Validation of an Arabic Version of the Adherence to Refills and Medications Scale (ARMS)
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Population and Data Source
2.4. Research Instrument Translation and Validation
2.5. Study Variables
2.6. Sample Size Estimation
2.7. Statistical Analysis
2.8. Ethical Approval
3. Results
3.1. Participants’ Characteristics
3.2. Reliability and Internal Consistency
3.3. Factor Analysis
3.4. Adherence Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
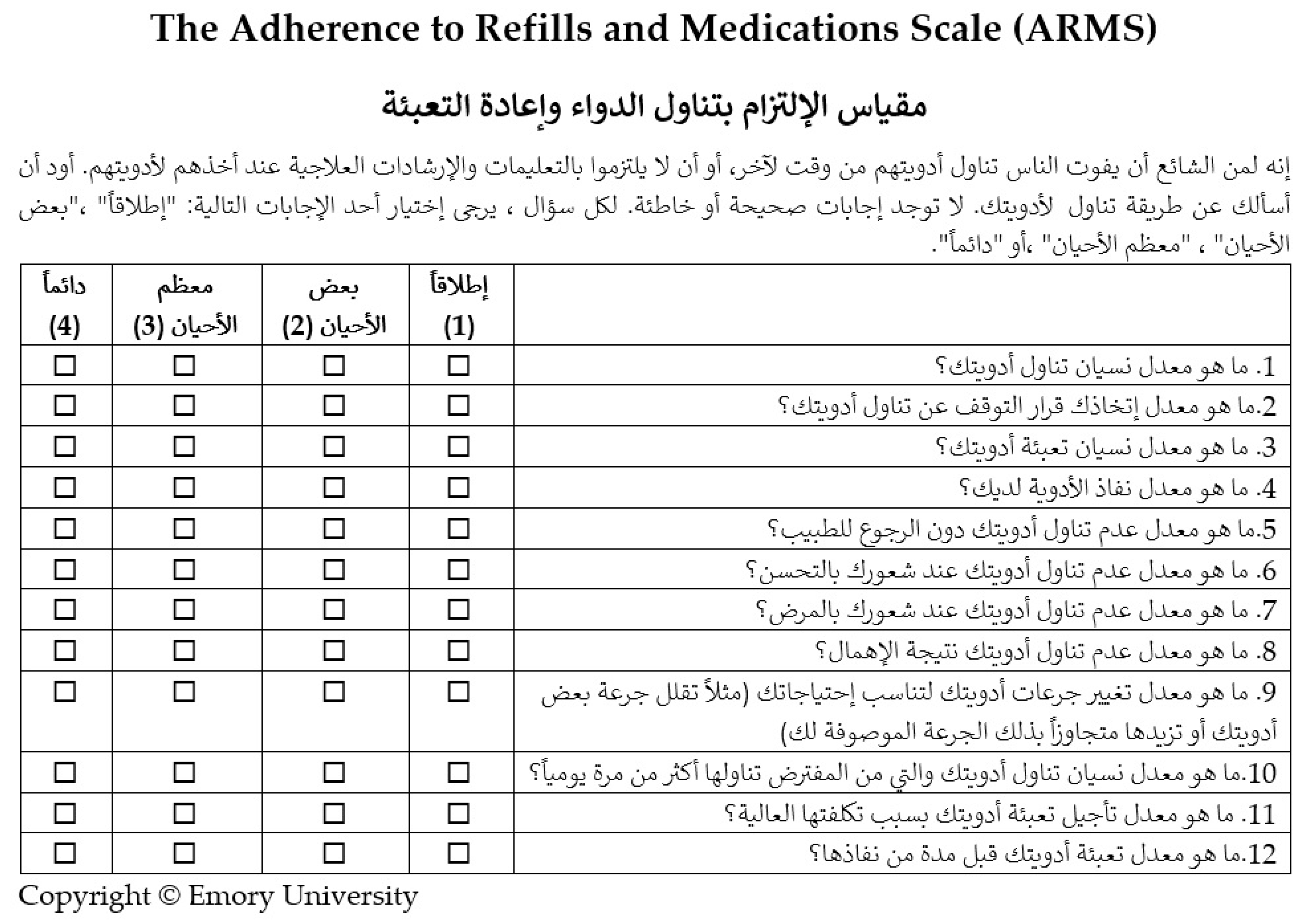
Appendix A
References
- Organization, W.H. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Mohiuddin, A.K. Risks and reasons associated with medication non-adherence. J. Clin. Pharm. 2019, 1, 50–53. [Google Scholar]
- Marcum, Z.A.; Sevick, M.A.; Handler, S.M. Medication nonadherence: A diagnosable and treatable medical condition. JAMA 2013, 309, 2105–2106. [Google Scholar] [CrossRef] [PubMed]
- Kini, V.; Ho, P.M. Interventions to improve medication adherence: A review. JAMA 2018, 320, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.M.; Bryson, C.L.; Rumsfeld, J.S. Medication adherence: Its importance in cardiovascular outcomes. Circulation 2009, 119, 3028–3035. [Google Scholar] [CrossRef]
- Iuga, A.O.; McGuire, M.J. Adherence and health care costs. Risk Manag. Healthc. Policy 2014, 7, 35. [Google Scholar]
- Zyoud, S.H.; Al-Jabi, S.W.; Sweileh, W.M.; Morisky, D.E. Relationship of treatment satisfaction to medication adherence: Findings from a cross-sectional survey among hypertensive patients in Palestine. Health Qual. Life Outcomes 2013, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ménard, S.; Jbilou, J.; Lauzier, S. Family caregivers’ reported nonadherence to the controller medication of asthma in children in Casablanca (Morocco): Extent and associated factors. J. Asthma 2018, 55, 1362–1372. [Google Scholar] [CrossRef]
- Altuwairqi, H.B. Barriers to medication adherence among cardiac patients following at King Fahad Medical City, Riyadh, Saudi Arabia. Saudi J. Health Sci. 2016, 5, 20. [Google Scholar] [CrossRef]
- Khan, A.R.; Lateef, Z.N.A.-A.; Al Aithan, M.A.; Bu-Khamseen, M.A.; Al Ibrahim, I.; Khan, S.A. Factors contributing to non-compliance among diabetics attending primary health centers in the Al Hasa district of Saudi Arabia. J. Fam. Community Med. 2012, 19, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, D.; Kekwaletswe, T.C.; Hosek, S.; Martinez, J.; Rodriguez, F. Stigma and social barriers to medication adherence with urban youth living with HIV. AIDS Care 2007, 19, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Blashill, A.J.; Perry, N.; Safren, S.A. Mental health: A focus on stress, coping, and mental illness as it relates to treatment retention, adherence, and other health outcomes. Curr. HIV/AIDS Rep. 2011, 8, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Lazaro, C.I.; Adams, D.P.; Fernandez-Lazaro, D.; Garcia-González, J.M.; Caballero-Garcia, A.; Miron-Canelo, J.A. Medication adherence and barriers among low-income, uninsured patients with multiple chronic conditions. Res. Soc. Adm. Pharm. 2019, 15, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Pasina, L.; Brucato, A.; Falcone, C.; Cucchi, E.; Bresciani, A.; Sottocorno, M.; Taddei, G.C.; Casati, M.; Franchi, C.; Djade, C.D.; et al. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging 2014, 31, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Fresco, P. Medication adherence measures: An overview. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kreys, E. Measurements of medication adherence: In search of a gold standard. J. Clin. Pathw. 2016, 2, 43–47. [Google Scholar]
- Anghel, L.A.; Farcas, A.M.; Oprean, R.N. An overview of the common methods used to measure treatment adherence. Med. Pharm. Rep. 2019, 92, 117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-M.-U.; Caze, A.L.; Cottrell, N. What are validated self-report adherence scales really measuring?: A systematic review. Br. J. Clin. Pharmacol. 2014, 77, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Zullig, L.L.; Mendys, P.; Bosworth, H.B. Medication adherence: A practical measurement selection guide using case studies. Patient Educ. Couns. 2017, 100, 1410–1414. [Google Scholar] [CrossRef]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.; Czajkowski, S.; Hilliard, M.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Ashur, S.; Shamsuddin, K.; Shah, S.; Bosseri, S.; Morisky, D. Reliability and known-group validity of the Arabic version of the 8-item Morisky Medication Adherence Scale among type 2 diabetes mellitus patients. East. Mediterr. Health J. 2015, 21, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lavsa, S.M.; Holzworth, A.; Ansani, N.T. Selection of a validated scale for measuring medication adherence. J. Am. Pharm. Assoc. 2011, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Karademir, M.; Koseoglu, I.H.; Vatansever, K.; Van Den Akker, M. Validity and reliability of the Turkish version of the Hill–Bone compliance to high blood pressure therapy scale for use in primary health care settings. Eur. J. Gen. Pract. 2009, 15, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Jayawardena, R.; Katulanda, P.; Constantine, G.R.; Ramanayake, V.; Galappatthy, P. Translation and validation of the sinhalese version of the brief medication questionnaire in patients with diabetes mellitus. J. Diabetes Res. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.-F.; Liu, Y.-J.; Wang, A.-X.; Lv, P.-H. Psychometric properties of the Chinese version of the self-efficacy for appropriate medication use scale in patients with stroke. Patient Prefer. Adherence 2016, 10, 321. [Google Scholar]
- Kripalani, S.; Risser, J.; Gatti, M.E.; Jacobson, T.A. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009, 12, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Gökdoğan, F.; Kes, D. Validity and reliability of the Turkish adherence to refills and medications scale. Int. J. Nurs. Pract. 2017, 23, e12566. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Park, E.; Schlenk, E.A.; Kim, M.; Kim, D.J. Psychometric evaluation of a Korean version of the Adherence to Refills and Medications Scale (ARMS) in adults with type 2 diabetes. Diabetes Educ. 2016, 42, 188–198. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, J.; Yang, S.-Y. Psychometric evaluation of Chinese version of Adherence to Refills and Medications Scale (ARMS) and blood-pressure control among elderly with hypertension. Patient Prefer. Adherence 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Lomper, K.; Chabowski, M.; Chudiak, A.; Białoszewski, A.; Dudek, K.; Jankowska-Polańska, B. Psychometric evaluation of the Polish version of the Adherence to Refills and Medications Scale (ARMS) in adults with hypertension. Patient Prefer. Adherence 2018, 12, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, N.S.; MacLean, C.D.; Chew, L.D.; Littenberg, B. The Single Item Literacy Screener: Evaluation of a brief instrument to identify limited reading ability. BMC Fam. Pract. 2006, 7, 1–7. [Google Scholar] [CrossRef]
- Al-Jumaili, A.A.; Al-Rekabi, M.D.; Sorofman, B. Evaluation of instruments to assess health literacy in Arabic language among Iraqis. Res. Soc. Adm. Pharm. 2015, 11, 803–813. [Google Scholar] [CrossRef]
- Anthoine, E.; Moret, L.; Regnault, A.; Sébille, V.; Hardouin, J.-B. Sample size used to validate a scale: A review of publications on newly-developed patient reported outcomes measures. Health Qual. Life Outcomes 2014, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.W.; Costello, A.B. Sample size and subject to item ratio in principal components analysis. Pract. Assess. Res. Eval. 2004, 9, 11. [Google Scholar]
- Ledesma, R.D.; Valero-Mora, P. Determining the number of factors to retain in EFA: An easy-to-use computer program for carrying out parallel analysis. Pract. Assess. Res. Eval. 2007, 12, 2. [Google Scholar]
- Xia, Y.; Yang, Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: The story they tell depends on the estimation methods. Behav. Res. Methods 2019, 51, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Onsman, A.; Brown, T. Exploratory factor analysis: A five-step guide for novices. Australas. J. Paramed. 2010, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liljequist, D.; Elfving, B.; Skavberg Roaldsen, K. Intraclass correlation—A discussion and demonstration of basic features. PLoS ONE 2019, 14, e0219854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinetti, G. StaTips Part IV: Selection, interpretation and reporting of the intraclass correlation coefficient. South Eur. J. Orthod. Dentofac. Res. 2018, 5, 3–5. [Google Scholar] [CrossRef]
- Ursachi, G.; Horodnic, I.A.; Zait, A. How reliable are measurement scales? External factors with indirect influence on reliability estimators. Procedia Econ. Financ. 2015, 20, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Fornell, C.; Larcker, D.F. Evaluating structural equation models with unobservable variables and measurement error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Ab Hamid, M.; Sami, W.; Sidek, M.M. Discriminant validity assessment: Use of Fornell & Larcker criterion versus HTMT criterion. Paper presented at: J. Phys. Conf. Ser. 2017, 890, 012163. [Google Scholar]
- Association WM. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Kamusella, T.D. The Arabic language: A Latin of modernity? J. Natl. Mem. Lang. Politics 2017, 11, 117–145. [Google Scholar] [CrossRef] [Green Version]
- Abuyassin, B.; Laher, I. Diabetes epidemic sweeping the Arab world. World J. Diabetes 2016, 7, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tyrovolas, S.; El Bcheraoui, C.; Alghnam, S.A.; Alhabib, K.F.; Almadi, M.A.H.; Al-Raddadi, R.M.; Bedi, N.; Tantawi, M.E.; Krish, V.S.; Memish, Z.A.; et al. The burden of disease in Saudi Arabia 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Planet. Health 2020, 4, e195–e208. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Jaber, S.; Aziz, M.I.A.; AlBuhairan, F.; AlGhaithi, A.; AlHAmad, N.M.; Al-Hooti, S.N.; Al-Jasari, A.; AlMazroa, M.A.; AlQasmi, A.M.; et al. The state of health in the Arab world, 1990–2010: An analysis of the burden of diseases, injuries, and risk factors. Lancet 2014, 383, 309–320. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Cutillo, L. Parametric and multivariate methods. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Nakai, K., Schonbach, C., Eds.; Elsevier: Amsterdam, Netherlands, 2019; pp. 738–746. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Yu, H.Y.; You, M.-A.; Son, Y.-J. Impact of health literacy on medication adherence in older people with chronic diseases. Collegian 2017, 24, 11–18. [Google Scholar] [CrossRef]
- Tian, C.Y.; Xu, R.H.; Mo, P.K.-H.; Dong, D.; Wong, E.L.-Y. Generic health literacy measurements for adults: A scoping review. Int. J. Environ. Res. Public Health 2020, 17, 7768. [Google Scholar] [CrossRef] [PubMed]

| Gender | |
| Male | 141 (69.8) |
| Female | 61 (30.2) |
| Age group (years) | |
| 18–25 | 22 (10.8) |
| 26–33 | 11 (5.4) |
| 34–41 | 14 (6.9) |
| 42–49 | 23 (11.3) |
| 50–57 | 34 (16.8) |
| 58–65 | 58 (28.7) |
| 66–73 | 31 (15.3) |
| ≥74 | 9 (4.4) |
| Marital status | |
| Single | 30 (14.8) |
| Married | 152 (75.2) |
| Divorced | 7 (3.4) |
| Widowed | 13 (6.4) |
| Educational level | |
| No official education | 24 (11.8) |
| Completed few years of elementary school | 8 (3.9) |
| Elementary school diploma | 13 (6.4) |
| Middle school diploma | 15 (7.4) |
| Secondary school diploma or equivalent (industrial or commercial diplomas) | 39 (19.3) |
| Post-secondary school diploma or technical college | 15 (7.4) |
| University degree | 76 (37.6) |
| Graduate degree or equivalent (masters, doctorate, medical fellowship) | 12 (5.9) |
| Employment status | |
| Government sector employee | 51 (25.2) |
| Private sector employee | 11 (5.4) |
| Freelancer | 8 (3.9) |
| Retired | 70 (34.6) |
| Unemployed | 62 (30.6) |
| Monthly income * | |
| Less than 5000 | 49 (24.2) |
| 5000–10,000 | 81 (40.1) |
| 10,000–15,000 | 37 (18.3) |
| 15,000–20,000 | 22 (10.8) |
| More than 20,000 | 13 (6.4) |
| Health literacy | |
| Adequate | 160 (79.2) |
| Marginal\limited | 42 (20.7) |
| Number of Medications Taken | |
| One | 14 (6.9) |
| Two | 18 (8.9) |
| Three | 13 (6.4) |
| Four | 22 (10.8) |
| Five | 18 (8.9) |
| Six and more | 117 (57.9) |
| Chronic Diseases | |
| Hypertension | 99 (49.01) |
| Hypothyroidism | 21 (10.4) |
| Cardiovascular disease | 12 (5.9) |
| Dyslipidemia | 97 (48) |
| Diabetes mellitus type I | 69 (34.1) |
| Diabetes mellitus type II (no insulin use) | 122 (60.4) |
| Diabetes mellitus type II (with insulin use) | 4 (1.9) |
| Psychiatric disorders | 3 (1.4) |
| Pulmonary diseases | 15 (7.4) |
| Other | 26 (12.9) |
| Number of chronic health conditions | |
| 1–2 | 113(55.94) |
| 3–4 | 80(39.60) |
| 5–6 | 9(4.45) |
| Medical insurance | |
| Yes | 26 (12.8) |
| No | 176 (87.1) |
| Mean ± SD | Item–Total Correlation | Cronbach’s Alpha if Item Removed | |
|---|---|---|---|
| 1. How often do you forget to take your medicine? | 1.50 ± 0.74 | 0.510 | 0.78 |
| 2. How often do you decide not to take your medicine? | 1.29 ± 0.63 | 0.532 | 0.78 |
| 3. How often do you forget to get prescriptions filled? | 1.35 ± 0.61 | 0.473 | 0.78 |
| 4. How often do you run out of medicine? | 1.72 ± 0.89 | 0.459 | 0.78 |
| 5. How often do you skip a dose of your medicine before you go to the doctor? | 1.25 ± 0.61 | 0.524 | 0.78 |
| 6. How often do you miss taking your medicine when you feel better? | 1.29 ± 0.64 | 0.529 | 0.78 |
| 7. How often do you miss taking your medicine when you feel sick? | 1.31 ± 0.74 | 0.528 | 0.78 |
| 8. How often do you miss taking your medicine when you are careless? | 1.28 ± 0.65 | 0.612 | 0.77 |
| 9. How often do you change the dose of your medicines to suit your needs (like when you take more or less pills than you’re supposed to)? | 1.66 ± 0.78 | 0.385 | 0.80 |
| 10. How often do you forget to take your medicine when you are supposed to take it more than once a day? | 1.38 ± 0.69 | 0.572 | 0.77 |
| 11. How often do you put off refilling your medicines because they cost too much money? | 1.58 ± 0.82 | 0.363 | 0.79 |
| 12. How often do you plan ahead and refill your medicines before they run out? * | 2.25 ± 1.22 | 0.217 | 0.82 |
| Factor 1 | Factor 2 | |
|---|---|---|
| % variance explained | 52.94 | 47.06 |
| Items | ||
| 1. How often do you forget to take your medicine? | 0.808 | |
| 2. How often do you decide not to take your medicine? | 0.588 | |
| 3. How often do you forget to get prescriptions filled? | 0.337 | |
| 4. How often do you run out of medicine? | 0.639 | |
| 5. How often do you skip a dose of your medicine before you go to the doctor? | 0.607 | |
| 6. How often do you miss taking your medicine when you feel better? | 0.648 | |
| 7. How often do you miss taking your medicine when you feel sick? | 0.646 | |
| 8. How often do you miss taking your medicine when you are careless? | 0.803 | |
| 9. How often do you change the dose of your medicines to suit your needs (like when you take more or less pills than you’re supposed to)? | 0.338 | |
| 10. How often do you forget to take your medicine when you are supposed to take it more than once a day? | 0.755 | |
| 11. How often do you put off refilling your medicines because they cost too much money? | 0.402 | |
| 12. How often do you plan ahead and refill your medicines before they run out? | 0.446 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alammari, G.; Alhazzani, H.; AlRajhi, N.; Sales, I.; Jamal, A.; Almigbal, T.H.; Batais, M.A.; Asiri, Y.A.; AlRuthia, Y. Validation of an Arabic Version of the Adherence to Refills and Medications Scale (ARMS). Healthcare 2021, 9, 1430. https://doi.org/10.3390/healthcare9111430
Alammari G, Alhazzani H, AlRajhi N, Sales I, Jamal A, Almigbal TH, Batais MA, Asiri YA, AlRuthia Y. Validation of an Arabic Version of the Adherence to Refills and Medications Scale (ARMS). Healthcare. 2021; 9(11):1430. https://doi.org/10.3390/healthcare9111430
Chicago/Turabian StyleAlammari, Ghaida, Hawazin Alhazzani, Nouf AlRajhi, Ibrahim Sales, Amr Jamal, Turky H. Almigbal, Mohammed A. Batais, Yousif A. Asiri, and Yazed AlRuthia. 2021. "Validation of an Arabic Version of the Adherence to Refills and Medications Scale (ARMS)" Healthcare 9, no. 11: 1430. https://doi.org/10.3390/healthcare9111430
APA StyleAlammari, G., Alhazzani, H., AlRajhi, N., Sales, I., Jamal, A., Almigbal, T. H., Batais, M. A., Asiri, Y. A., & AlRuthia, Y. (2021). Validation of an Arabic Version of the Adherence to Refills and Medications Scale (ARMS). Healthcare, 9(11), 1430. https://doi.org/10.3390/healthcare9111430







