Relationship between Oral Health Status and Postoperative Fever among Patients with Lung Cancer Treated by Surgery: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
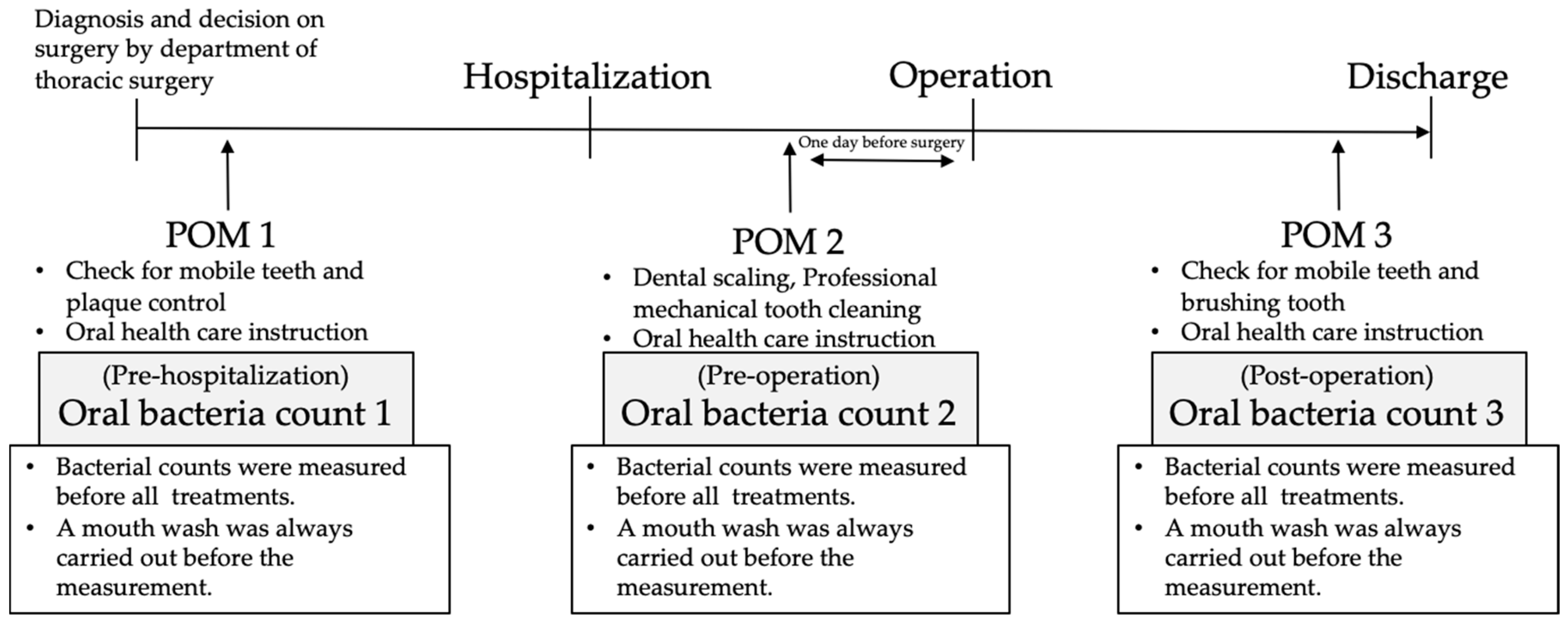
2.1. Regular Perioperative Oral Management in Kagawa Prefectural Central Hospital
2.2. Data Sources and Search Strategy
2.3. Study Variables
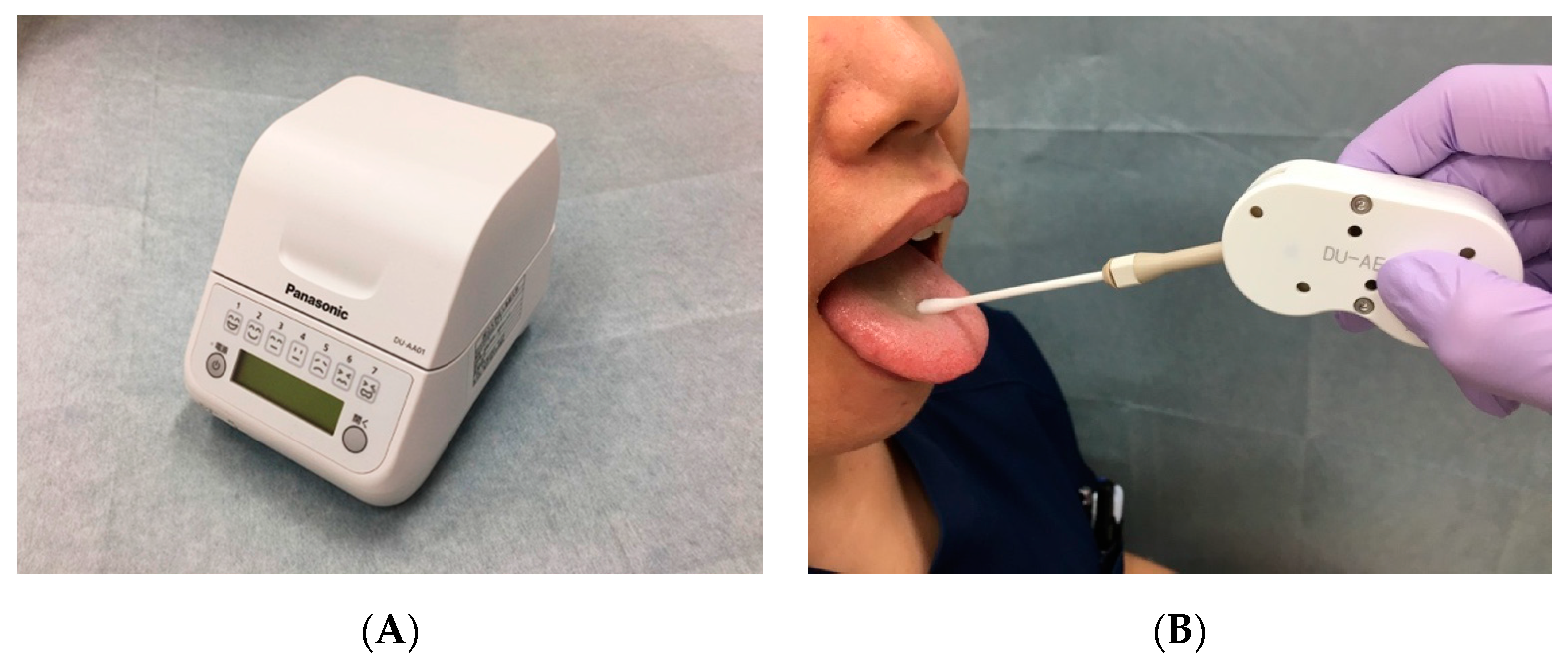
2.4. Oral Bacteria Count
2.5. Study Outcomes
2.6. Statistical Analyses
3. Results
3.1. Patient Demographics and Characteristics
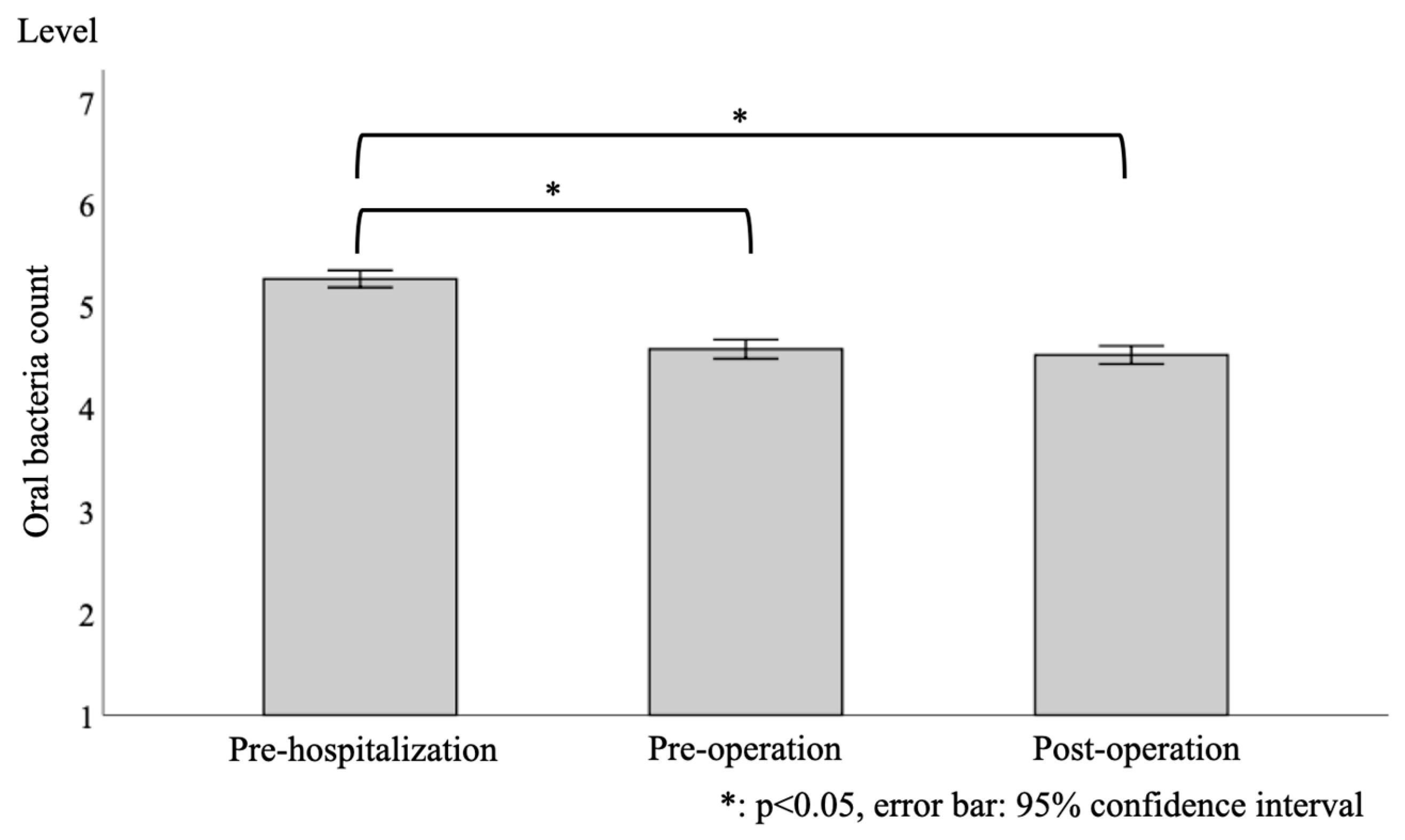
3.2. Longitudinal Changes in the Level of Oral Bacterial Counts
3.3. Risk Factors for Postoperative Fever Duration by Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurasawa, Y.; Maruoka, Y.; Sekiya, H.; Negishi, A.; Mukohyama, H.; Shigematsu, S.; Sugizaki, J.; Karakida, K.; Ohashi, M.; Ueno, M.; et al. Pneumonia prevention effects of perioperative oral management in approximately 25,000 patients following cancer surgery. Clin. Exp. Dent. Res. 2020, 6, 165–173. [Google Scholar] [CrossRef]
- Mori, K.; Horinouchi, M.; Domitsu, A.; Shimotahira, T.; Soutome, S.; Yamaguchi, T.; Oho, T. Proper oral hygiene protocols decreased inflammation of gingivitis in a patient during chemotherapy with bevacizumab: A case report. Clin. Case Rep. 2017, 5, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Yoshida, M.; Matsui, T.; Sasaki, H. Oral care and pneumonia. Oral Care Working Group. Lancet 1999, 354, 515. [Google Scholar] [CrossRef]
- Mori, H.; Hirasawa, H.; Oda, S.; Shiga, H.; Matsuda, K.; Nakamura, M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006, 32, 230–236. [Google Scholar] [CrossRef]
- Nobuhara, H.; Yanamoto, S.; Funahara, M.; Matsugu, Y.; Hayashida, S.; Soutome, S.; Kawakita, A.; Ikeda, S.; Itamoto, T.; Umeda, M. Effect of perioperative oral management on the prevention of surgical site infection after colorectal cancer surgery: A multicenter retrospective analysis of 698 patients via analysis of covariance using propensity score. Medicine 2018, 97, e12545. [Google Scholar] [CrossRef]
- Soutome, S.; Yanamoto, S.; Funahara, M.; Hasegawa, T.; Komori, T.; Oho, T.; Umeda, M. Preventive Effect on Post-Operative Pneumonia of Oral Health Care among Patients Who Undergo Esophageal Resection: A Multi-Center Retrospective Study. Surg. Infect. (Larchmt) 2016, 17, 479–484. [Google Scholar] [CrossRef]
- Nishikawa, M.; Honda, M.; Kimura, R.; Kobayashi, A.; Yamaguchi, Y.; Kobayashi, H.; Kawamura, H.; Nakayama, Y.; Todate, Y.; Takano, Y.; et al. Clinical impact of periodontal disease on postoperative complications in gastrointestinal cancer patients. Int. J. Clin. Oncol. 2019, 24, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, M.; Matsui, H.; Ono, S.; Hagiwara, Y.; Morita, K.; Yasunaga, H. Preoperative oral care and effect on postoperative complications after major cancer surgery. Br. J. Surg. 2018, 105, 1688–1696. [Google Scholar] [CrossRef]
- Kamiyoshihara, M.; Igai, H.; Ibe, T.; Kawatani, N.; Uchiyama, T.; Gomi, A.; Takahashi, S.; Otake, H.; Shimizu, K.; Mogi, A.; et al. Perioperative Oral Management of Lung Cancer Patients; Medical, Dental, and Regional Dental Clinic Collaboration. Jpn. J. Thorac. Surg. 2016, 69, 4–11. [Google Scholar]
- Nishino, T.; Yoshida, T.; Inoue, S.; Aoyama, M.; Takizawa, H.; Tangoku, A.; Yamamura, Y.; Azuma, M. Perioperative Oral Management for Esophageal Cancer and Lung Cancer Surgery. Nihon Geka Gakkai Zasshi 2017, 118, 155–160. [Google Scholar] [PubMed]
- Safdar, N.; Crnich, C.J.; Maki, D.G. The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care 2005, 50, 725–739, discussion 739–741. [Google Scholar] [PubMed]
- Sato, S.; Nakamura, M.; Shimizu, Y.; Goto, T.; Kitahara, A.; Koike, T.; Tsuchida, M. Impact of postoperative complications on outcomes of second surgery for second primary lung cancer. Surg. Today 2020. (Online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Hendry, M.; Din, N.; Stanciu, M.A.; Nafees, S.; Hendry, A.; Teoh, Z.H.; Lloyd, T.; Parsonage, R.; Neal, R.D.; et al. Pragmatic methods for reviewing exceptionally large bodies of evidence: Systematic mapping review and overview of systematic reviews using lung cancer survival as an exemplar. Syst. Rev. 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Agostini, P.; Cieslik, H.; Rathinam, S.; Bishay, E.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Singh, S.; Naidu, B. Postoperative pulmonary complications following thoracic surgery: Are there any modifiable risk factors? Thorax 2010, 65, 815–818. [Google Scholar] [CrossRef]
- Im, Y.; Park, H.Y.; Shin, S.; Shin, S.H.; Lee, H.; Ahn, J.H.; Sohn, I.; Cho, J.H.; Kim, H.K.; Zo, J.I.; et al. Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer. Respir. Res. 2019, 20, 136. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Y.; Yao, L.; Xu, J.; Zhang, S.; Du, C.; Chen, F. Risk factors of postoperative pulmonary complications after minimally invasive anatomic resection for lung cancer. Ther. Clin. Risk Manag. 2019, 15, 223–231. [Google Scholar] [CrossRef]
- Yadav, S.R.; Kini, V.V.; Padhye, A. Inhibition of Tongue Coat and Dental Plaque Formation by Stabilized Chlorine Dioxide vs. Chlorhexidine Mouthrinse: A Randomized, Triple Blinded Study. J. Clin. Diagn. Res. 2015, 9, ZC69. [Google Scholar] [CrossRef]
- Hamada, R.; Suehiro, J.; Nakano, M.; Kikutani, T.; Konishi, K. Development of rapid oral bacteria detection apparatus based on dielectrophoretic impedance measurement method. IET Nanobiotechnol. 2011, 5, 25–31. [Google Scholar] [CrossRef]
- Kikutani, T.; Tamura, F.; Tashiro, H.; Yoshida, M.; Konishi, K.; Hamada, R. Relationship between oral bacteria count and pneumonia onset in elderly nursing home residents. Geriatr. Gerontol. Int. 2015, 15, 417–421. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsuo, K.; Okamoto, M.; Nakata, H.; Sakamoto, H.; Fujita, M. Perioperative changes in oral bacteria number in patients undergoing cardiac valve surgery. J. Oral Sci. 2019, 61, 526–528. [Google Scholar] [CrossRef]
- Inai, Y.; Nomura, Y.; Takarada, T.; Hanada, N.; Wada, N. Risk factors for postoperative pneumonia according to examination findings before surgery under general anesthesia. Clin. Oral Investig. 2020, 24, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ihara, S.; Nishimatsu, K.; Komuta, K. Low Body Mass Index Is an Independent Prognostic Factor in Patients With Non-Small Cell Lung Cancer Treated With Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. World J. Oncol. 2019, 10, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Yoshida, N.; Kawai, H.; Obuchi, S.; Yoshida, H.; Mataki, S.; Hirano, H.; Watanabe, Y. Development of an oral health-related self-efficacy scale for use with older adults. Geriatr. Gerontol. Int. 2017, 17, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, T.; Tamura, F.; Takahashi, Y.; Konishi, K.; Hamada, R. A novel rapid oral bacteria detection apparatus for effective oral care to prevent pneumonia. Gerodontology 2012, 29, e560–e565. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, Y.; Hiraki, T.; Gobara, H.; Iguchi, T.; Fujiwara, H.; Matsui, Y.; Toyooka, S.; Soh, J.; Kiura, K.; Kanazawa, S. Fever after lung radiofrequency ablation: Prospective evaluation of its incidence and associated factors. Eur. J. Radiol. 2015, 84, 2202–2209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef]
- Gubens, M.A.; Davies, M. NCCN Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 574–578. [Google Scholar] [CrossRef]
- Zhong, L.; Suo, J.; Wang, Y.; Han, J.; Zhou, H.; Wei, H.; Zhu, J. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J. Surg. Oncol. 2020, 18, 27. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Loo, B.W.; Akerley, W.; Attia, A.; Bassetti, M.; Boumber, Y.; Decker, R.; Dobelbower, M.C.; Dowlati, A.; Downey, R.J.; et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 1171–1182. [Google Scholar] [CrossRef]
- Bunetel, L.; Tamanai-Shacoori, Z.; Martin, B.; Autier, B.; Guiller, A.; Bonnaure-Mallet, M. Interactions between oral commensal Candida and oral bacterial communities in immunocompromised and healthy children. J. Mycol. Med. 2019, 29, 223–232. [Google Scholar] [CrossRef]
- Inoue, M.; Okumura, M.; Sawabata, N.; Miyaoka, E.; Asamura, H.; Yoshino, I.; Tada, H.; Fujii, Y.; Nakanishi, Y.; Eguchi, K.; et al. Clinicopathological characteristics and surgical results of lung cancer patients aged up to 50 years: The Japanese Lung Cancer Registry Study 2004. Lung Cancer 2014, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ozcaka, O.; Basoglu, O.K.; Buduneli, N.; Tasbakan, M.S.; Bacakoglu, F.; Kinane, D.F. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: A randomized clinical trial. J. Periodontal Res. 2012, 47, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.M.; Rose, L.; Carbone, S.; Smith, O.M.; Burry, L.; Fan, E.; Amaral, A.C.K.; McCredie, V.A.; Pinto, R.; Quinonez, C.R.; et al. Protocol for a multi-centered, stepped wedge, cluster randomized controlled trial of the de-adoption of oral chlorhexidine prophylaxis and implementation of an oral care bundle for mechanically ventilated critically ill patients: The CHORAL study. Trials 2019, 20, 603. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Matsuo, K.; Suzuki, H.; Yoshihara, A. Perioperative changes in knowledge and attitude toward oral health by oral health education. Oral Dis. 2019, 25, 1214–1220. [Google Scholar] [CrossRef]
- Tanda, N.; Hoshikawa, Y.; Sato, T.; Takahashi, N.; Koseki, T. Exhaled acetone and isoprene in perioperative lung cancer patients under intensive oral care: Possible indicators of inflammatory responses and metabolic changes. Biomed. Res. 2019, 40, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rotman, J.A.; Plodkowski, A.J.; Hayes, S.A.; de Groot, P.M.; Shepard, J.A.; Munden, R.F.; Ginsberg, M.S. Postoperative complications after thoracic surgery for lung cancer. Clin. Imaging 2015, 39, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M. Correction to: Preoperative predictors of postoperative complications after gastric cancer resection. Surg. Today 2020, 50, 321. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.; Tokmak, A.; Guzel, A.I.; Aksoy, R.T.; Ozer, I.; Yilmaz, N.; Doganay, M. Association of clinical outcomes and complications with obesity in patients who have undergone abdominal myomectomy. J. Chin. Med. Assoc. 2016, 79, 435–439. [Google Scholar] [CrossRef]
- Aghi, M.K.; Eskandar, E.N.; Carter, B.S.; Curry, W.T., Jr.; Barker, F.G., 2nd. Increased prevalence of obesity and obesity-related postoperative complications in male patients with meningiomas. Neurosurgery 2007, 61, 754–760, discussion 751–760. [Google Scholar] [CrossRef]
- Harmanli, O.; Esin, S.; Knee, A.; Jones, K.; Ayaz, R.; Tunitsky, E. Effect of obesity on perioperative outcomes of laparoscopic hysterectomy. J. Reprod. Med. 2013, 58, 497–503. [Google Scholar]
- Walid, M.S.; Sahiner, G.; Robinson, C.; Robinson, J.S., 3rd; Ajjan, M.; Robinson, J.S., Jr. Postoperative fever discharge guidelines increase hospital charges associated with spine surgery. Neurosurgery 2011, 68, 945–949, discussion 949. [Google Scholar] [CrossRef]
- Ikebe, K.; Matsuda, K.; Morii, K.; Nokubi, T.; Ettinger, R.L. The relationship between oral function and body mass index among independently living older Japanese people. Int. J. Prosthodont. 2006, 19, 539–546. [Google Scholar]
- Munro, J.; Booth, A.; Nicholl, J. Routine preoperative testing: A systematic review of the evidence. Health Technol. Assess. 1997, 1, i. [Google Scholar] [CrossRef]
- Farup, P.G. Are the Results of a Combined Behavioural and Surgical Treatment of Morbid Obesity Satisfactory and Predictable? Nutrients 2020, 12, 1997. [Google Scholar] [CrossRef]
- Sakashita, R.; Sato, T.; Ono, H.; Hamaue, A.; Hamada, M. Impact of the Consistency of Food Substances on Health and Related Factors of Residents in Welfare Facilities for Seniors in Japan. Dent. J. 2020, 8, 9. [Google Scholar] [CrossRef]
- Hollaar, V.; van der Maarel-Wierink, C.; van der Putten, G.J.; de Swart, B.; de Baat, C. Effect of daily application of a 0.05% chlorhexidine solution on the incidence of (aspiration) pneumonia in care home residents: Design of a multicentre cluster randomised controlled clinical trial. BMJ Open 2015, 5, e007889. [Google Scholar] [CrossRef]
- Suma, S.; Naito, M.; Wakai, K.; Naito, T.; Kojima, M.; Umemura, O.; Yokota, M.; Hanada, N.; Kawamura, T. Tooth loss and pneumonia mortality: A cohort study of Japanese dentists. PLoS ONE 2018, 13, e0195813. [Google Scholar] [CrossRef]
| Characteristics | N (%), Mean (SD) or Median (IQR) | ||||
|---|---|---|---|---|---|
| All Data (n = 441) | Body Mass Index Categories | ||||
| Underweight (n = 54) BMI < 18.5 kg/m2 | Normal Weight (n = 286) 18.5 kg/m2 ≤ BMI < 25.0 kg/m2 | Overweight (n = 101) 25.0 kg/m2 ≤ BMI | |||
| Gender | Male | 276 (62.6) | 35 (64.8) | 171 (59.8) | 70 (69.3) |
| Female | 165 (37.4) | 19 (35.2) | 115 (40.2) | 31 (30.7) | |
| Age (years) | 71.0 (64.0–76.0) | 73.0 (68.0–78.0) | 70.5 (64–75.3) | 69.0 (62.5–76.0) | |
| Body mass index (kg/m2) | 22.6 (20.3–24.8) | 17.3 (16.2–17.9) | 22.1 (20.6–23.5) | 26.6 (25.6–28.1) | |
| Performance status | 0 | 422 (95.7) | 49 (90.7) | 277 (96.9) | 96 (95.0) |
| 1 | 11 (2.5) | 3 (5.6) | 4 (1.4) | 4 (4.0) | |
| 2 | 5 (1.1) | 1 (1.9) | 4 (1.4) | 0 (0) | |
| 3 | 2 (0.5) | 0 (0) | 1 (0.3) | 1 (1.0) | |
| 4 | 1 (0.2) | 1 (1.9) | 0 (0) | 0 (0) | |
| Brinkman index | 450.0 (0–1000) | 380.0 (0–1025.0) | 420.0 (0–936.3) | 600.0 (0–1157.5) | |
| Housemate (number) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | |
| Type of lung cancer | Non-small cell carcinoma | 364 (82.5) | 46 (85.2) | 241 (84.3) | 78 (77.2) |
| Small cell carcinoma | 8 (1.8) | 0 (0) | 2 (0.7) | 6 (5.9) | |
| Other | 69 (15.6) | 8 (14.8) | 43 (15.0) | 17 (16.8) | |
| Cancer stage | 1 | 312 (70.7) | 35 (64.8) | 204 (71.3) | 73 (72.3) |
| 2 | 68 (15.4) | 11 (20.4) | 44 (15.4) | 13 (12.9) | |
| 3 | 57 (12.9) | 8 (14.8) | 36 (12.6) | 13 (12.9) | |
| 4 | 4 (0.9) | 0 (0) | 2 (0.7) | 2 (2.0) | |
| Number of teeth | 22.0 (10.0–28.0) | 19.5 (4.5–27.0) | 23.5 (10.0–28.0) | 22.0 (7.5–28.0) | |
| Dentures (yes) | 198 (44.9) | 29 (53.7) | 124 (43.4) | 45 (44.6) | |
| Home dentist (yes) | 72 (16.3) | 35 (64.8) | 176 (61.5) | 53 (52.5) | |
| Oral bacteria count at pre-hospitalization | (106 CFU/mL) | 33.5 (25.0) | 31.6 (24.2) | 34.6 (25.2) | 31.2 (24.9) |
| Level | 5.3 (0.9) | 5.2 (0.9) | 5.3 (0.9) | 5.3 (0.8) | |
| Oral bacteria count at pre-operation | (106 CFU/mL) | 17.9 (16.8) | 17.0 (18.2) | 18.6 (18.0) | 16.1 (11.5) |
| Level | 4.6 (1.0) | 4.5 (1.0) | 4.6 (1.1) | 4.6 (0.8) | |
| Oral bacteria count at post-operation | (106 CFU/mL) | 15.8 (15.0) | 17.9 (19.2) | 16.2 (14.9) | 13.5 (12.3) |
| Level | 4.5 (1.0) | 4.5 (1.1) | 4.6 (1.0) | 4.5 (0.8) | |
| White blood cell count at pre-operation | 103/μL | 6.0 (4.9–7.1) | 5.6 (4.6–6.8) | 6.0 (4.9–7.1) | 6.1 (5.0–7.0) |
| Serum albumin value at pre-operation | g/dL | 4.2 (3.9–4.4) | 4.1 (3.9–4.4) | 4.2 (3.9–4.4) | 4.2 (3.9–4.4) |
| Estimated duration of hospitalization (days) | 14.0 (7.0–14.0) | 14.0 (7.0–14.0) | 14.0 (7.0–14.0) | 14.0 (7.0–14.0) | |
| Duration of hospitalization (days) | 10.0 (9.0–14.0) | 11.0 (8.0–15.0) | 10.0 (9.0–14.0) | 10.0 (8.0–13.0) | |
| Operation time (minutes) | 223.0 (142.0–272.0) | 218.5 (153.3–260.8) | 226.0 (143.0–273.0) | 211.0 (132.5–276.0) | |
| Fever (yes) | 367 (83.2) | 47 (87.0) | 244 (85.3) | 76 (75.2) | |
| Duration of fever (days) | 2.0 (1.0–4.0) | 3.0 (2.0–6.0) | 2.5 (1.0–4.0) | 2.0 (1.0–3.5) | |
| Univariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Explanatory Variable | All Data | Body Mass Index Categories | ||||||
| Underweight BMI < 18.5 kg/m2 | Normal Weight 18.5 kg/m2 ≤ BMI < 25.0 kg/m2 | Overweight 25.0 kg/m2 ≤ BMI | ||||||
| Odds Ratio (CI) | p-Values | Odds Ratio (CI) | p-Values | Odds Ratio (CI) | p-Values | Odds Ratio (CI) | p-Values | |
| Age | 0.96 (0.93–1.00) | 0.05 | ||||||
| Body mass index (kg/m2) | 0.92 (0.85–0.99) | 0.02 | ||||||
| PS | 0.39 (0.22–0.70) | <0.01 | 0.38 (0.12–1.10) | 0.08 | 0.49 (0.23–1.06) | 0.07 | 0.09 (0.009–0.90) | 0.04 |
| Housemate (number) | 0.64 (0.46–0.90) | 0.01 | 0.59 (0.38–0.92) | 0.02 | ||||
| Number of teeth | 0.91 (0.82–1.02) | 0.10 | 1.03 (1.00–1.06) | 0.09 | 0.96 (0.91–1.00) | 0.06 | ||
| Oral bacteria count at pre-hospitalization | 0.72 (0.53–0.99) | 0.04 | ||||||
| White blood cell count at pre-operation | 0.83 (0.74–0.94) | <0.01 | 0.79 (0.69–0.92) | <0.01 | ||||
| Serum albumin value at pre-operation | 0.08 (0.005–1.24) | 0.07 | ||||||
| Operation time (minutes) | 1.00 (0.999–1.01) | 0.10 | ||||||
| Multivariate analysis | ||||||||
| Body mass index (kg/m2) | 0.92 (0.85–0.99) | 0.03 | ||||||
| PS | 0.38 (0.21–0.70) | <0.01 | 0.04 (0.003–0.67) | 0.02 | 0.05 (0.004–0.62) | 0.02 | ||
| Housemate (number) | 0.60 (0.42–0.86) | <0.01 | 0.58 (0.37–0.92) | 0.02 | ||||
| Number of teeth | 0.73 (0.53–0.99) | 0.05 | 0.94 (0.89–0.99) | 0.03 | ||||
| White blood cell count at pre-operation | 0.86 (0.75–0.98) | 0.02 | 0.78 (0.67–0.91) | <0.01 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itohara, C.; Matsuda, Y.; Sukegawa-Takahashi, Y.; Sukegawa, S.; Furuki, Y.; Kanno, T. Relationship between Oral Health Status and Postoperative Fever among Patients with Lung Cancer Treated by Surgery: A Retrospective Cohort Study. Healthcare 2020, 8, 405. https://doi.org/10.3390/healthcare8040405
Itohara C, Matsuda Y, Sukegawa-Takahashi Y, Sukegawa S, Furuki Y, Kanno T. Relationship between Oral Health Status and Postoperative Fever among Patients with Lung Cancer Treated by Surgery: A Retrospective Cohort Study. Healthcare. 2020; 8(4):405. https://doi.org/10.3390/healthcare8040405
Chicago/Turabian StyleItohara, Chieko, Yuhei Matsuda, Yuka Sukegawa-Takahashi, Shintaro Sukegawa, Yoshihiko Furuki, and Takahiro Kanno. 2020. "Relationship between Oral Health Status and Postoperative Fever among Patients with Lung Cancer Treated by Surgery: A Retrospective Cohort Study" Healthcare 8, no. 4: 405. https://doi.org/10.3390/healthcare8040405
APA StyleItohara, C., Matsuda, Y., Sukegawa-Takahashi, Y., Sukegawa, S., Furuki, Y., & Kanno, T. (2020). Relationship between Oral Health Status and Postoperative Fever among Patients with Lung Cancer Treated by Surgery: A Retrospective Cohort Study. Healthcare, 8(4), 405. https://doi.org/10.3390/healthcare8040405






