External Validation of a Population-Based Prediction Model for High Healthcare Resource Use in Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Context and Setting
2.2. Data Sources
2.3. Participants
2.4. High Resource User Outcome
2.5. Predictors
2.6. External Validation of the Model
2.7. General Statistical Analyses
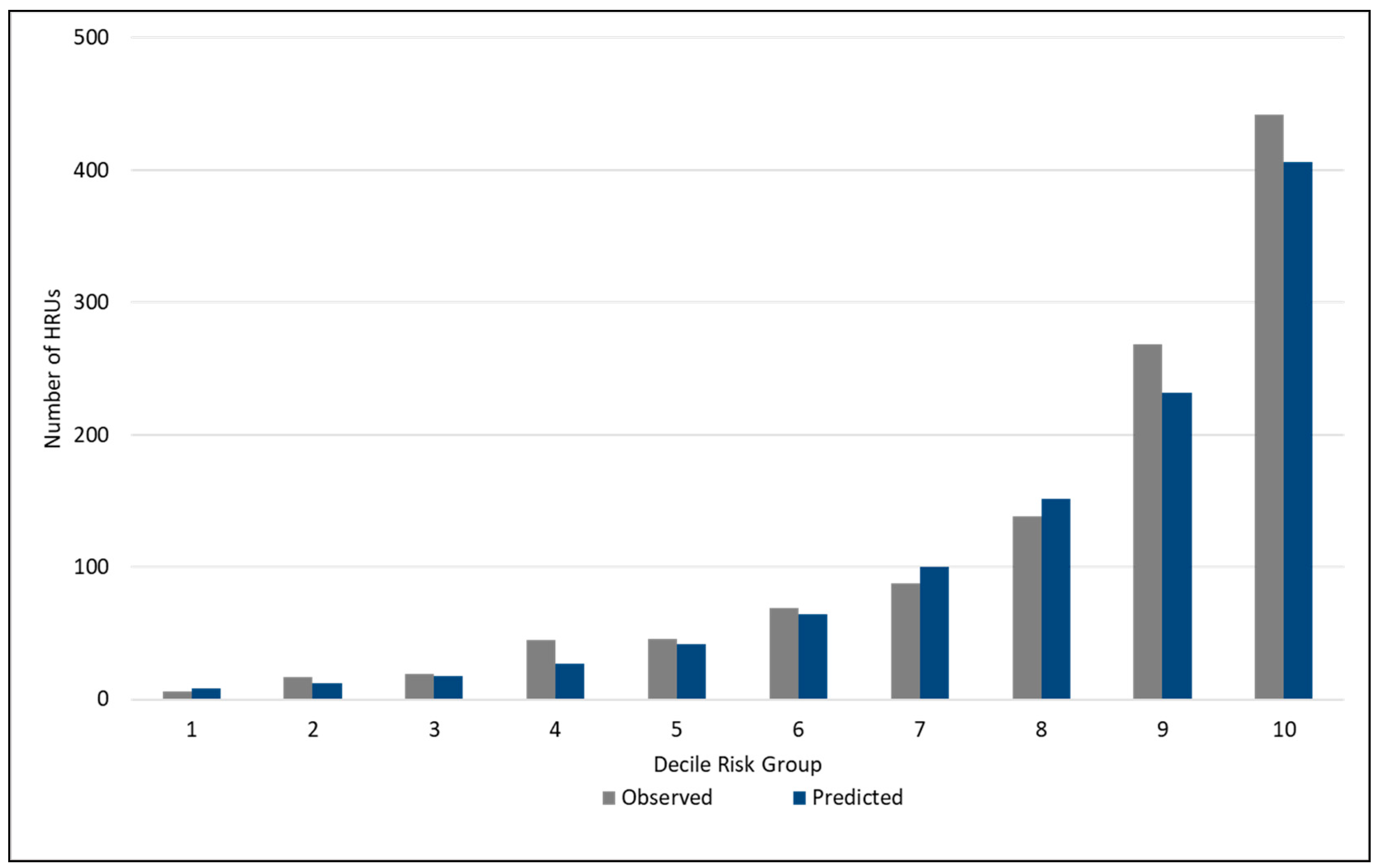
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing
Appendix A. Description of Health Administrative Data Sources
| Predictor | Original Model Regression Coefficients | Updated Model * Regression Coefficients |
|---|---|---|
| Intercept | −5.0742 | −3.1746 |
| Male | 0.2425 | 0.2094 |
| Female | 0 | 0 |
| Aged <30 | 0 | 0 |
| Aged 30–39 | 0.1202 | 0.1779 |
| Aged 40–49 | 0.9213 | 0.838 |
| Aged 50–59 | 1.6306 | 1.5334 |
| Aged 60–69 | 2.3558 | 2.2518 |
| Aged 70–79 | 2.8083 | 2.839 |
| Aged 80+ | 3.6188 | 3.6104 |
| Bottom Income Quintile | 0.5256 | 0.3785 |
| Income Quintile 2 | 0.4035 | 0.3409 |
| Income Quintile 3 | 0.2000 | 0.1276 |
| Income Quintile 4 | 0.2267 | 0.2167 |
| Highest Income Quintile | 0 | 0 |
| Missing Income Quintile | 0.3841 | 0.264 |
| Non-white ethnicity | −0.2426 | −0.2397 |
| White Ethnicity | 0 | 0 |
| Missing Ethnicity | 0.00983 | 0.0823 |
| Former Heavy Smoker | 0.2926 | 0.3547 |
| Former Light Smoker | 0.1296 | 0.168 |
| Current Heavy Smoker | 0.4579 | 0.4607 |
| Current Light Smoker | 0.2569 | 0.2301 |
| Non-Smoker | 0 | 0 |
| Missing Smoking Status | −0.0661 | −0.0692 |
| Food Secure | −0.2661 | −0.3816 |
| Food Insecure | 0 | 0 |
| Missing Food Security | −0.7921 | −0.7942 |
| Poor General Health | 1.0628 | 0.9877 |
| Fair General Health | 0.4080 | 0.3513 |
| Excellent/Very good/good general health | 0 | 0 |
| Missing General Health | 0.1470 | −0.0384 |
| Has Chronic Condition | 0.3617 | 0.4285 |
| No Chronic Condition | 0 | 0 |
| BMI <18.5 | 0.0174 | 0.1401 |
| BMI 18.5–24.9 | 0 | 0 |
| BMI 25.0–29.9 | 0.0753 | 0.0745 |
| BMI 30.0–34.9 | 0.1131 | 0.1786 |
| BMI 35.0–39.9 | 0.3624 | 0.2816 |
| BMI ≥ 40.0 | 0.6384 | 0.6653 |
| BMI Missing | 0.0959 | 0.0345 |
| Heavy Drinker | −0.0105 | 0.018 |
| Moderate Drinker | −0.0757 | −0.0439 |
| Light Drinker | 0 | 0 |
| Non-Drinker | 0.0420 | 0.0875 |
| Missing alcohol consumption | 0.0619 | 0.0693 |
| Bottom Physical Activity Quartile | 0.1766 | 0.1071 |
| Physical Activity Quartile 2 | −0.0110 | −0.0822 |
| Physical Activity Quartile 3 | 0.0172 | −0.0331 |
| Highest Physical Activity Quartile | 0 | 0 |
| Missing Physical Activity | 0.3490 | 0.2548 |
| Immigrant <10 years | −0.2358 | −0.2852 |
| Immigrant ≥10 years | −0.0375 | −0.0572 |
| Non-Immigrant | 0 | 0 |
| Missing Immigrant Status | 0.1781 | 0.3221 |
References
- Canadian Institute for Health Information. National Health Expenditure Trends, 1975 to 2018; CIHI: Ottawa, ON, Canada, 2017. [Google Scholar]
- French, E.; Kelly, E. Medical Spending around the Developed World. Fisc. Stud. 2016, 37, 327–344. [Google Scholar] [CrossRef]
- Wodchis, W.P.; Austin, P.C.; Henry, D.A. A 3-year study of high-cost users of health care. Can. Med Assoc. J. 2016, 188, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Vergouwe, Y.; Royston, P.; Moons, K.G.M. Prognosis and prognostic research: Validating a prognostic model. BMJ 2009, 338, b605. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Harrell, F.E. Prediction models need appropriate internal, internal–external, and external validation. J. Clin. Epidemiol. 2016, 69, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; Kengne, A.P.; E Grobbee, D.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Rosella, L.C.; Kornas, K.; Yao, Z.; Manuel, D.G.; Bornbaum, C.; Fransoo, R.; Stukel, T. Predicting High Health Care Resource Utilization in a Single-payer Public Health Care System: Development and Validation of the High Resource User Population Risk Tool. Med Care 2018, 56, e61. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Census Profile, 2016 Census. 2019. Available online: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=46&Geo2=PR&Code2=01&SearchText=Manitoba&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=46&type=0 (accessed on 2 December 2020).
- Beland, Y. Canadian Community Health Survey—Methodological Overview. Health Rep. 2002, 13, 9–14. [Google Scholar] [PubMed]
- Thomas, S.; Wannell, B. Combining cycles of the Canadian Community Health Survey. Stat. Can. Health Rep. 2009, 20, 55–60. [Google Scholar]
- Wodchis, W.P.; Bushmeneva, K.; Nikitovic, M.; McKillop, I. Guidelines on Person-Level Costing Using Administrative Databases in Ontario, in Working Paper Series; Health System Performance Research Network: Toronto, ON, Canada, 2013; pp. 1–70. [Google Scholar]
- Thomas, G. Levels and Patterns of Alcohol Use in Canada; Alcohol Price Policy Series: Report 1; Canadian Centre on Substance Abuse: Ottawa, ON, Canada, 2012. [Google Scholar]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for some traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Rosella, L.C.; Fu, L.; Alberga, A. The relationship between life satisfaction and healthcare utilization: A longitudinal study. Am. J. Prev. Med. 2018, 55, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Rosella, L.C.; Calzavara, A.; Petch, J.; Pinto, A.D.; Manson, H.; Goel, V.; Wodchis, W.P. Looking beyond income and education: Socioeconomic status gradients among future high-cost users of health care. Am. J. Prev. Med. 2015, 49, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Rosella, L.C.; Fitzpatrick, T.; Wodchis, W.P.; Calzavara, A.; Manson, H.; Goel, V. High-cost health care users in Ontario, Canada: Demographic, socio-economic, and health status characteristics. BMC Health Serv. Res. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.W.; Vembu, S.; Wang, J.; Tu, K.; Morris, Q.; Abrams, H.B. Using the electronic medical record to identify patients at high risk for frequent emergency department visits and high system costs. Am. J. Med. 2017, 130, 601.e17–601.e22. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Boyd, C.M.; Leff, B.; Lemke, K.W.; Bodycombe, D.P.; Weiner, J.P. Identifying Consistent High-cost Users in a Health Plan. Med. Care 2016, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Newman, J.F. Societal and individual determinants of medical care utilization in the United States. Milbank Meml. Fund Q. Health Soc. 1973, 51, 95–124. [Google Scholar] [CrossRef]
- Region of Peel—Public Health. The Changing Landscape of Health in Peel: A Comprehensive Health Status Report; Peel Public Health: Mississaugua, ON, Canada, 2019. [Google Scholar]
| Risk Factor | HRU 2, Top 5% % (95% CI) n = 1145 | Non-HRU, Bottom 95% % (95% CI) n = 9359 |
|---|---|---|
| Sex | ||
| Male | 49.7 (44.5, 54.8) | 49.3 (48.5, 50.1) |
| Female | 50.3 (45.2, 55.5) | 50.7 (49.9, 51.5) |
| Age group | ||
| <30 | 3.5 (1.8, 5.2) | 22.9 (22.1, 23.6) |
| 30–39 | 5.4 (2.8, 8.0) | 18.0 (17.1, 19.0) |
| 40–49 | 10.7 (7.3, 14.1) | 19.5 (18.0, 21.0) |
| 50–59 | 17.2 (12.2, 22.1) | 17.8 (16.5,19.0) |
| 60–69 | 21.1 (16.3, 25.8) | 12.3 (11.3, 13.4) |
| 70–79 | 18.2 (15.4, 21.0) | 6.6 (6.1, 7.1) |
| ≥80 | 23.9 (20.5, 27.3) | 2.8 (2.5, 3.2) |
| Ethnicity | ||
| White | 86.3 (82.0, 90.6) | 79.3 (77.6, 80.9) |
| Non-white | Suppressed | 9.9 (8.5, 11.3) |
| Missing | 8.0 (5.5, 10.5) | 10.8 (9.8, 11.9) |
| Immigrant status | ||
| Non-immigrant | 84.0 (79.6, 88.4) | 84.4 (82.9, 85.8) |
| Immigrant (<10 years) | Suppressed | 4.5 (3.7, 5.3) |
| Immigrant (≥10 years) | 15.4 (11.0, 19.9) | 11.0 (9.8, 12.2) |
| Missing | Suppressed | 0.2 (0.1, 0.3) |
| Household income | ||
| Q1 (lowest) | 24.7 (20.5, 28.8) | 17.4 (15.9, 18.9) |
| Q2 | 22.5 (18.7, 26.3) | 17.2 (15.8, 18.6) |
| Q3 | 16.8 (12.5, 21.2) | 18.0 (16.4, 19.6) |
| Q4 | 11.9 (7.8, 16.0) | 18.0 (16.6, 19.5) |
| Q5 (highest) | 8.7 (6.1, 11.4) | 18.8 (17.4, 20.1) |
| Missing | 15.4 (11.3, 19.5) | 10.6 (9.5, 11.6) |
| Food Security | ||
| Food Secure | 92.4 (89.3, 95.5) | 92.7 (91.8, 93.7) |
| Food Insecure | 5.0 (2.6, 7.4) | 5.9 (5.0, 6.7) |
| Missing | Suppressed | 1.4 (1.0, 1.8) |
| Chronic Condition | ||
| Yes | 84.8 (81.0, 88.6) | 56.5 (54.8, 58.2) |
| No | 13.6 (10.2, 17.1) | 37.5 (35.7, 39.2) |
| Missing | Suppressed | 6.1 (5.2, 6.9) |
| General Health | ||
| Excellent/very good | 30.2 (25.4, 35.0) | 59.5 (57.7, 61.2) |
| Good | 33.4 (28.2, 38.4) | 29.8 (28.2, 31.5) |
| Fair | 24.7 (20.9, 28.5) | 8.8 (7.8, 9.7) |
| Poor | 11.7 (8.5, 14.9) | 1.9 (1.5, 2.3) |
| Missing | Suppressed | Suppressed |
| Body Mass Index | ||
| <18.5 kg/m2 | Suppressed | 2.0 (1.4, 2.5) |
| 18.5–24.9 kg/m2 | 32.0 (27.4, 36.5) | 38.4 (36.8, 40.0) |
| 25.0–29.9 kg/m2 | 34.6 (29.6, 39.7) | 34.4 (32.8, 36.0) |
| 30.0–34.9 kg/m2 | 15.8 (11.8, 19.8) | 13.5 (12.3, 14.7) |
| 35.0–39.9 kg/m2 | 4.2 (2.3, 6.2) | 4.6 (3.9, 5.3) |
| ≥40.0 kg/m2 | Suppressed | 2.0 (1.5, 2.5) |
| Missing | 7.6 (5.0, 10.1) | 5.1 (4.3, 5.9) |
| Smoking Status | ||
| Heavy smoker | 7.45 (3.4, 11.5) | 3.1 (2.5, 3.7) |
| Light smoker | 15.4 (12.0, 18.8) | 18.9 (17.6, 20.2) |
| Former heavy smoker | 13.1 (9.0, 17.1) | 7.0 (6.2, 7.9) |
| Former light smoker | 22.8 (18.6, 27.0) | 16.2 (14.9, 17.5) |
| Non-smoker | 37.0 (32.3, 41.7) | 51.2 (49.4, 53.0) |
| Missing | 4.3 (2.8, 5.8) | 3.5 (2.9, 4.2) |
| Physical activity | ||
| Q1 (lowest) | 35.4 (30.5, 40.3) | 24.1 (22.5, 25.6) |
| Q2 | 26.1 (21.7, 30.4) | 23.2 (21.9, 24.6) |
| Q3 | 19.9 (15.5, 24.3) | 25.1 (23.5, 26.6) |
| Q4 (highest) | 13.6 (10.3, 17.0) | 25.9 (24.3, 27.4) |
| Missing | 5.0 (2.6, 7.3) | 1.7 (1.2, 2.3) |
| Alcohol consumption | ||
| Heavy drinker | 4.9 (2.7, 7.0) | 8.0 (6.9, 9.1) |
| Moderate drinker | 10.7 (8.1, 13.3) | 19.3 (17.9, 20.7) |
| Light drinker | 12.7 (8.8, 16.6) | 14.7 (13.6, 15.8) |
| Non-drinker | 71.3 (66.6, 76.1) | 57.4 (55.7, 59.1) |
| Missing | Suppressed | 0.6 (0.4, 0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosella, L.C.; Kornas, K.; Sarkar, J.; Fransoo, R. External Validation of a Population-Based Prediction Model for High Healthcare Resource Use in Adults. Healthcare 2020, 8, 537. https://doi.org/10.3390/healthcare8040537
Rosella LC, Kornas K, Sarkar J, Fransoo R. External Validation of a Population-Based Prediction Model for High Healthcare Resource Use in Adults. Healthcare. 2020; 8(4):537. https://doi.org/10.3390/healthcare8040537
Chicago/Turabian StyleRosella, Laura C., Kathy Kornas, Joykrishna Sarkar, and Randy Fransoo. 2020. "External Validation of a Population-Based Prediction Model for High Healthcare Resource Use in Adults" Healthcare 8, no. 4: 537. https://doi.org/10.3390/healthcare8040537
APA StyleRosella, L. C., Kornas, K., Sarkar, J., & Fransoo, R. (2020). External Validation of a Population-Based Prediction Model for High Healthcare Resource Use in Adults. Healthcare, 8(4), 537. https://doi.org/10.3390/healthcare8040537






