A Combined Approach for Health Assessment in Adolescent Endurance Runners
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Protocols
2.3. Graded Treadmill Run for the Determination of Aerobic Performance
2.4. ECG Measurements
2.5. Blood Analysis
2.6. Statistical Analyses
3. Results
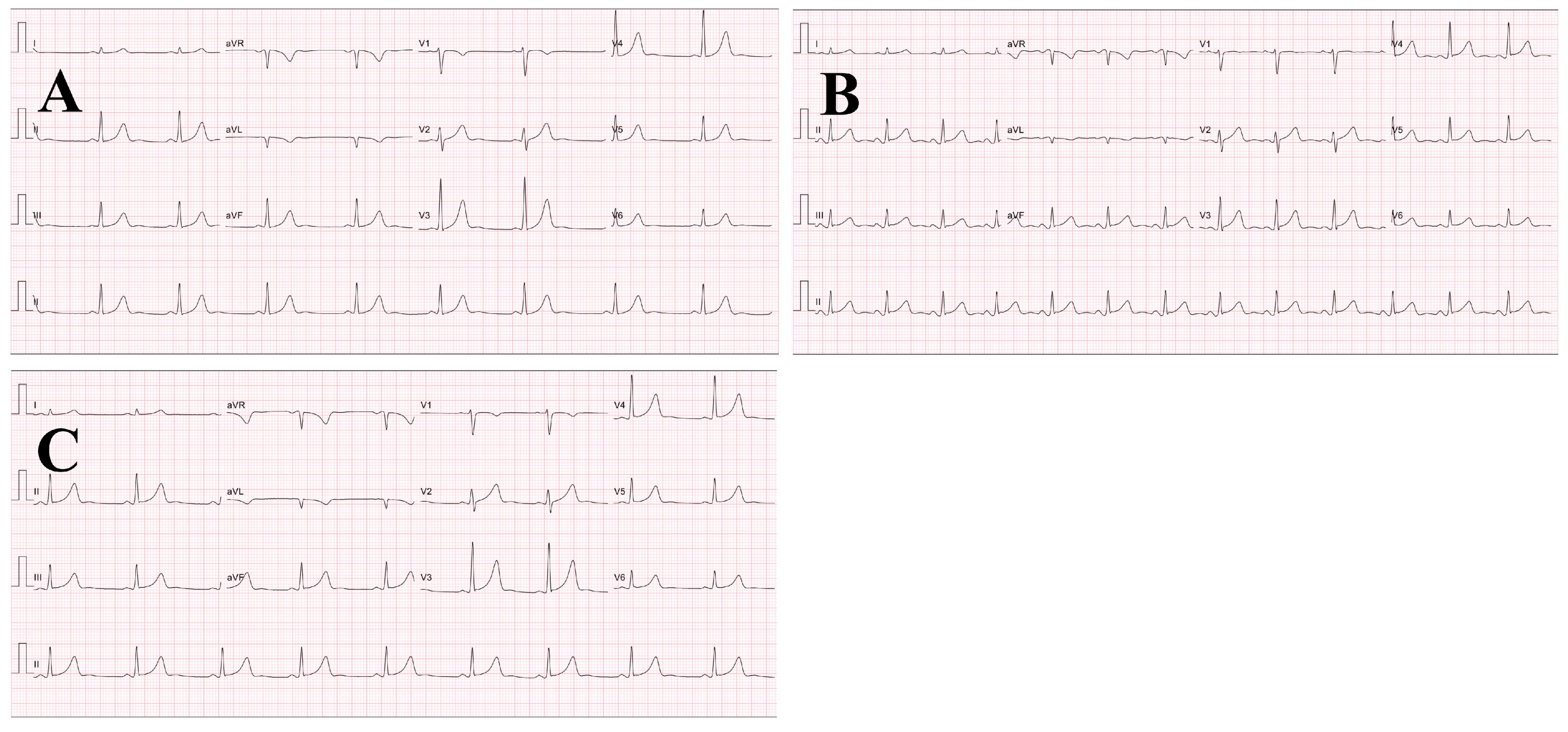
3.1. ECG Outcome
3.2. Blood Analysis
4. Discussion
4.1. Testosterone
4.2. Cortisol
4.3. Immunoglobulin Levels
4.4. Limitations and Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myer, G.D.; Jayanthi, N.; Difiori, J.P.; Faigenbaum, A.D.; Kiefer, A.W.; Logerstedt, D.; Micheli, L.J. Sport specialization, part I: Does early sports specialization increase negative outcomes and reduce the opportunity for success in young athletes? Sports Health 2015, 7, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Thomas, J.; Côté, J.; Deakin, J. Youth sport programs: An avenue to foster positive youth development. Phys. Educ. Sport Peda. 2005, 10, 19–40. [Google Scholar] [CrossRef]
- Firth, J.; Schuch, F.; Mittal, V.A. Using exercise to protect physical and mental health in youth at risk for psychosis. Res. Psychother. 2020, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Grubic, N.; Puskas, J.; Phelan, D.; Fournier, A.; Martin, L.J.; Johri, A.M. Shock to the heart: Psychosocial implications and applications of sudden cardiac death in the young. Curr. Cardiol. Rep. 2020, 22, 168. [Google Scholar] [CrossRef]
- Gustafsson, H.; Hassmén, P.; Kenttä, G.; Johansson, M. A qualitative analysis of burnout in elite Swedish athletes. Psychol. Sport Exerc. 2008, 9, 800–816. [Google Scholar] [CrossRef]
- Williams, C.; Nute, M.L. Some physiological demands of a half-marathon race on recreational runners. Br. J. Sports Med. 1983, 17, 152–161. [Google Scholar] [CrossRef]
- Tian, Y.; Nie, J.; Tong, T.K.; Baker, J.S.; Thomas, N.E.; Shi, Q. Serum oxidant and antioxidant status during early and late recovery periods following an all-out 21-km run in trained adolescent runners. Eur. J. Appl. Physiol. 2010, 110, 971–976. [Google Scholar] [CrossRef]
- Tian, Y.; Tong, T.K.; Lippi, G.; Huang, C.; Shi, Q.; Nie, J. Renal function parameters during early and late recovery periods following an all-out 21-km run in trained adolescent runners. Clin. Chem. Lab. Med. 2011, 49, 993–997. [Google Scholar] [CrossRef]
- Nie, J.; George, K.P.; Tong, T.K.; Gaze, D.; Tian, Y.; Lin, H.; Shi, Q. The influence of a half-marathon race upon cardiac troponin T release in adolescent runners. Curr. Med. Chem. 2011, 18, 3452–3456. [Google Scholar] [CrossRef]
- Kong, Z.; Nie, J.; Lin, H.; George, K.; Zhao, G.; Zhang, H.; Tong, T.K.; Shi, Q. Sex differences in release of cardiac troponin T after endurance exercise. Biomarkers 2017, 22, 345–350. [Google Scholar]
- MacKinnon, L.T. Special feature for the Olympics: Effects of exercise on the immune system: Overtraining effects on immunity and performance in athletes. Immunol. Cell Biol. 2000, 78, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Padgett, D.A.; Glaser, R. How stress influences the immune response. Trends Immunol. 2003, 24, 444–448. [Google Scholar] [CrossRef]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone: A vascular hormone in health and disease. J. Endocrinol. 2013, 217, R47–R71. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018; pp. 441–448. [Google Scholar]
- Tremblay, M.S.; Copeland, J.L.; van Helder, W. Influence of exercise duration on post-exercise steroid hormone responses in trained males. Eur. J. Appl. Physiol. 2005, 94, 505–513. [Google Scholar] [CrossRef]
- Hayes, L.D.; Grace, F.M.; Baker, J.S.; Sculthorpe, N. Exercise-induced responses in salivary testosterone, cortisol and their ratios in men: A meta-analysis. Sports Med. 2015, 45, 713–726. [Google Scholar] [CrossRef]
- O’Leary, C.B.; Lehman, C.; Koltun, K.; Smith-Ryan, A.; Hackney, A.C. Response of testosterone to prolonged aerobic exercise during different phases of the menstrual cycle. Eur. J. Appl. Physiol. 2013, 113, 2419–2424. [Google Scholar] [CrossRef]
- Manna, I.; Jana, K.; Samanta, P.K. Effect of intensive exercise-induced testicular gametogenic and steroidogenic disorders in mature male Wistar strain rats: A correlative approach to oxidative stress. Acta Physiol. Scand. 2003, 178, 33–40. [Google Scholar] [CrossRef]
- Raastad, T.; Bjøro, T.; Hallén, J. Hormonal responses to high- and moderate-intensity strength exercise. Eur. J. Appl. Physiol. 2000, 82, 121–128. [Google Scholar] [CrossRef]
- Hiruntrakul, A.; Nanagara, R.; Emasithi, A.; Borer, K.T. Effect of endurance exercise on resting testosterone levels in sedentary subjects. Cent. Eur. J. Public Health 2010, 18, 169–172. [Google Scholar] [CrossRef]
- Venckunas, T.; Krusnauskas, R.; Snieckus, A.; Eimantas, N.; Baranauskiene, N.; Skurvydas, A.; Brazaitis, M.; Kamandulis, S. Acute effects of very low-volume high-intensity interval training on muscular fatigue and serum testosterone level vary according to age and training status. Eur. J. Appl. Physiol. 2019, 119, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J.; Corrigan, D.L. Influence of short-term cycling on salivary cortisol levels. Med. Sci. Sports Exerc. 1987, 19, 224–228. [Google Scholar] [PubMed]
- Cook, N.J.; Ng, A.; Read, G.F.; Harris, B.; Riad-Fahmy, D. Salivary cortisol for monitoring adrenal activity during marathon runs. Horm. Res. 1987, 25, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and growth hormone responses to exercise at different times of day. J. Clin. Endocrinol. Metab. 2001, 86, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Debono, M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129–138. [Google Scholar] [CrossRef]
- Fragala, M.S.; Kraemer, W.J.; Mastro, A.M.; Denegar, C.R.; Volek, J.S.; Kupchak, B.R.; Häkkinen, K.; Anderson, J.M.; Maresh, C.M. Glucocorticoid receptor expression on human B cells in response to acute heavy resistance exercise. Neuroimmunomodulation 2011, 18, 156–164. [Google Scholar] [CrossRef]
- Hug, M.; Mullis, P.E.; Vogt, M.; Ventura, N.; Hoppeler, H. Training modalities: Over-reaching and over training in athletes, including a study of the role of hormones. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 191–209. [Google Scholar] [CrossRef]
- Ronsen, O.; Kjeldsen-Kraugh, J.; Haug, E.; Bahr, R.; Pedersen, B.K. Recovery time affects immunoendocrince responses to a second bout of endurance exercise. Am. J. Physiol Cell Physiol. 2002, 283, C1612–C1620. [Google Scholar] [CrossRef]
- Tibana, R.A.; Prestes, J.; De Sousa, N.M.F.; de Souza, V.C.; Nobrega, O.d.; Baffi, M.; Ferreira, C.E.S.; Cunha, G.V.; Navalta, J.w.; Trombeta, J.C.d.S.; et al. Time-course of changes in physiological, psychological, and performance markers following a functional-fitness competition. Int. J. Exerc. Sci. 2019, 12, 904–918. [Google Scholar]
- Fink, H.A.; Litwack-Harrison, S.; Taylor, B.C.; Bauer, D.C.; Orwoll, E.S.; Lee, C.G.; Barrett-Connor, E.; Schousboe, J.T.; Kado, D.M.; Garimella, P.S.; et al. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: The osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2016, 27, 331–338. [Google Scholar] [CrossRef]
- Bishop, N.C.; Gleeson, M.; Nicholas, C.W.; Ali, A. Influence of carbohydrate supplementation on plasma cytokine and neutrophil degranulation responses to high intensity intermittent exercise. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.A.; Von Duvillard, S.P. Influence of carbohydrate delivery on the immune response during exercise and recovery from exercise. Nutrition 2004, 20, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Steerenberg, P.A.; van Asperen, I.A.; van Nieuw Amerongen, A.; Biewenga, J.; Mol, D.; Medema, G.J. Salivary levels of immunoglobulin A in triathletes. Eur. J. Oral Sci. 1997, 105, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen-Cannarella, S.L.; Nieman, D.C.; Balk-Lamberton, A.J.; A Markoff, P.; Chritton, D.B.; Gusewitch, G.; Lee, J.W. The effects of moderate exercise training on immune response. Med. Sci. Sports Exerc. 1991, 23, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.J.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. There is limited existing evidence to support the common assumption that strenuous endurance exercise bouts impair immune competency. Expert Rev. Clin. Immunol. 2019, 15, 105–109. [Google Scholar] [CrossRef]
- Andrew, M.; Smith, L.; Wadee, A. Complement, immunoglobulin and creatine kinase response in black and white males after muscle-damaging exercise. SAJSM 2009, 21, 47–52. [Google Scholar]
- Hejazi, K.; Hosseini, S.R. Influence of selected exercise on serum immunoglobulin, testosterone and cortisol in semi-endurance elite runners. Asian J. Sports Med. 2012, 3, 185–192. [Google Scholar] [CrossRef]
- Karacabey, K.; Peker, I.; Saygın, Ö.; Cıloglu, F.; Ozmerdivenli, R.; Bulut, V. Effects of acute aerobic and aAnaerobic exercise on humoral immune factors in elite athletes. Biotechnol. Biotechnol. Equip. 2005, 19, 175–180. [Google Scholar] [CrossRef]
- Gunzer, W.; Konrad, M.; Pail, E. Exercise-induced immunodepression in endurance athletes and nutritional intervention with carbohydrate, protein and fat—What is possible, What is not? Nutrients 2012, 4, 1187–1212. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Can nutrition limit exercise-induced immunodepression? Nutr. Rev. 2006, 64, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Pizza, F.X.; Paquet, A.; Davis, B.J.; Forrest, M.B.; Braun, W.A. Influence of carbohydrate status on immune responses before and after endurance exercise. J. Appl. Physiol. 1998, 84, 1917–1925. [Google Scholar] [CrossRef]
- Bishop, N.C.; Walker, G.J.; Bowley, L.A.; Evans, K.F.; Molyneux, K.; Wallace, F.A.; Smith, A.C. Lymphocyte responses to influenza and tetanus toxoid in vitro following intensive exercise and carbohydrate ingestion on consecutive days. J. Appl. Physiol. 2005, 99, 1327–1335. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Khan, Q.; Drysdale, P.T.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol. 2005, 98, 565–571. [Google Scholar] [CrossRef]
| Variables | Mean ± SD | Range |
|---|---|---|
| Age (year) | 16.2 ± 0.6 | 15.1–17.1 |
| Body height (cm) | 173.8 ± 5.2 | 165.5–182.2 |
| Body weight (kg) | 60.3 ± 5.7 | 51.6–70.2 |
| Percentage of body fat (%) | 10.9 ± 2.6 | 5.9–16.9 |
| Fat free mass (kg) | 50.9 ± 5.5 | 40.5–58.7 |
| Training years (year) | 3.2 ± 1.8 | 1.3–8.3 |
| VO2max (mL kg−1·min−1) | 59.5 ± 5.3 | 50.6–68.9 |
| Personal best in half-marathon (min) | 73.9 ± 2.2 | 71.1–77.0 |
| Personal best in full-marathon (min) | 175.5 ± 8.0 | 163.7–187.3 |
| Variables | Pre-ex | 2-h | 4-h | 24-h |
|---|---|---|---|---|
| Testosterone (ng dL−1) | 410.5 ± 102.2 | 374.6 ± 131.9 | 331.3 ± 110.4 * | 474.6 ± 148.6 |
| Cortisol (μg dL−1) | 13.3 ± 2.8 | 9.9 ± 2.0 ** | 7.8 ± 3.2 *** | 8.9 ± 3.5 * |
| Albumin (g L−1) | 44.5 ± 1.2 | 45.2 ± 1.8 | 44.8 ± 1.1 | 44.3 ± 1.4 |
| IgA (g L−1) | 1.77 ± 0.66 | 1.68 ± 0.61 ** | 1.70 ± 0.63 * | 1.67 ± 0.63 * |
| IgG (g L−1) | 10.70 ± 2.61 | 9.91 ± 2.30 * | 9.80 ± 2.18 * | 9.60 ± 2.23 ** |
| Hematocrit (%) | 43.5 ± 3.1 | 42.9 ± 2.8 | 42.2 ± 2.3 | 42.4 ± 2.0 |
| Hemoglobin (g dL−1) | 14.2 ± 0.8 | 13.9 ± 0.9 | 14.0 ± 0.8 | 14.1 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.K.; Baker, J.S.; Henriquez, F.L.; Shi, Q.; Zhang, H.; Kong, Z.; Nie, J. A Combined Approach for Health Assessment in Adolescent Endurance Runners. Healthcare 2021, 9, 163. https://doi.org/10.3390/healthcare9020163
Tong TK, Baker JS, Henriquez FL, Shi Q, Zhang H, Kong Z, Nie J. A Combined Approach for Health Assessment in Adolescent Endurance Runners. Healthcare. 2021; 9(2):163. https://doi.org/10.3390/healthcare9020163
Chicago/Turabian StyleTong, Tomas K., Julien S. Baker, Fiona L. Henriquez, Qingde Shi, Haifeng Zhang, Zhaowei Kong, and Jinlei Nie. 2021. "A Combined Approach for Health Assessment in Adolescent Endurance Runners" Healthcare 9, no. 2: 163. https://doi.org/10.3390/healthcare9020163
APA StyleTong, T. K., Baker, J. S., Henriquez, F. L., Shi, Q., Zhang, H., Kong, Z., & Nie, J. (2021). A Combined Approach for Health Assessment in Adolescent Endurance Runners. Healthcare, 9(2), 163. https://doi.org/10.3390/healthcare9020163







