Relationships between Gait Regularity and Cognitive Function, including Cognitive Domains and Mild Cognitive Impairment, in Community-Dwelling Older People
Abstract
1. Introduction
2. Materials and Methods
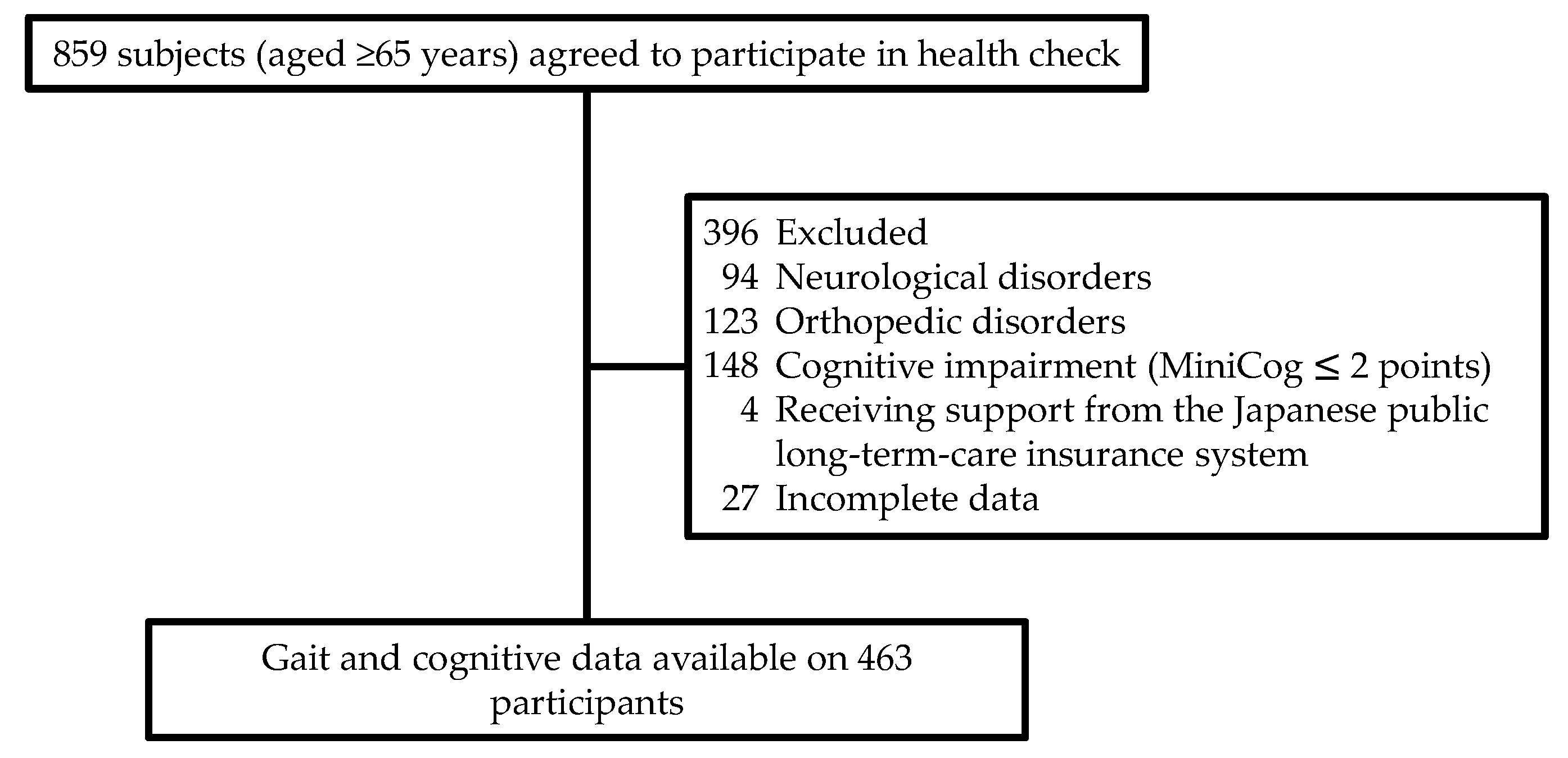
2.1. Participants
2.2. Gait Measurement
2.3. Cognitive Assessment
2.4. Mild Cognitive Impairment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants According to MCI Status
3.2. Association between Gait Regularity and Cognitive Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valkanova, V.; Ebmeier, K.P. What can gait tell us about dementia? Review of epidemiological and neuropsychological evidence. Gait Posture 2017, 53, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Combined effects of mild cognitive impairment and slow gait on risk of dementia. Exp. Gerontol. 2018, 110, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Tsutsumimoto, K.; Nakakubo, S.; Kim, M.J.; Kurita, S.; Shimada, H. Rethinking the relationship between spatiotemporal gait variables and dementia: A prospective study. J. Am. Med. Dir. Assoc. 2019, 20, 899–903. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kitamura, A.; Seino, S.; Murayama, H.; Amano, H.; Nofuji, Y.; Nishi, M.; Yokoyama, Y.; Shinozaki, T.; Yokota, I.; et al. Gait performance trajectories and incident disabling dementia among community-dwelling older Japanese. J. Am. Med. Dir. Assoc. 2017, 18, 192.e13–192.e20. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.F.; Olsson, E.; Wahlund, L.O. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2005, 19, 299–304. [Google Scholar] [CrossRef]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The impact of mild cognitive impairment on gait and balance: A systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2016, 63, 67–83. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Mild cognitive impairment, slow gait, and risk of disability: A prospective study. J. Am. Med. Dir. Assoc. 2015, 16, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Robbins, M.; Holtzer, R.; Zimmerman, M.; Wang, C.; Xue, X.; Lipton, R.B. Gait dysfunction in mild cognitive impairment syndromes. J. Am. Geriatr. Soc. 2008, 56, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: “walking speed: The sixth vital sign”. J. Geriatr. Phys. Ther. 2009, 32, 46–49. [Google Scholar] [CrossRef]
- Byun, S.; Han, J.W.; Kim, T.H.; Kim, K.; Kim, T.H.; Park, J.Y.; Suh, S.W.; Seo, J.Y.; So, Y.; Lee, K.H.; et al. Gait variability can predict the risk of cognitive decline in cognitively normal older people. Dement. Geriatr. Cogn. Disord. 2018, 45, 251–261. [Google Scholar] [CrossRef]
- Gillain, S.; Dramé, M.; Lekeu, F.; Wojtasik, V.; Ricour, C.; Croisier, J.L.; Salmon, E.; Petermans, J. Gait speed or gait variability, which one to use as a marker of risk to develop Alzheimer disease? A pilot study. Aging Clin. Exp. Res. 2016, 28, 249–255. [Google Scholar] [CrossRef]
- Kijima, Y.; Kiyama, R.; Sekine, M.; Tamura, T.; Fujimoto, T.; Maeda, T.; Ohshige, T. Estimation of gait independence using a tri-axial accelerometer in stroke patients. J. Aging Phys. Act. 2018, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Allali, G.; Thiery, S.; Gautier, J.; Fantino, B.; Annweiler, C. Association between high variability of gait speed and mild cognitive impairment: A cross-sectional pilot study. J. Am. Geriatr. Soc. 2011, 59, 1973–1974. [Google Scholar] [CrossRef]
- Kobsar, D.; Olson, C.; Paranjape, R.; Hadjistavropoulos, T.; Barden, J.M. Evaluation of age-related differences in the stride-to-stride fluctuations, regularity and symmetry of gait using a waist-mounted tri-axial accelerometer. Gait Posture 2014, 39, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Mignardot, J.B.; Deschamps, T.; Barrey, E.; Auvinet, B.; Berrut, G.; Cornu, C.; Constans, T.; De Decker, L. Gait disturbances as specific predictive markers of the first fall onset in elderly people: A two-year prospective observational study. Front. Aging Neurosci. 2014, 6, 22. [Google Scholar] [CrossRef]
- Kikkert, L.H.J.; Vuillerme, N.; Van Campen, J.P.; Appels, B.A.; Hortobágyi, T.; Lamoth, C.J.C. The relationship between gait dynamics and future cognitive decline: A prospective pilot study in geriatric patients. Int. Psychogeriatr. 2018, 30, 1301–1309. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J. Gait and Dementia. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 167, pp. 419–427. ISBN 978-012-804-766-8. [Google Scholar]
- Valkanova, V.; Esser, P.; Demnitz, N.; Sexton, C.E.; Zsoldos, E.; Mahmood, A.; Griffanti, L.; Kivimäki, M.; Singh-Manoux, A.; Dawes, H.; et al. Association between gait and cognition in an elderly population based sample. Gait Posture 2018, 65, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Y.; Tao, S.; Huang, S.; Zhang, C.; Lv, Z. Wearable sensor-based daily life walking assessment of gait for distinguishing individuals with amnestic mild cognitive impairment. Front. Aging Neurosci. 2019, 11, 285. [Google Scholar] [CrossRef]
- Choi, J.S.; Oh, H.S.; Kang, D.W.; Mun, K.R.; Choi, M.H.; Lee, S.J.; Yang, J.W.; Chung, S.C.; Mun, S.W.; Tack, G.R. Comparison of gait and cognitive function among the elderly with alzheimer’s disease, mild cognitive impairment and healthy. Int. J. Precis. Eng. Manuf. 2011, 12, 169–173. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Scanlan, J.; Brush, M.; Vitaliano, P.; Dokmak, A. The mini-cog: A cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int. J. Geriatr. Psychiatry 2000, 15, 1021–1027. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kawada, M.; Nakai, Y.; Kiyama, R.; Yone, K. Validity of measurement for trailing limb angle and propulsion force during gait using a magnetic inertial measurement unit. BioMed Res. Int. 2019, 2019, 8123467. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.P.; Cully, J.A.; Snow, A.L.; Massman, P.; Doody, R. The Alzheimer’s disease assessment scale—Cognitive subscale: Normative data for older adult controls. Alzheimer Dis. Assoc. Disord. 2004, 18, 236–240. [Google Scholar]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T. Comorbid mild cognitive impairment and depressive symptoms predict future dementia in community older adults: A 24-month follow-up longitudinal study. J. Alzheimer’s Dis. 2016, 54, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Assal, F.; Kressig, R.W.; Dubost, V.; Herrmann, F.R.; Beauchet, O. Impact of impaired executive function on gait stability. Dement. Geriatr. Cogn. Disord. 2008, 26, 364–369. [Google Scholar] [CrossRef]
- Kraan, C.M.; Tan, A.H.J.; Cornish, K.M. The developmental dynamics of gait maturation with a focus on spatiotemporal measures. Gait Posture 2017, 51, 208–217. [Google Scholar] [CrossRef]
- Doi, T.; Hirata, S.; Ono, R.; Tsutsumimoto, K.; Misu, S.; Ando, H. The harmonic ratio of trunk acceleration predicts falling among older people: Results of a 1-year prospective study. J. Neuroeng. Rehabil. 2013, 10, 7. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Effects of white matter lesions on trunk stability during dual-task walking among older adults with mild cognitive impairment. Age 2015, 37, 120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cosentino, E.; Palmer, K.; Della Pietà, C.; Mitolo, M.; Meneghello, F.; Levedianos, G.; Iaia, V.; Venneri, A. Association between gait, cognition, and gray matter volumes in mild cognitive impairment and healthy controls. Alzheimer Dis. Assoc. Disord. 2020, 34, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.; Arai, T.; Fujita, H. Brain activity in the prefrontal cortex during cognitive tasks and dual tasks in community-dwelling elderly people with pre-frailty: A pilot study for early detection of cognitive decline. Healthcare 2021, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Montembeault, M.; Barden, J.M.; Szturm, T.; Bherer, L.; Liu-Ambrose, T.; Chester, V.L.; Li, K.; Helbostad, J.L.; Allali, G. Brain gray matter volume associations with gait speed and related structural covariance networks in cognitively healthy individuals and in patients with mild cognitive impairment: A cross-sectional study. Exp. Gerontol. 2019, 122, 116–122. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall (n = 463) | No MCI (n = 342) | MCI (n = 121) | p-Value |
|---|---|---|---|---|
| Age (year) | 74.1 ± 5.8 | 73.5 ± 5.6 | 75.6 ± 6.0 | <0.001 |
| Female, n (%) | 294 (63.4) | 217 (63.4) | 77 (63.6) | 0.971 |
| Height (cm) | 155.0 ± 8.3 | 155.2 ± 8.2 | 154.4 ± 8.7 | 0.371 |
| Weight (kg) | 55.8 ± 9.8 | 56.1 ± 9.8 | 54.7 ± 9.7 | 0.170 |
| Body mass index | 23.1 ± 3.2 | 23.2 ± 3.1 | 22.9 ± 3.2 | 0.310 |
| Current medication | 2.73 ± 2.7 | 2.65 ± 2.6 | 2.97 ± 2.9 | 0.263 |
| Educational history (year) | 11.4 ± 2.2 | 11.5 ± 2.2 | 10.9 ± 2.2 | 0.009 |
| Gait speed (m/s) | 1.30 ± 0.2 | 1.32 ± 0.2 | 1.24 ± 0.2 | 0.001 |
| Cognitive functions | ||||
| Attention (s) | 22.9 ± 8.1 | 20.4 ± 4.7 | 29.9 ± 11.2 | <0.001 |
| Executive (s) | 47.7 ± 31.7 | 36.9 ± 11.9 | 78.2 ± 46.9 | <0.001 |
| Process speed (score) | 41.4 ± 10.6 | 44.2 ± 9.3 | 33.5 ± 10.3 | <0.001 |
| Memory (score) | 7.56 ± 1.4 | 7.86 ± 1.1 | 6.71 ± 1.6 | <0.001 |
| Component | Overall (n = 463) | no MCI (n = 342) | MCI (n = 121) | p-Value |
|---|---|---|---|---|
| Stride regularity | ||||
| Anteroposterior | 0.84 ± 0.09 | 0.84 ± 0.09 | 0.83 ± 0.09 | 0.111 |
| Mediolateral | 0.66 ± 0.13 | 0.67 ± 0.13 | 0.65 ± 0.14 | 0.127 |
| Vertical | 0.82 ± 0.09 | 0.83 ± 0.09 | 0.80 ± 0.11 | 0.017 |
| Step regularity | ||||
| Anteroposterior | 0.81 ± 0.09 | 0.82 ± 0.09 | 0.80 ± 0.09 | 0.007 |
| Mediolateral | −0.56 ± 0.14 | −0.58 ± 0.14 | −0.54 ± 0.15 | 0.058 |
| Vertical | 0.77 ± 0.11 | 0.78 ± 0.10 | 0.73 ± 0.12 | <0.001 |
| Component | Attention | Executive Function | Processing Speed | Memory |
|---|---|---|---|---|
| Stride regularity | ||||
| Anteroposterior | −0.060 | −0.036 | −0.007 | 0.023 |
| Mediolateral | −0.068 | −0.016 | 0.015 | 0.026 |
| Vertical | −0.139 * | −0.109 * | 0.006 | −0.003 |
| Step regularity | ||||
| Anteroposterior | −0.094 * | −0.066 | 0.073 | 0.056 |
| Mediolateral | 0.078 | 0.059 | 0.073 | 0.056 |
| Vertical | −0.165 ** | −0.176 ** | 0.152 ** | 0.084 |
| Component | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Stride regularity | ||
| Anteroposterior | 0.365 (0.015–8.827) | 0.535 |
| Mediolateral | 2.273 (0.012–435.6) | 0.759 |
| Vertical | 2.496 (0.033–189.5) | 0.679 |
| Step regularity | ||
| Anteroposterior | 0.684 (0.081–5.753) | 0.726 |
| Mediolateral | 0.728 (0.015–36.49) | 0.874 |
| Vertical | 0.019 (0.001–0.473) | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, T.; Kiyama, R.; Nakai, Y.; Kawada, M.; Takeshita, Y.; Araki, S.; Makizako, H. Relationships between Gait Regularity and Cognitive Function, including Cognitive Domains and Mild Cognitive Impairment, in Community-Dwelling Older People. Healthcare 2021, 9, 1571. https://doi.org/10.3390/healthcare9111571
Miyazaki T, Kiyama R, Nakai Y, Kawada M, Takeshita Y, Araki S, Makizako H. Relationships between Gait Regularity and Cognitive Function, including Cognitive Domains and Mild Cognitive Impairment, in Community-Dwelling Older People. Healthcare. 2021; 9(11):1571. https://doi.org/10.3390/healthcare9111571
Chicago/Turabian StyleMiyazaki, Takasuke, Ryoji Kiyama, Yuki Nakai, Masayuki Kawada, Yasufumi Takeshita, Sota Araki, and Hyuma Makizako. 2021. "Relationships between Gait Regularity and Cognitive Function, including Cognitive Domains and Mild Cognitive Impairment, in Community-Dwelling Older People" Healthcare 9, no. 11: 1571. https://doi.org/10.3390/healthcare9111571
APA StyleMiyazaki, T., Kiyama, R., Nakai, Y., Kawada, M., Takeshita, Y., Araki, S., & Makizako, H. (2021). Relationships between Gait Regularity and Cognitive Function, including Cognitive Domains and Mild Cognitive Impairment, in Community-Dwelling Older People. Healthcare, 9(11), 1571. https://doi.org/10.3390/healthcare9111571







