic significance of soft tissue extension, International Prognostic Index, and multifocality in primary bone lymphoma: A single institutional experience. Br. J. Haematol. 2014, 166, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Zeynalova, S.; Nickelsen, M.; Kansara, R.; Villa, D.; Sehn, L.H.; Glass, B.; Scott, D.W.; Gascoyne, R.D.; Connors, J.M.; et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. J. Clin. Oncol. 2016, 34, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.; Sagnelli, C.; Carlomagno, N.; Tammaro, V.; Candida, M.; Vernillo, A.; Peluso, G.; Minieri, G.; Sica, A.; Ciccozzi, M.; et al. Familial polyposis coli: The management of desmoid tumor bleeding. Open Med. 2019, 14, 572–576. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Wu, L.; Visco, C.; Tai, Y.C.; Tzankov, A.; Liu, W.-M.; Montes-Moreno, S.; Dybkaer, K.; Chiu, A.; Orazi, A.; et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: Report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012, 120, 3986–3996. [Google Scholar] [CrossRef]
- Wang, X.J.; Medeiros, L.J.; E Bueso-Ramos, C.; Tang, G.; Wang, S.; Oki, Y.; Desai, P.; Khoury, J.D.; Miranda, R.N.; Tang, Z.; et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod. Pathol. 2017, 30, 194–203. [Google Scholar] [CrossRef]
- Petrich, A.M.; Gandhi, M.; Jovanovic, B.; Castillo, J.J.; Rajguru, S.; Yang, D.T.; Shah, K.A.; Whyman, J.D.; Lansigan, F.; Hernandez-Ilizaliturri, F.J.; et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood 2014, 124, 2354–2361. [Google Scholar] [CrossRef]
- Karube, K.; Enjuanes, A.; Dlouhy, I.; Jares, P.; Martin-Garcia, D.; Nadeu, F.; Ordóñez, G.R.; Rovira, J.; Clot, G.; Royo, C.; et al. Integrating genomic alterations in diffuse large B-cell lymphoma identifies new relevant pathways and potential therapeutic targets. Leukemia 2018, 32, 675–684. [Google Scholar] [CrossRef]
- Niitsu, N.; Okamoto, M.P.; Miura, I.; Hirano, M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 2009, 23, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Johnson, N.A.; Ben-Neriah, S.; Connors, J.M.; Sehn, L.H.; Farinha, P.; Horsman, D.E.; Gascoyne, R.D. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009, 114, 3533–3537. [Google Scholar] [CrossRef]
- Tomita, N.; Tokunaka, M.; Nakamura, N.; Takeuchi, K.; Koike, J.; Motomura, S.; Miyamoto, K.; Kikuchi, A.; Hyo, R.; Yakushijin, Y.; et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica 2009, 94, 935–943. [Google Scholar] [CrossRef]
- Hu, S.; Xu-Monette, Z.Y.; Tzankov, A.; Green, T.; Wu, L.; Balasubramanyam, A.; Liu, W.-M.; Visco, C.; Li, Y.; Miranda, R.N.; et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: A report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013, 121, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Slack, G.W.; Mottok, A.; Sehn, L.H.; Villa, D.; Kansara, R.; Kridel, R.; Steidl, C.; Ennishi, D.; Tan, K.L.; et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016, 127, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.; Ziepert, M.; Becher, C.; Barth, T.F.E.; Bernd, H.-W.; Feller, A.C.; Klapper, W.; Hummel, M.; Stein, H.; Hansmann, M.-L.; et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013, 121, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Staiger, A.M.; Ziepert, M.; Horn, H.; Scott, D.W.; Barth, T.F.; Bernd, H.-W.; Feller, A.C.; Klapper, W.; Szczepanowski, M.; Hummel, M.; et al. Clinical Impact of the Cell-of-Origin Classification and the MYC/BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2017, 35, 2515–2526. [Google Scholar] [CrossRef]
- Green, T.M.; Young, K.H.; Visco, C.; Xu-Monette, Z.Y.; Orazi, A.; Go, R.S.; Nielsen, O.; Gadeberg, O.V.; Mourits-Andersen, T.; Frederiksen, M.; et al. Immunohistochemical Double-Hit Score Is a Strong Predictor of Outcome in Patients with Diffuse Large B-Cell Lymphoma Treated with Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J. Clin. Oncol. 2012, 30, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Boehme, V.; Schmitz, N.; Zeynalova, S.; Loeffler, M.; Pfreundschuh, M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 2009, 113, 3896–3902. [Google Scholar] [CrossRef]
- Møller, M.B.; Pedersen, N.T.; Christensen, B.E. Diffuse large B-cell lymphoma: Clinical implications of extranodal versus nodal presentation—A population-based study of 1575 cases. Br. J. Haematol. 2003, 124, 151–159. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.-L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; Villa, D.; Michaelsen, T.Y.; Hutchings, M.; Mikhaeel, N.G.; Savage, K.J.; Sehn, L.H.; Barrington, S.; Hansen, J.W.; Smith, D.; et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: An international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur. J. Cancer 2017, 75, 195–203. [Google Scholar] [CrossRef]
- Récher, C.; Coiffier, B.; Haioun, C.; Molina, T.J.; Fermé, C.; Casasnovas, O.; Thiéblemont, C.; Bosly, A.; Laurent, G.; Morschhauser, F.; et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. Lancet 2011, 378, 1858–1867. [Google Scholar] [CrossRef]
- Stein, S.A.; Wartofsky, L. Primary Thyroid Lymphoma: A Clinical Review. J. Clin. Endocrinol. Metab. 2013, 98, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Yeh, K.-H.; Chen, L.-T.; Lin, C.-W.; Hsu, P.-N.; Hsu, C.; Wu, M.-S.; Tzeng, Y.-S.; Tsai, H.-J.; Wang, H.-P.; et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: A distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014, 4, e220. [Google Scholar] [CrossRef]
- Lee, G.-W.; Go, S.-I.; Kim, S.-H.; Hong, J.; Kim, Y.R.; Oh, S.; Kim, S.-Y.; Do, Y.R.; Lee, H.; Lee, S.I.; et al. Clinical outcome and prognosis of patients with primary sinonasal tract diffuse large B-cell lymphoma treated with rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy: A study by the Consortium for Improving Survival of Lymphoma. Leuk. Lymphoma 2015, 56, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- de Leval, L.; Bonnet, C.; Copie-Bergman, C.; Seidel, L.; Baia, M.; Brière, J.; Molina, T.J.; Fabiani, B.; Petrella, T.; Bosq, J.; et al. Diffuse large B-cell lymphoma of Waldeyer’s ring has distinct clinicopathologic features: A GELA study. Ann. Oncol. 2012, 23, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sica, A.; Santagata, M.; Sagnelli, C.; Rambaldi, P.; Franco, R.; Creta, M.; Vitiello, P.; Caccavale, S.; Tammaro, V.; Sagnelli, E.; et al. Primary Extra-Nodal DLBCL of Glands: Our Experiences outside Guidelines of Treatment. Healthcare 2021, 9, 286. https://doi.org/10.3390/healthcare9030286
Sica A, Santagata M, Sagnelli C, Rambaldi P, Franco R, Creta M, Vitiello P, Caccavale S, Tammaro V, Sagnelli E, et al. Primary Extra-Nodal DLBCL of Glands: Our Experiences outside Guidelines of Treatment. Healthcare. 2021; 9(3):286. https://doi.org/10.3390/healthcare9030286
Chicago/Turabian StyleSica, Antonello, Mario Santagata, Caterina Sagnelli, Piero Rambaldi, Renato Franco, Massimiliano Creta, Paola Vitiello, Stefano Caccavale, Vincenzo Tammaro, Evangelista Sagnelli, and et al. 2021. "Primary Extra-Nodal DLBCL of Glands: Our Experiences outside Guidelines of Treatment" Healthcare 9, no. 3: 286. https://doi.org/10.3390/healthcare9030286
APA StyleSica, A., Santagata, M., Sagnelli, C., Rambaldi, P., Franco, R., Creta, M., Vitiello, P., Caccavale, S., Tammaro, V., Sagnelli, E., & Ronchi, A. (2021). Primary Extra-Nodal DLBCL of Glands: Our Experiences outside Guidelines of Treatment. Healthcare, 9(3), 286. https://doi.org/10.3390/healthcare9030286








