Laser Photobiomodulation (PBM)—A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Search Strategy
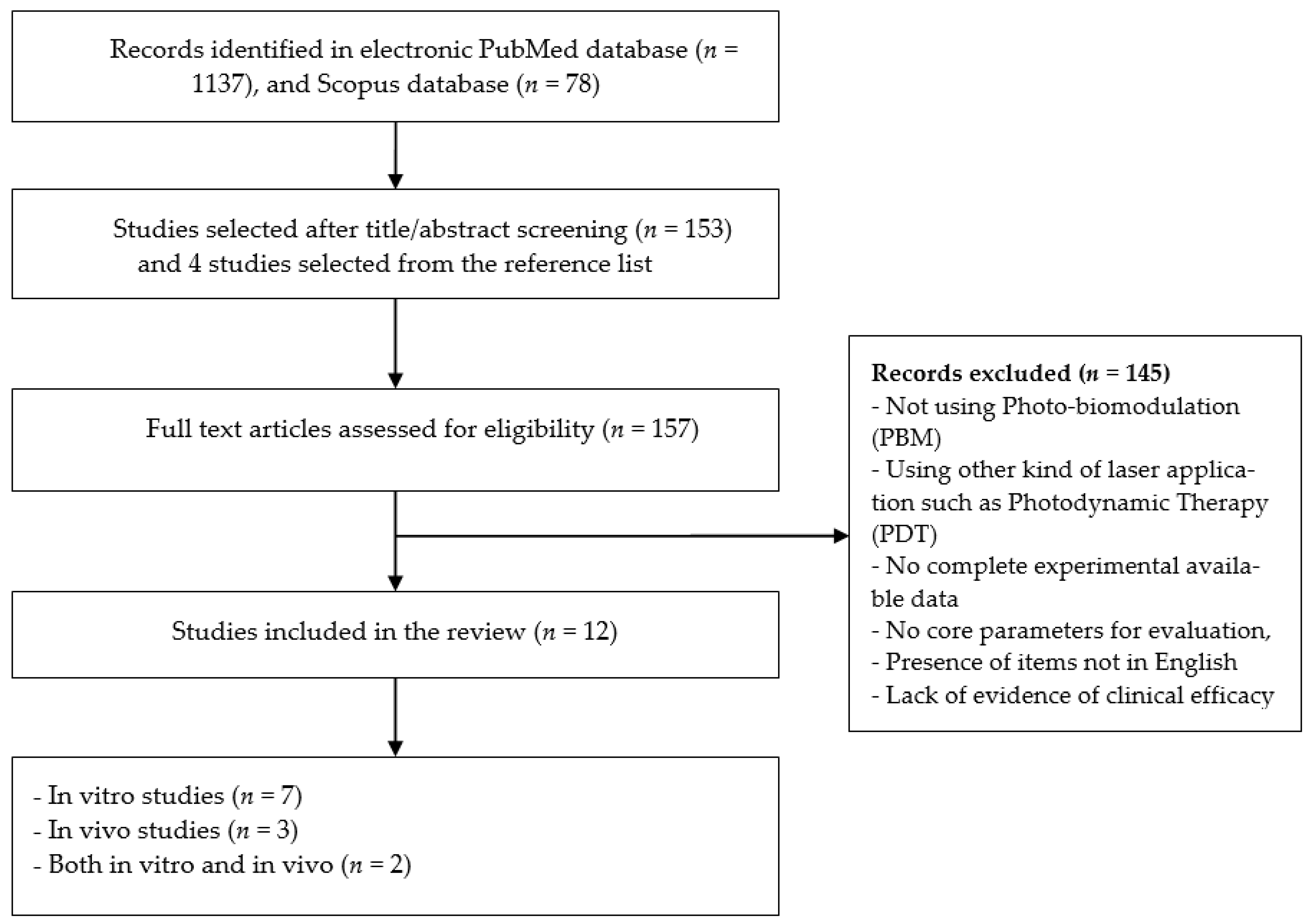
2.3. Study Selection
2.4. Data-Collection and Synthesis Process
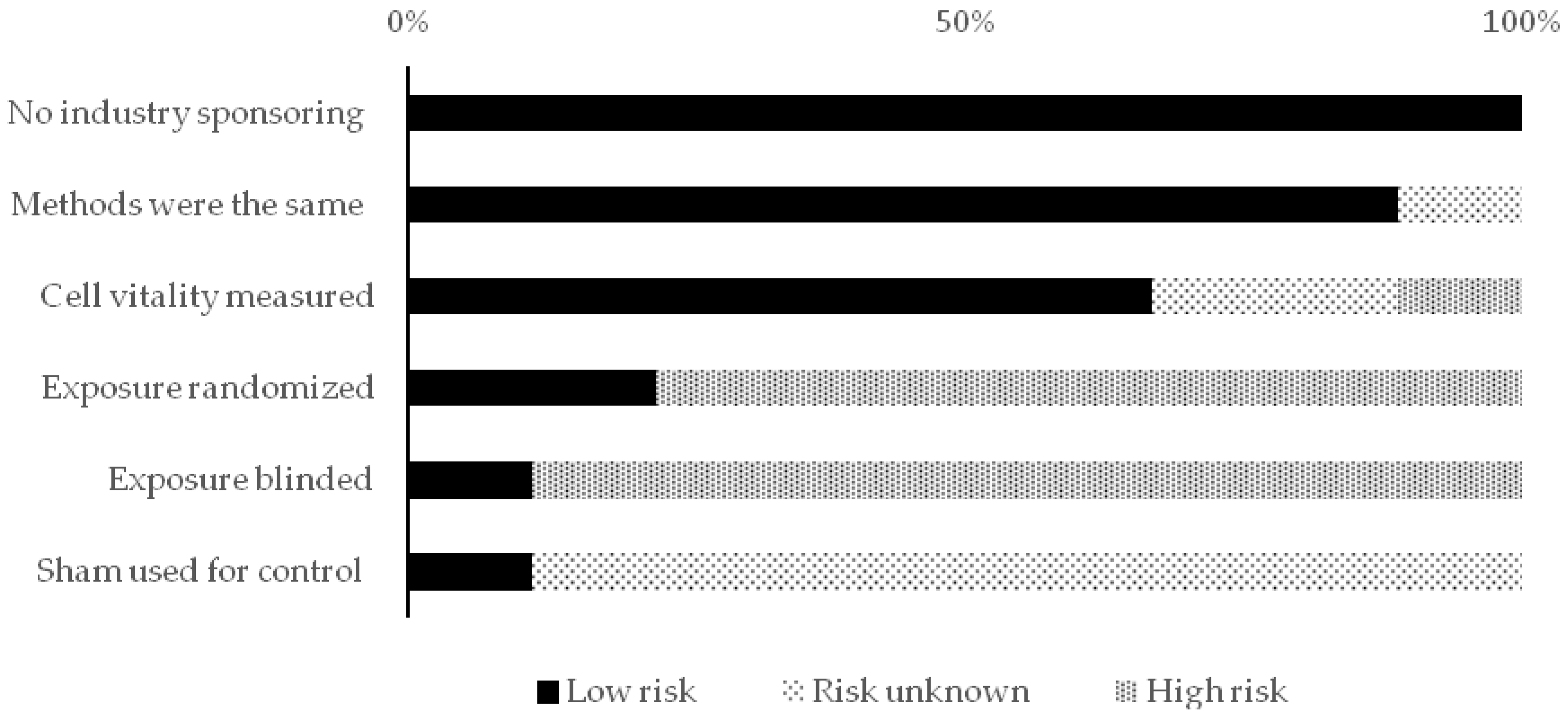
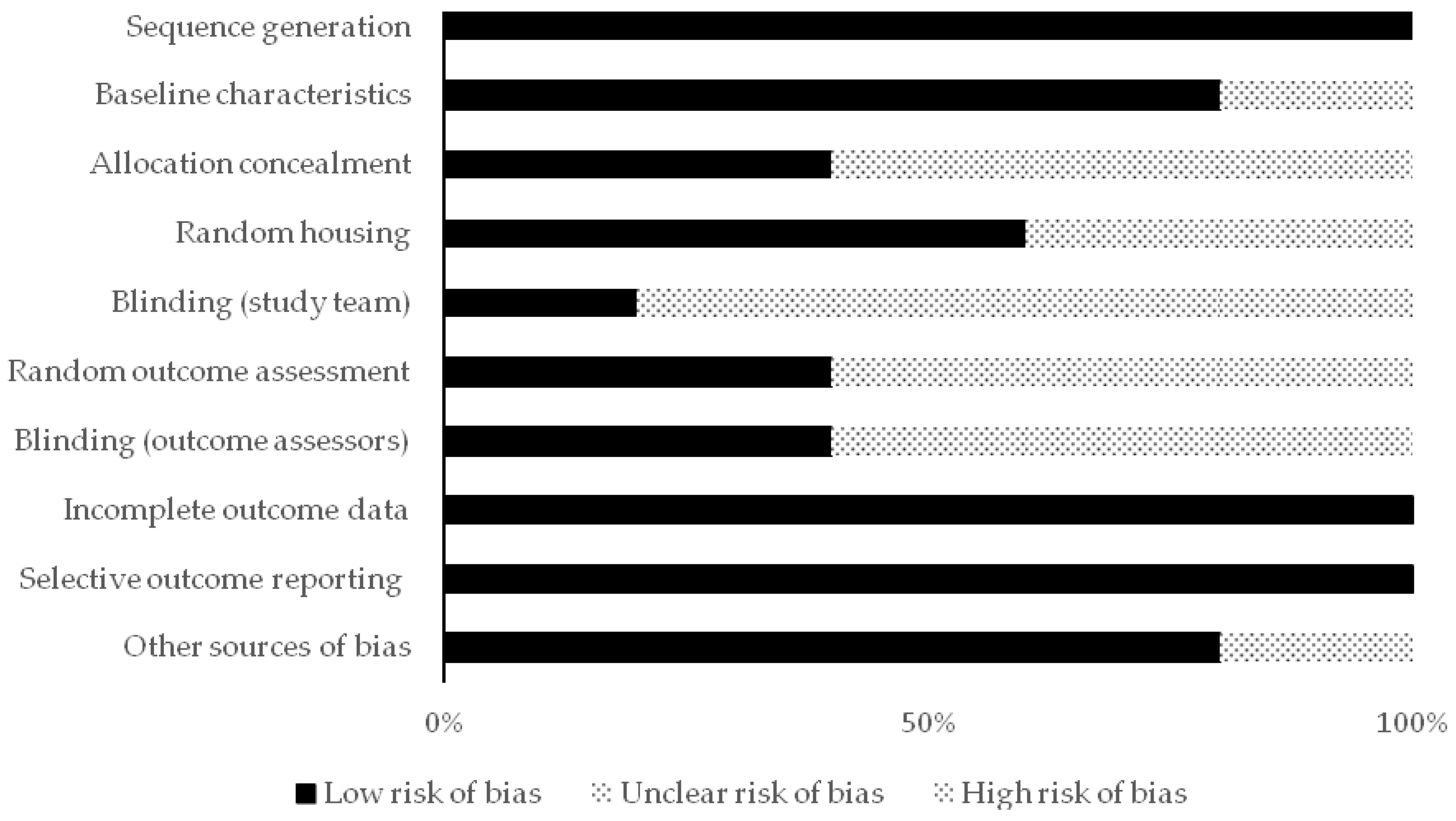
2.5. Reporting Quality and Risk of Bias Assessments
3. Results
3.1. Studies that Demonstrate Inhibitory Effect with PBM (Positive Outcome)
3.2. Studies that Demonstrate a Stimulatory Effect of PBM (Negative Outcome)
3.3. Laser Parameters of the Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Ob-servatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 6 December 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of inci-dence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Nelson, S.T.; Strahan, J.R. Photobiomodulation and Cancer: What Is the Truth? Photomed. Laser Surg. 2018, 36, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Mester, A.F. The biomedical effects of laser application. Lasers Surg. Med. 1985, 5, 31–39. [Google Scholar] [CrossRef]
- Karu, T.I.; Afanas’Eva, N.I. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light. Dokl. Akad. Nauk 1995, 342, 693–695. [Google Scholar]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy—An Update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: A Generalizable and Unifying Hypothesis. Crit. Rev. Toxicol. 2001, 31, 353–424. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Silvestri, M.; Tamburi, F.; Sicilia, C.; Del Duca, E.; Scali, E.; Bennardo, L.; Nisticò, S.P. A new 675-nm laser device in the treatment of acne scars: An observational study. Lasers Med. Sci. 2021, 36, 227–231. [Google Scholar] [CrossRef]
- Sroka, R.; Schaffer, M.; Fuchs, C.; Pongratz, T.; Schrader-Reichard, U.; Busch, M.; Schaffer, P.M.; Baumgartner, R. Effects on the mitosis of normal and tumor cells induced by light treatment of different wavelengths. Lasers Surg. Med. 1999, 25, 263–271. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Giudice, F.S.; Corrêa, L.; Pinto, D.S., Jr.; Hamblin, M.R.; De Sousa, S.C. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J. Biophotonics 2013, 6, 839–847. [Google Scholar] [CrossRef]
- Navrátil, L.; Kymplová, J. Contraindications in Noninvasive Laser Therapy: Truth and Fiction. J. Clin. Laser Med. Surg. 2002, 20, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; Genot, M.-T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Kiro, N.E.; Hamblin, M.R.; Abrahamse, H. Photobiomodulation of breast and cervical cancer stem cells using low-intensity la-ser irradiation. Tumor Biol. 2017, 39, 1010428317706913. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef]
- Ottaviani, G.; Martinelli, V.; Rupel, K.; Caronni, N.; Naseem, A.; Zandonà, L.; Perinetti, G.; Gobbo, M.; Di Lenarda, R.; Bussani, R.; et al. Laser Therapy Inhibits Tumor Growth in Mice by Promoting Immune Surveillance and Vessel Normalization. EBioMedicine 2016, 11, 165–172. [Google Scholar] [CrossRef]
- Mohsen, A.; Tenore, G.; Rocchetti, F.; Del Vecchio, A.; Ricci, R.; Barberi, W.; Cartoni, C.; Iori, A.P.; Pippi, R.; Polimeni, A.; et al. Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Un-dergoing Allogeneic Hematopoietic Stem Cell Transplantation. Appl. Sci. 2020, 10, 7479. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Golbach, L.A.; Portelli, L.A.; Savelkoul, H.F.J.; Terwel, S.R.; Kuster, N.; de Vries, R.B.M.; Verburg-van Kemenade, B.M.L. Calcium homeostasis and low-frequency magnetic and electric field expo-sure: A systematic review and meta-analysis of in vitro studies. Environ. Int. 2016, 92–93, 695–706. [Google Scholar]
- De Castro, J.L.; Pinheiro, A.L.; Werneck, C.E.; Soares, C.P. The effect of laser therapy on the proliferation of oral KB carcinoma cells: An in vitro study. Photomed. Laser Surg. 2005, 23, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Frigo, L.; Luppi, J.S.; Favero, G.M.; Maria, D.A.; Penna, S.C.; Bjordal, J.; Bensadoun, R.J.; Lopes-Martins, R.A.B. The effect of low-level laser irradiation (In-Ga-Al-AsP—660 nm) on melanoma in vitro and in vivo. BMC Cancer 2009, 9, 404. [Google Scholar] [CrossRef]
- De CMonteiro, J.S.; Pinheiro, A.N.; de Oliveira, S.C.; Aciole, G.T.S.; Sousa, J.A.C.; Cangussú, M.C.T.; dos Santos, J.N. Influence of laser phototherapy (lambda 660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: Histological study. Photomed. Laser Surg. 2011, 29, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Myakishev-Rempel, M.; Stadler, I.; Brondon, P.; Axe, D.R.; Friedman, M.; Nardia, F.B.; Lanzafame, R. A Preliminary Study of the Safety of Red Light Phototherapy of Tissues Harboring Cancer. Photomed. Laser Surg. 2012, 30, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Schartinger, V.H.; Galvan, O.; Riechelmann, H.; Dudás, J. Differential responses of fibroblasts, non-neoplastic epithelial cells, and oral carcinoma cells to low-level laser therapy. Support. Care Cancer 2011, 20, 523–529. [Google Scholar] [CrossRef]
- Gomes Henriques, Á.C.; Ginani, F.; Oliveira, R.M.; Barboza, C.A.G.; Rocha, H.A.O.; de Castro, J.F.L.; della Coletta, R.; de Almeida Freitas, R. Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med. Sci. 2014, 29, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.H.; Moon, J.H.; Choi, S.H.; Ahn, J.C. Low-level laser therapy promoted aggressive proliferation and angiogenesis through decreasing of transforming growth factor-beta1 and increasing of Akt/hypoxia inducible factor-1alpha in anaplastic thyroid cancer. Photomed. Laser Surg. 2016, 34, 229–235. [Google Scholar]
- Takemoto, M.M.; Garcez, A.S.; Sperandio, M. High energy density LED-based photobiomodulation inhibits squamous cell car-cinoma progression in co-cultures in vitro. J. Photochem. Photobiol. B 2019, 199, 111592. [Google Scholar] [CrossRef]
- Shirazian, S.; Keykha, E.; Pourshahidi, S.; Ebrahimi, H. Effects of 660 nm and 810 nm Low-Power Diode Laser on Proliferation and Invasion of Oral Cancer Cells in Cell Culture Media. Photochem. Photobiol. 2020. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Pavia, J.M.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Wu, S.; Xing, D.; Gao, X.; Chen, W.R. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J. Cell. Physiol. 2008, 218, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, S.; Xing, D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3β signaling pathway. J. Cell. Physiol. 2010, 226, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xing, D. Intracellular signaling cascades following light irradiation. Laser Photonics Rev. 2014, 8, 115–130. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, F.; Wei, Y.; Chen, W.R.; Chen, Q.; Xing, D. Cancer Phototherapy via Selective Photoinactivation of Respiratory Chain Oxidase to Trigger a Fatal Superoxide Anion Burst. Antioxid. Redox Signal. 2014, 20, 733–746. [Google Scholar] [CrossRef]
- Abrahamse, H.; Crous, A. Biochemical Responses of Isolated Lung CSCs after Application of Low Intensity Laser Irradiation. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 13–14 February 2016; Volume 9596. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy. Dose Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of low level light therapy—An introduction. Proc. SPIE 2006, 6140, 61001–61012. [Google Scholar]
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta BBA Bioenerg. 2008, 1777, 877–881. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumors: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Haddad, R.I.; Shin, D.M. Recent Advances in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef]
- Hang, C.Y.; Chia, C.Y. Photodynamic therapy with 5-aminolevulinic acid (ALA) impairs tumor initiating and chemo-resistance property in head and neck cancer-derived cancer stem cells. PLoS ONE 2014, 9, e87129. [Google Scholar]
- Malik, F.; Korkaya, H.; Clouthier, S.G.; Wicha, M.S. Regulation of breast cancer stem cells by mesenchymal stem cells in the metastatic niche. In Principles of Stem Cell Biology and Cancer: Future Applications and Therapeutics, 1st ed.; Regad, T., Sayers, T.J., Rees, R.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 123–143. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell pro-liferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012, 209, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Tumor metabolism: Cancer cells give and take lactate. J. Clin. Investig. 2008, 118, 3835–3837. [Google Scholar] [CrossRef]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar]
- Tsai, S.R.; Yin, R.; Huang, Y.Y.; Sheu, B.C.; Lee, S.C.; Hamblin, M.R. Low-level light therapy potentiates npe6-mediated photodynam-ic therapy in a human osteosarcoma cell line via increased ATP. Photodiagnosis Photodyn. Ther. 2015, 12, 123–130. [Google Scholar] [CrossRef]
- Reiners, J.J., Jr.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.-M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal pho-todamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, M.; Zhou, C.; Yin, F.; Wang, Z.; Hua, Y.; Cai, Z. Hematoporphyrin Monomethyl Ether-Mediated Photodynamic Therapy Selectively Kills Sarcomas by Inducing Apoptosis. PLoS ONE 2013, 8, e77727. [Google Scholar] [CrossRef] [PubMed]
- Djavid, G.E.; Bigdeli, B.; Goliaei, B.; Nikoofar, A.; Hamblin, M.R. Photobiomodulation leads to enhanced radiosensitivity through induction of apoptosis and autophagy in human cervical cancer cells. J. Biophotonics 2017, 10, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemis-try and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Jans, D.A.; Sobolev, A.S. Targeted intracellular delivery of photosensitizers to enhance photodynamic effi-ciency. Immunol. Cell Biol. 2000, 78, 452–464. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int. J. Cancer 2007, 120, 2527–2537. [Google Scholar] [CrossRef]
- Ying, R.; Liang, H.L.; Whelan, H.T.; Eells, J.T.; Wong-Riley, M.T.T. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Res. 2008, 1243, 167–173. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Xing, D. Inhibition of Abeta(25–35)-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012, 24, 224–232. [Google Scholar] [CrossRef]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; van den Bossche, J.; Mack, M.; Pipeleers, D.; Veld, P.I.; de Baetselier, P.; et al. Different tumor microenvironments contain functionally distinct macrophage subsets derived from Ly6C monocytes (high). Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.H.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Petrellis, M.C.; Frigo, L.; Marcos, R.L.; Pallotta, R.C.; de Carvalho, M.H.C.; Muscará, M.N.; Maria, D.A.; Lopes-Martins, R.Á.B. Laser photo-biomodulation of pro-inflammatory mediators on Walker Tumor 256 induced rats. J. Photochem. Photobiol. B 2017, 177, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Santana-Blank, L.A.; Castes, M.; Rojas, M.E.; Vargas, F.; Scott-Algara, D. Evaluation of serum levels of tumor necrosis factor alpha (TNF-alpha) and soluble receptor IL-2 (sIL-2R) and of CD4, CD8 and Natural Killer (NK) populations during treatment with infrared pulsed laser device (IPLD). Clin. Exp. Immunol. 1992, 90, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Santana-Blank, L.A.; Rodriguez-Santana, E.; Scott-Algara, D.; Hunger, M.; Santana-Rodriguez, K.E.; Orellana, R. Short term bioef-fects of an infrared pulsed laser device on burned rat skin monitored by transverse relaxation times (NMR). Lasers Surg. Med. 2000, 27, 411–419. [Google Scholar] [CrossRef]
- Santana-Blank, L.A.; Rodríguez-Santana, E.; Vargas, F.; Santana-Rodríguez, K.E. Photo-induced cytomorphologic changes in an advanced cancer phase I clinical trial. Lasers Surg. Med. 2002, 30, 19–26. [Google Scholar] [CrossRef]
- Antunes, H.S.; Herchenhorn, D.; Small, I.A.; Araújo, C.M.M.; Viégas, C.M.P.; Ramos, G.A.; Dias, F.L.; Ferreira, C.G. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol. 2017, 71, 11–15. [Google Scholar] [CrossRef]
- Santana-Blank, L.A.; Rodríguez-Santana, E.; Vargas, F.; Reyes, H.; Fernández-Andrade, P.; Rukos, S.; Santana-Rodríguez, K.E. Phase I Trial of an Infrared Pulsed Laser Device in Patients with Advanced Neoplasias. Clin Cancer Res. 2002, 8, 3082–3091. [Google Scholar]
- Balwill, F.; Osbourne, R.; Burke, F.; Naylor, S.; Talbot, D.; Durbin, H.; Tavernier, J.; Fiers, W. Evidence for tumor necrosis factor/cachectin production in cancer. Lancet 1987, 2, 1229–1232. [Google Scholar] [CrossRef]

| Assessment Type | Item |
|---|---|
| Reporting quality (both in vivo and in vitro studies) | - Is the cell origin and cell type used reported? * |
| - Are the type of emitter and wavelength reported? | |
| - Are the total energy and energy density reported? | |
| - Is the radiation duration of PBM reported? | |
| - Is the application technique adequately reported? | |
| Risk of bias scheme (only in vitro studies) | |
| - Performance bias | - Is a sham used for control treatment? |
| - Was the exposure blinded? | |
| - Was the exposure randomized? | |
| - Selection bias | - Is the cell vitality scored/measured? |
| - Detection bias | - Were the methods the same for control and exposure treatment? |
| - Other bias | - Was there no industry sponsoring involved? |
| Author, Year | Study Type | Sample | Laser | Method of Evaluation | Main Outcomes | Conclusions | PBM Effect |
|---|---|---|---|---|---|---|---|
| 1. Sroka et al., 1999 [11] | In vitro | Human SCC 1 of gingival mucosa (ZMK1) and other human cells | Different lasers; 410, 488, 630, 635, 640, 805, and 1064 nm | Mitosis rate by orcein-staining and cell proliferation by BrdU-test | A slight decrease in the mitotic rate of ZMK1 was observed with the increase in the irradiation energy independently with the wavelength. At irradiation of 20 J/cm2, a slight decrease in mitosis rate was observed when compared to controls without dependence on wavelength. | At specific parameters, an inhibitory effect of PBM was observed on human SCC when compared to controls. | Inhibitory |
| 2. de Castro et al., 2005 [22] | In vitro | KB cells | Diode lasers 685 and 830 nm | Cellular viability by MTT spectroscopy assay | The time significantly influenced the cellular viability in both control and test groups (for both wavelengths). The PBM in both test groups significantly influenced the cellular viability when compared to control. The test group irradiated with 830 nm showed a significant increase in proliferation when compared to the other test group (685 nm). | PBM had a significant bio-stimulatory effect on KB cells proliferation influenced by the wavelength. | Stimulatory |
| 3. Frigo et al., 2009 [23] | In vitro/In vivo | Melanoma cells (B16F10)/Melanoma cells in mouse model | InGaAlAsP 2 laser 660 nm | In vitro: cell viability and cell cycle changes by Tripan Blue, MTT, and cell quest histogram. In vivo: tumor volume and histological characteristics. | In vitro: The high irradiance (2.5 W/cm2) combined with high dose (1050 J/cm2) stimulated melanoma tumor growth. In vivo: A significant increase in the tumor volume, blood vessels and cell abnormalities was observed in the group of does 1050 J/cm2. | PBM over melanoma showed a stimulative effect and increase in tumor growth when applied in high irradiance and dose. | Stimulatory |
| 4. de C Monteiro et al., 2011 [24] | In vivo | Cancerous lesions on hamster’s cheek induced by chemical carcinogenesis | Diode laser 660 nm | Histological analysis | The test group (with PBM) showed a significant difference in the amount of poorly differentiated tumors when compared to other groups without PBM. | PBM with these parameters may cause a progression of the severity of oral SCC in hamsters. | Stimulatory |
| 5. Myakishev-Rempel et al., 2012 [25] | In vivo | SKH mouse nonmelanoma UV 3-induced skin cancer model | NASA LED 4 670 nm | Photographic measurements of tumor growth | PBM didn’t show a measurable effect on tumor growth. | PBM with these parameters may be safe in case of application in presence of malignant lesions. | Inhibitory |
| 6. Schartinger et al., 2012 [26] | In vitro | Human SCC cells (SCC25) and human normal cells | GaAlAs 5 660 nm | Cell proliferation assay by MTT, cell cycle analysis, and apoptosis assay | In SCC25 cells, PBM showed a significant decrease in cell proliferation and in the percentage of G1-phase cells, and a significant increase in the percentage of S-phase cells when compared to the control. PBM showed a proapoptotic effect in SCC25. | PBM with these parameters did not show a stimulative effect. | Inhibitory |
| 7. Sperandio et al., 2013 [12] | In vitro | Oral dysplastic cells (DOK) and oral cancer cells (SCC9 and SCC25) | GaAlAs laser 660 nm and 780 nm | Cellular viability by 3-h MTS assay, the apoptosis rate by TUNEL assay, and proteins analysis by Western blot and immunofluorescence | In SCC9, PBM showed inhibition of growth with 660 nm and stimulative effect with 780 nm. In SCC25, PBM showed a stimulative effect with both wavelengths. At 72 h evaluation time, PBM showed the lower levels of stimulation. PBM showed an effect on proteins and in particular caused an increased expression of p-Akt 6, pS6 and cyclin D1 proteins producing an aggressive isoform of Hsp90. Only SCC25 showed apoptosis when irradiated with 780 nm at 48 h (6.15 J/cm2) and at 72 h (3.07 J/cm2). | PBM with these parameters can aggravate oral cancer cellular behavior and modify the expression of proteins related to the progression and invasion of cancer cells. | Stimulatory |
| 8. Gomes Henriques et al., 2014 [27] | In vitro | Human SCC of tongue (SCC25) | InGaAlP 7 laser 660 | Cell growth assay, cell invasion analysis by Matrigel assay, and protein expression analysis | PBM on SCC25 with energy density of 1.0 J/cm2 showed a significant increase in proliferation, and expression of cyclin D1 and nuclear β-catenin, and a promotion of invasion through the reduction of E-cadherin and induction of MMP-9 8 expression. | PBM stimulated the proliferation and invasion of SCC25 and caused alterations on proteins expression. | Stimulatory |
| 9. Ottaviani et al., 2016 [18] | In vitro/In vivo | In vitro: Mouse melanoma cells (B16F10) and other human cells In vivo: Oral carcinogenesis model with 4-NQO 9 on mouse tongue | GaAs 10 and InGaAlAsP lasers 660, 800, and 970 nm | In vitro: ATP production assay In vivo: Histological evaluation and Immunofluorescence, real time PCR 11 and Flow Cytometry | In vitro: PBM showed an increase in cellular metabolism. In vivo: PBM reduced the tumor progression and this was associated with secretion of type I interferons from T lymphocytes and dendritic cells. A decrease in the angiogenic macrophages was observed in the tumor mass with a promotion of the vessel’s normalization. | PBM reduced tumor growth and was a safe procedure. | Inhibitory |
| 10. Rhee et al., 2016 [28] | In vivo | Anaplastic thyroid cancer cell line FRO in mouse model | Diode laser 650 nm | Tumor volume, histological evaluation, and IHC 12 staining analysis | PBM caused an elevation of HIF-1α 13 and p-Akt, and a decrease in TGF-β1 14 expression that play a role in the cell cycle regulation. | These effects may cause an over-proliferation and angiogenesis of cancer cells. PBM may cause aggressiveness of cancer through TGF-β1 and Akt/HIF-1α cascades. | Stimulatory |
| 11. Takemoto et al., 2019 [29] | In vitro | Human SCC cell line (CAL27) seeded over normal stromal gingival fibroblasts | LED 660 nm | Expansion of colonies and cell counts, viability and apoptosis after PBM with 36 J/cm2 | After 72 h of treatment, PBM inhibited the expansion of colonies. At high dose (36 J/cm2), PBM showed a general advantage with regard to the cell viability, apoptosis, and death assays on the stromal fibroblasts over cancer cells. | PBM (LED) at high doses inhibited in vitro the progression and number of cancer cells colonies without affecting the surrounding fibroblasts. | Inhibitory |
| 12. Shirazian et al., 2020 [30] | In vitro | SCC cells originated from tongue (TSCC-1) | Diode lasers 660 and 810 nm | Cell proliferation by MTT assay, and flow cytometry to assess cyclin D1, β-catenin, E-cadherin, and MMP-9 markers. RT-PCR 15 to assess Ki67 and VEGF 16 expression levels | At 24 h evaluation, the cell proliferation was generally lower in PBM groups. In 810 nm groups (100 and 200 mW), higher percentages of cyclin D1and MMP-9 were observed, and a significant decrease in VEGF marker in the 810 nm group of 200 mW. In 660 nm groups (40 and 80 mW), higher percentages of β-catenin and E-cadherin were observed. No differences were observed among groups for the Ki67 marker. | PBM with 660 nm (80 mW) and 810 nm (200 mW) showed a significant inhibitory effect on cell proliferation at 0 and 24 h. | Inhibitory |
| Author; Tear | Wavelength | Type of Emitter | Power (mW) | Energy per Point (J) | Irradiation Time (sec) | Spot Size (cm2) | Energy Density (J/cm2) | Total Energy (J) | PBM Technique | PBM Schedule |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sroka et al., 1999 [11] | 410, 488, 630, 635, 640, 805, and 1064 nm | Kr+, Ar+- pumped tunable dye, GaAlAs 1, and Nd:YAG 2 lasers | – | – | – | – | 0–20 | – | – | – |
| 2. de Castro et al., 2005 [22] | 685 nm 830 nm | Diode lasers | 31 34.5 | – | – | 0.8 | 4 | – | – | 2 sessions with 48 intervals |
| 3. Frigo et al., 2009 [23] | 660 nm | InGaAlAsP 3 | 50 | 3 21 | 60 420 | 0.02 | 150 1050 | 9 63 | CW 4 | 3 sessions with 24 h intervals |
| 4. de C Monteiro et al., 2011 [24] | 660 nm | Diode laser | 30 | 4 | 133 | 0.07 | 56.4 | – | CW | Every other day for 4 Weeks |
| 5. Myakishev-Rempel et al., 2012 [25] | 670 nm | NASA LED 5 | – | – | 312 | – | 2.5 | – | – | 2 sessions daily for 37 days |
| 6. Schartinger et al., 2012 [26] | 660 nm | GaAlAs | 350 | – | 900 | – | – | – | – | 3 sessions with 24 h intervals |
| 7. Sperandio et al., 2013 [12] | 660 nm 780 nm | GaAlAs | 40 | – | – | 0.039 | 2.05 3.07 6.15 (for each) | – | In contact | One session |
| 8. Gomes Henriques et al., 2014 [27] | 660 nm | InGaAlP 6 | 30 | 0.48 0.99 | 16 33 | 0.03 | 0.5 1.0 | – | CW | 2 sessions with 48 h intervals |
| 9. Ottaviani et al. 2016 [18] | 660 nm 800 nm 970 nm | GaAs 7 InGaAlAsP | 100 1 W 2.5 W | – | 60 30 30 | – | 3 6 6 | – | CW | In vivo: one session a day for 4 days |
| 10. Rhee et al., 2016 [28] | 650 nm | Diode laser | – | 0.3 0.6 | 150 300 | 0.02 | 15 30 | 0.3 0.6 | In contact and CW | One session |
| 11. Takemoto et al., 2019 [29] | 660 nm | LED | 100 | – | – | – | 3 6 9 12 24 36 | – | – | 3 times with 24 h intervals |
| 12. Shirazian et al., 2020 [30] | 660 nm 810 nm | Diode laser | 40, 80 100, 200 | – | 30, 15 12, 6 | 0.3 | 4 | – | Non-contact and CW | 4 session with 0, 24, 72, and 168 h intervals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Vecchio, A.; Tenore, G.; Luzi, M.C.; Palaia, G.; Mohsen, A.; Pergolini, D.; Romeo, U. Laser Photobiomodulation (PBM)—A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies. Healthcare 2021, 9, 134. https://doi.org/10.3390/healthcare9020134
Del Vecchio A, Tenore G, Luzi MC, Palaia G, Mohsen A, Pergolini D, Romeo U. Laser Photobiomodulation (PBM)—A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies. Healthcare. 2021; 9(2):134. https://doi.org/10.3390/healthcare9020134
Chicago/Turabian StyleDel Vecchio, Alessandro, Gianluca Tenore, Maria Clotilde Luzi, Gaspare Palaia, Ahmed Mohsen, Daniele Pergolini, and Umberto Romeo. 2021. "Laser Photobiomodulation (PBM)—A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies" Healthcare 9, no. 2: 134. https://doi.org/10.3390/healthcare9020134
APA StyleDel Vecchio, A., Tenore, G., Luzi, M. C., Palaia, G., Mohsen, A., Pergolini, D., & Romeo, U. (2021). Laser Photobiomodulation (PBM)—A Possible New Frontier for the Treatment of Oral Cancer: A Review of In Vitro and In Vivo Studies. Healthcare, 9(2), 134. https://doi.org/10.3390/healthcare9020134











