An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants
2.3. Instruments and Procedure
2.4. Self-Report Questionnaires
2.5. Somatosensory Sensitivity
2.6. Motor Function
2.7. Static and Dynamic Balance
2.8. Statistical Analyses
3. Results
3.1. Self-Report Questionnaires
3.2. Somatosensory Sensitivity
3.3. Motor Function
3.4. Static and Dynamic Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goubert, D.; Danneels, L.; Graven-Nielsen, T.; Descheemaeker, F.; Meeus, M. Differences in pain processing between patients with chronic low back pain, recurrent low back pain, and fibromyalgia. Pain Phys. 2017, 20, 307–318. [Google Scholar]
- Chaves, D. Actualización en fibromialgia. Med. Leg. Costa Rica 2013, 30, 83–88. [Google Scholar]
- Vora, A.J.; Doerr, K.D.; Wolfer, L.R. Functional anatomy and pathophysiology of axial low back pain: Disc, posterior elements, sacroiliac joint, and associated pain generators. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 679–709. [Google Scholar] [CrossRef]
- Berenshteyn, Y.; Gibson, K.; Hackett, G.C.; Trem, A.B.; Wilhelm, M. Is standing balance altered in individuals with chronic low back pain? A systematic review. Disabil. Rehabil. 2019, 41, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, D.C.; Driusso, P.; Avila, M.A.; Gramani-Say, K.; Moreira, F.M.A.; Parizotto, N.A. Static postural sway of women with and without fibromyalgia syndrome: A cross-sectional study. Clin. Biomech. 2017, 44, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Heredia, M.; Huertas, E.; Martínez, R.; Palacios, D.; Alegre, J.; Santamaría, M.; Fernández, C. Balance deficiencies in women with fibromyalgia assessed using computerised dynamic posturography: A cross-sectional study in Spain. BMJ Open 2017, 7, e016239. [Google Scholar] [CrossRef]
- Rasouli, O.; Vasseljen, O.; Fors, E.A.; Lorås, H.W.; Stensdotter, A.K. Lower regulatory frequency for postural control in patients with fibromyalgia and chronic fatigue syndrome. PLoS ONE 2018, 13, e0195111. [Google Scholar] [CrossRef]
- Jones, K.D.; King, L.A.; Mist, S.D.; Bennet, R.M.; Horak, F.B. Postural control deficits in people with fibromyalgia: A pilot study. Arthritis Res. Ther. 2011, 13, R127. [Google Scholar] [CrossRef]
- Leinonen, V.; Kankaanpaa, M.; Luukkonen, M.; Kansanen, M.; Hanninen, O.; Airaksinen, O.; Taimela, S. Lumbar paraspinal muscle function, perception of lumbar position and postural control in back pain related to herniated disc. Spine 2003, 28, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, O.; Stensdotter, A.K.; Van der Meer, A.L.H. TauG-guidance of dynamic balance control during gait initiation in patients with chronic fatigue syndrome and fibromyalgia. Clin. Biomech. 2016, 37, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Gallego, J.M.; Adsuar, J.C.; Domínguez, F.J.; Olivares, P.R.; Gusi, N. Fear of falling in women with fibromyalgia and its relation with number of falls and balance performance. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Da Silva, R.A.; Vieira, E.R.; Fernandes, K.B.P.; Andraus, R.A.; Oliveira, M.R.; Sturion, L.A.; Calderon, M.G. People with chronic low back pain have poorer balance than controls in challenging tasks. Disabil. Rehabil. 2018, 40, 1294–1300. [Google Scholar] [CrossRef]
- Hicks, G.; Sions, J.; Coyle, P.C.; Pohlig, R. Altered spatiotemporal characteristics of gait in older adults with chronic low back pain. Gait Posture 2017, 55, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Tsigkanos, C.; Gaskell, L.; Smirniotou, A.; Tsigkanos, G. Static and dynamic balance deficiencies in chronic low back pain. J. Back Musculoskelet. Rehabil. 2016, 29, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Auvinet, B.; Bileckot, R.; Alix, A.S.; Chaleil, D.; Barrey, E. Gait disorders in patients with fibromyalgia. Joint Bone Spine 2006, 73, 543–546. [Google Scholar] [CrossRef]
- Costa, I.D.; Gamundi, A.; Miranda, J.G.; França, L.G.; De Santana, C.N.; Montoya, P. Altered functional performance in patients with fibromyalgia. Front. Hum. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.M.; García, V.A.; Porres, J.M.; Delgado, M.; Soto, V.M. Spatial-temporal parameters of gait in women with fibromyalgia. Clin. Rheumatol. 2009, 28, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.J.; Carmona, L.; Valverde, M.; Ribas, B. EPISER study group. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a nationwide study in Spain. Clin. Exp. Rheumatol. 2008, 26, 519–526. [Google Scholar] [PubMed]
- Monterde, S.; Salvat, I.; Montull, S.; Fernández-Ballart, J. Validation of fibromyalgia impact questionnaire. Rev. Esp. Reumatol. 2004, 31, 507–513. [Google Scholar]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Kinser, A.M.; Sands, W.A.; Stone, M.H. Reliability and validity of a pressure algometer. J. Strength Cond. Res. 2009, 23, 312–314. [Google Scholar] [CrossRef]
- Deng, H.; He, F.; Zhang, S.; Calleman, C.J.; Costa, L.G. Quantitative measurements of vibration threshold in healthy adults and acrylamide workers. Int. Arch. Occup. Environ. Health 1993, 65, 53–56. [Google Scholar] [CrossRef]
- Frenette, B.; Mergler, D.; Ferraris, J. Measurement precision of a portable instrument to assess vibrotactile perception threshold. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 386–391. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1991, 83, S7–S11. [Google Scholar]
- Santo, A.S.E.; Mango, P.C.; Assumpçao, A.; Sauer, J.F.; Marques, A. Fibromyalgia: Is there association between balance and pain? A pilot study. Fisioter. Pesqui. 2014, 21, 27–33. [Google Scholar] [CrossRef]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006, 88, 21–24. [Google Scholar] [CrossRef]
- American Thoracic Society statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- King, S.; Wessel, J.; Bhambhani, Y.; Maikala, R.; Sholter, D.; Maksymowych, W. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J. Rheumatol. 1999, 26, 2233–2237. [Google Scholar]
- Peppin, J.F.; Marcum, S.; Kirsh, K.L. The chronic pain patient and functional assessment: Use of the 6-Minute Walk Test in a multidisciplinary pain clinic. Curr. Med. Res. Opin. 2014, 30, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Collado-Mateo, D.; Domínguez-Muñoz, F.J.; Adsuar, J.C.; Merellano-Navarro, E.; Olivares, P.R.; Gusi, N. Reliability of the Timed up and go test in fibromyalgia. Rehabil. Nurs. 2018, 43, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.C.; Velasco, T.; Sions, J.M.; Hicks, G.E. Lumbar mobility and performance-based function: An investigation in older adults with and without chronic low back pain. Pain Med. 2017, 18, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Imagama, S.; Matsuyama, Y.; Hasegawa, Y.; Sakai, Y.; Ito, Z.; Ishiguro, N.; Hamajima, N. Back muscle strength and spinal mobility are predictors of quality of life in middle-aged and elderly males. Eur. Spine J. 2011, 20, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S. Muscle strength testing: Use of normalisation for body size. Sports Med. 2002, 32, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceive dexertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Khasnis, A.; Gokula, R. Romberg’s test. J. Postgrad. Med. 2003, 49, 169–172. [Google Scholar] [PubMed]
- García-Pastor, C.; Álvarez, G.A. The Romberg test and Moritz Heinrich Romberg. Rev. Mex. Neurocienc. 2014, 15, 31–35. [Google Scholar]
- Gea, J.; Muñoz, M.A.; Costa, I.; Ciria, L.F.; Miranda, J.G.; Montoya, P. Viewing pain and happy faces elicited similar changes in postural body sway. PLoS ONE 2014, 9, e104381. [Google Scholar] [CrossRef] [PubMed]
- Peña, N.; Credidio, B.C.; Correa, L.P.; França, L.G.S.; Cunha, M.V.; Sousa, M.C. Free instrument for measurements of motion. Rev. Bras. Ensino Física 2013, 35, 1–5. [Google Scholar] [CrossRef]
- Claeys, K.; Brumagne, S.; Dankaerts, W.; Kiers, H.; Janssens, L. Decreased variability in postural control strategies in young people with non-specific low back pain is associated with altered proprioceptive reweighting. Eur. J. Appl. Physiol. 2011, 111, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Humbría, A.; Carmona, L.; Peña, J.L.; Ortiz, A.M. Impacto poblacional del dolor lumbar en España: Resultados del estudio EPISER. Rev. Esp. Reumatol. 2002, 29, 471–478. [Google Scholar]
- Nijs, J.; Van Houdenhove, B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: Application of pain neurophysiology in manual therapy practice. Man. Ther. 2009, 14, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Puta, C.; Schulz, B.; Schoeler, S.; Magerl, W.; Gabriel, B.; Gabriel, H.H.; Weiss, T. Somatosensory abnormalities for painful and innocuous stimuli at the back and at a site distinct from the region of pain in chronic back pain patients. PLoS ONE 2013, 8, e58885. [Google Scholar] [CrossRef] [PubMed]
- Sitges, C.; García-Herrera, M.; Pericás, M.; Collado, D.; Truyols, M.; Montoya, P. Abnormal brain processing of affective and sensory pain descriptors in chronic pain patients. J. Affect. Disord. 2007, 104, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Turk, D.C.; Flor, H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom. Med. 2004, 66, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Schneider, C. Revisiting the corticomotor plasticity in low back pain: Challenges and perspectives. Healthcare 2016, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Mendonca, M.; Fregni, F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med. Hypotheses 2014, 83, 332–333. [Google Scholar] [CrossRef]
- Pierrynowski, M.R.; Tiidus, P.M.; Galea, V. Women with fibromyalgia walk with an altered muscle synergy. Gait Posture 2005, 22, 210–218. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Wright, A. The effect of musculoskeletal pain on motor activity and control. J. Pain 2001, 2, 135–145. [Google Scholar] [CrossRef]
- Baliki, M.N.; Petre, B.; Torbey, S.; Hermann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef]
- Arendt, L.; Graven, T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Curr. Pain Headache Rep. 2003, 7, 355–361. [Google Scholar] [CrossRef]
- Friedrich, M.; Hahne, J.; Wepner, F.A. A controlled examination of medical and psychosocial factors associated with low back pain in combination with widespread musculoskeletal pain. Phys. Ther. 2009, 89, 786–803. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; Whalen, G.; Cunningham, J.; Claw, D.J. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet. Disord. 2004, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Cedraschi, C.; Luthy, C.; Girard, E.; Piguet, V.; Desmeules, J.; Allaz, A.F. Representations of symptom history in women with fibromyalgia vs chronic low back pain: A qualitative study. Pain Med. 2012, 13, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Tuzer, V.; Bulut, S.D.; Bastug, B.; Kalayar, G.; Göka, E.; Bestepe, E. Causal attributions and alexithymia in female patients with fibromyalgia or chronic low back pain. Nord. J. Psychiatry 2011, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Scherrer, J.F.; Salas, J.; Van den Berk-Clark, C.; Fernando, S.; Herndon, C.M. Differences in the association between depression and opioid misuse in chronic low back pain versus chronic pain at other locations. Healthcare 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.R.; Carlsson, S.G. Psychosocial vulnerability and maintaining forces related to fibromyalgia. In-depth interviews with twenty-two female patients. Scand. J. Caring Sci. 1998, 12, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Diatchenko, L.; Fillingim, R.B.; Smith, S.B.; Maixner, W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat. Rev. Rheumatol. 2013, 9, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, R.; Leeuw, R.; Zhu, H.; Nickerson, R.; Okeson, J.; Carlson, C. Prevalence of temporomandibular disorders in fibromyalgia and failed back syndrome patients: Blinded prospective comparison study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 2, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Griep, E.N.; Boersma, J.W.; Lentjes, E.G.; Prins, A.P.; van der Korst, J.K.; de Kloet, E.R. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. J. Rheumatol. 1998, 25, 1374–1381. [Google Scholar] [PubMed]
- Panerai, A.E.; Vecchiet, J.; Panzeri, P.; Meroni, P.; Scarone, S.; Pizzigallo, E.; Giamberardino, M.A.; Sacerdote, P. Peripheral blood mononuclear cell beta-endorphin concentration is decreased in chronic fatigue syndrome and fibromyalgia but not in depression: Preliminary report. Clin. J. Pain 2002, 18, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Sitges, C.; González-Roldán, A.M.; Duschek, S.; Montoya, P. Emotional influences on cognitive processing in fibromyalgia patients with different depression levels: An event-related potential study. Clin. J. Pain 2018, 34, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; Werner, N.S.; Limbert, N.; Winkelmann, A.; Montoya, P. Attentional bias toward negative information in patients with fibromyalgia syndrome. Pain Med. 2014, 15, 603–612. [Google Scholar] [CrossRef] [PubMed]

| Groups of Participants | FM (n = 60) | CLBP (n = 60) | Pain-Free Controls (n = 60) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± sd | Mean ± sd | Mean ± sd | p-values all groups | p-values FM—CLBP | p-values FM—pain-free controls | p-values CLBP—pain-free controls | |
| Age (years) | 52.57 ± 1.08 | 52.50 ± 1.42 | 49.87 ± 1.25 | 0.06 | 0.90 | 0.05 | 0.06 |
| Pain duration (years) | 7.38 ± 2.79 | 7.08 ± 4.07 | 0 | <0.001 | 0.81 | <0.001 | <0.001 |
| BMI (Kg/m2) | 23.79 ± 0.34 | 23.66 ± 0.30 | 23.15 ± 0.30 | 0.33 | 0.77 | 0.17 | 0.24 |
| Height (cm) | 168.1 ± 0.88 | 170.36 ± 0.83 | 168.65 ± 0.76 | 0.13 | 0.08 | 0.64 | 0.13 |
| Weight (kg) | 67.13 ± 0.89 | 68.65 ± 0.93 | 65.90 ± 1.00 | 0.12 | 0.24 | 0.33 | 0.08 |
| Gender | 54 ♀ 6 ♂ | 45 ♀ 15 ♂ | 45 ♀ 15 ♂ | 0.06 | 1 | 0.06 | 0.06 |
| Dependent Variables | FM (n = 60) | CLBP (n = 60) | Pain-Free Controls (n = 60) | p-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± sd (Range) | All Groups | FM—CLBP | FM—Pain-Free Controls | CLBP—Pain-Free Controls | η | Cohen’s d | |||
| Fibromyalgia impact questionnaire (0–100) | 80.3 ± 11.49 (77.49–83.11) | 59.66 ± 14.22 (56.85–62.47) | 13.36 ± 5.46 (10.55–16.16) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.868 | 1.597 |
| McGill Pain Questionnaire, Subscale A (0–30) | 210.05± 9.88 (19.41–22.68) | 12.85 ± 4.96 (11.21–14.28) | 0.53 ± 1.18 (–1.10–2.17) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.637 | 10.049 |
| McGill Pain Questionnaire, Subscale B (0–10) | 7.95± 0.85 (7.63–8.25) | 6.26 ± 1.42 (5.96–6.57) | 0.49 ± 1.26 (0.18–0.79) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.877 | 1.419 |
| McGill Pain Questionnaire, Subscale C (0–5) | 40.00± 0.37 (3.54–4.45) | 2.75 ± 0.70 (2.29–3.20) | 0.97 ± 2.97 (0.45–1.35) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.342 | 2.224 |
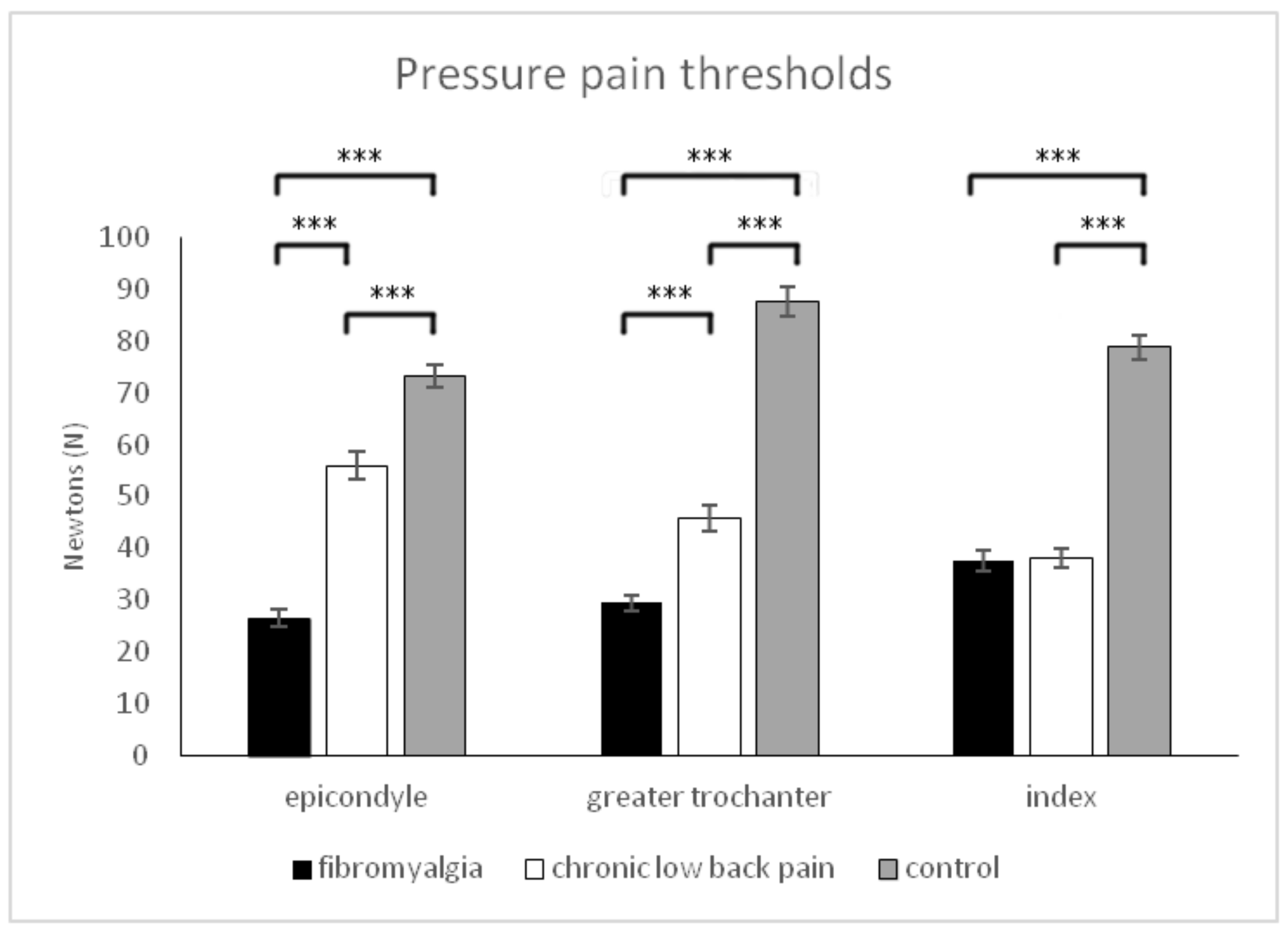
| Pressure pain sensitivity epicondyles (N) (0–100) | 26.43 ± 13.46 (220.02–30.86) | 55.95 ± 20.71 (51.53–60.37) | 73.26 ± 17.11 (68.84–77.68) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.558 | −1.690 |
| Pressure pain sensitivity greater trochanters (N) (0–100) | 29.47 ± 11.59 (24.77–34.17) | 45.78 ± 20.35 (410.08–50.48) | 87.59 ± 21.74 (82.89–92.29) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.641 | −0.985 |
| Pressure pain sensitivity index fingers (N) (0–100) | 37.64 ± 150.02 (33.49–41.78) | 380.06 ± 14.51 (33.91–42.20) | 78.78 ± 18.89 (74.64–82.93) | p < 0.001 | p>0.05 | p < 0.001 | p < 0.001 | 0.589 | −0.028 |
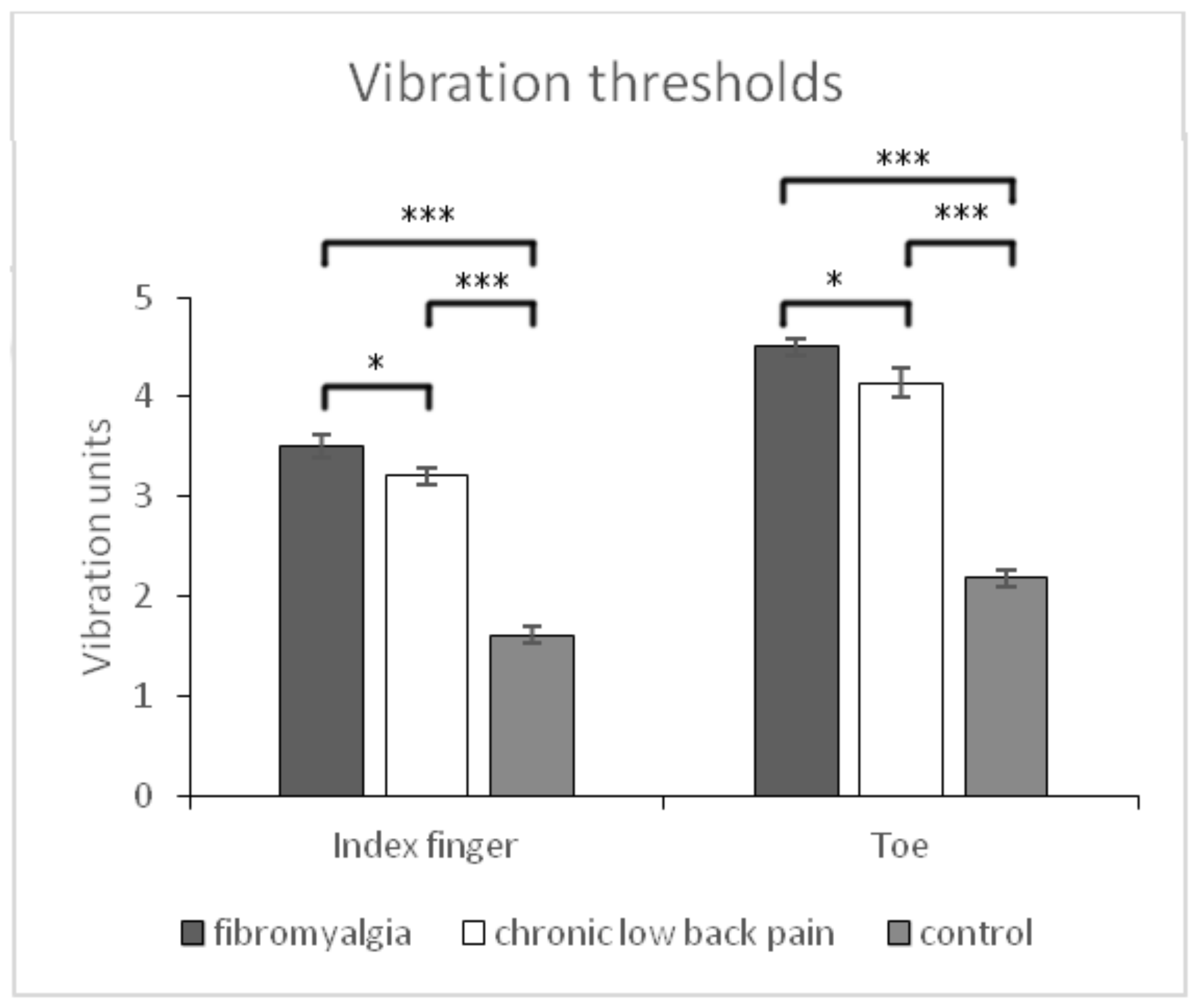
| Vibration thresholds index fingers (vibration units) | 3.52 ± 0.82 (3.33–3.70) | 3.21 ± 0.81 (30.02–3.39) | 1.61 ± 0.50 (1.42–1.79) | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 | 0.573 | 0.368 |
| Vibration thresholds toes (vibration units) | 4.51 ± 0.81 (4.29–4.71) | 4.14 ± 10.01 (3.94–4.35) | 2.18 ± 0.56 (1.97–2.38) | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 | 0.615 | 0.404 |
| Berg scale (0–56) | 28.12 ± 4.84 (26.77–29.46) | 33.15 ± 7.59 (31.80–34.49) | 55.57 ± 1.63 (54.22–56.91) | p < 0.001 | p < 0.005 | p < 0.001 | p < 0.001 | 0.838 | −0.792 |
| Six-minute walking test (m) | 363.8 ± 61.48 (344.3–383.2) | 401.3 ± 93.70 (381.8–420.7) | 611.7 ± 70.17 (592.2–631.1) | p < 0.001 | p < 0.005 | p < 0.001 | p < 0.001 | 0.675 | −0.473 |
| Timed Up and Go Test (s) | 17.58 ± 4.83 (16.70–18.46) | 13.18 ± 3.20 (12.30–140.06) | 7.70 ± 1.56 (6.82–8.58) | p < 0.001 | p < 0.005 | p < 0.005 | p < 0.005 | 0.580 | 10.074 |
| Isometric back muscle strength (kiloponds) | 29.25 ± 9.81 (23.59–34.90) | 420.08 ± 25.70 (36.43–47.73) | 120.4 ± 26.81 (114.7–1260.0) | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 | 0.770 | −0.660 |
| Borg scale (0–10) | 6.63 ± 1.48 (6.27–6.99) | 40.02 ± 1.67 (3.65–4.38) | 10.09 ± 10.06 (0.73–1.46) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.719 | 1.654 |
| Mean sway velocity (cm/s) | 0.019 ± 0.009 (0.017–0.020) | 0.011 ± 0.005 (0.010–0.013) | 0.007 ± 0.001 (0.006–0.009) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.363 | 1.143 |
| Mediolateral body sway (cm) | 0.013 ± 0.009 (0.011–0.014) | 0.007 ± 0.005 (0.005–0.008) | 0.003 ± 0.001 (0.001–0.005) | p < 0.001 | p < 0.001 | p < 0.01 | p < 0.01 | 0.261 | 0.714 |
| Anteroposterior body sway (cm) | 0.015 ± 0.008 (0.014–0.017) | 0.011 ± 0.006 (0.009–0.012) | 0.008 ± 0.002 (0.006–0.009) | p < 0.001 | p < 0.001 | p < 0.05 | p < 0.05 | 0.209 | 0.571 |
| Gait velocity (cm/s) | 2.78 ± 0.99 (2.50–30.05) | 2.81 ± 10.09 (2.53–30.09) | 3.67 ± 1.14 (3.40–3.95) | p < 0.001 | p < 0.05 | p < 0.02 | p < 0.02 | 0.130 | −0.038 |
| Stride length (cm) | 0.93 ± 0.33 (0.84–10.02) | 0.97 ± 0.41 (0.88–10.06) | 1.22 ± 0.34 (1.13–1.32) | p < 0.001 | p < 0.05 | p < 0.02 | p < 0.02 | 0.115 | −0.108 |
| Percentage of time in the stance phase (%) | 67.17 ± 10.42 (65.31–690.04) | 68.44 ± 5.49 (66.58–70.31) | 65.38 ± 4.62 (63.52–67.24) | p < 0.001 | p < 0.05 | p < 0.02 | p < 0.02 | 0.029 | −0.152 |
| Percentage of time in the swing phase (%) | 31.66 ± 5.87 (30.29–330.04) | 31.69 ± 5.62 (30.31–330.07) | 34.58 ± 4.64 (33.21–35.96) | p < 0.001 | p < 0.05 | p < 0.02 | p < 0.02 | 0.062 | −0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingorance, J.A.; Montoya, P.; Miranda, J.G.V.; Riquelme, I. An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance. Healthcare 2021, 9, 1533. https://doi.org/10.3390/healthcare9111533
Mingorance JA, Montoya P, Miranda JGV, Riquelme I. An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance. Healthcare. 2021; 9(11):1533. https://doi.org/10.3390/healthcare9111533
Chicago/Turabian StyleMingorance, José Antonio, Pedro Montoya, José García Vivas Miranda, and Inmaculada Riquelme. 2021. "An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance" Healthcare 9, no. 11: 1533. https://doi.org/10.3390/healthcare9111533
APA StyleMingorance, J. A., Montoya, P., Miranda, J. G. V., & Riquelme, I. (2021). An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance. Healthcare, 9(11), 1533. https://doi.org/10.3390/healthcare9111533







