Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients
Abstract
1. Introduction
2. Materials and Methods
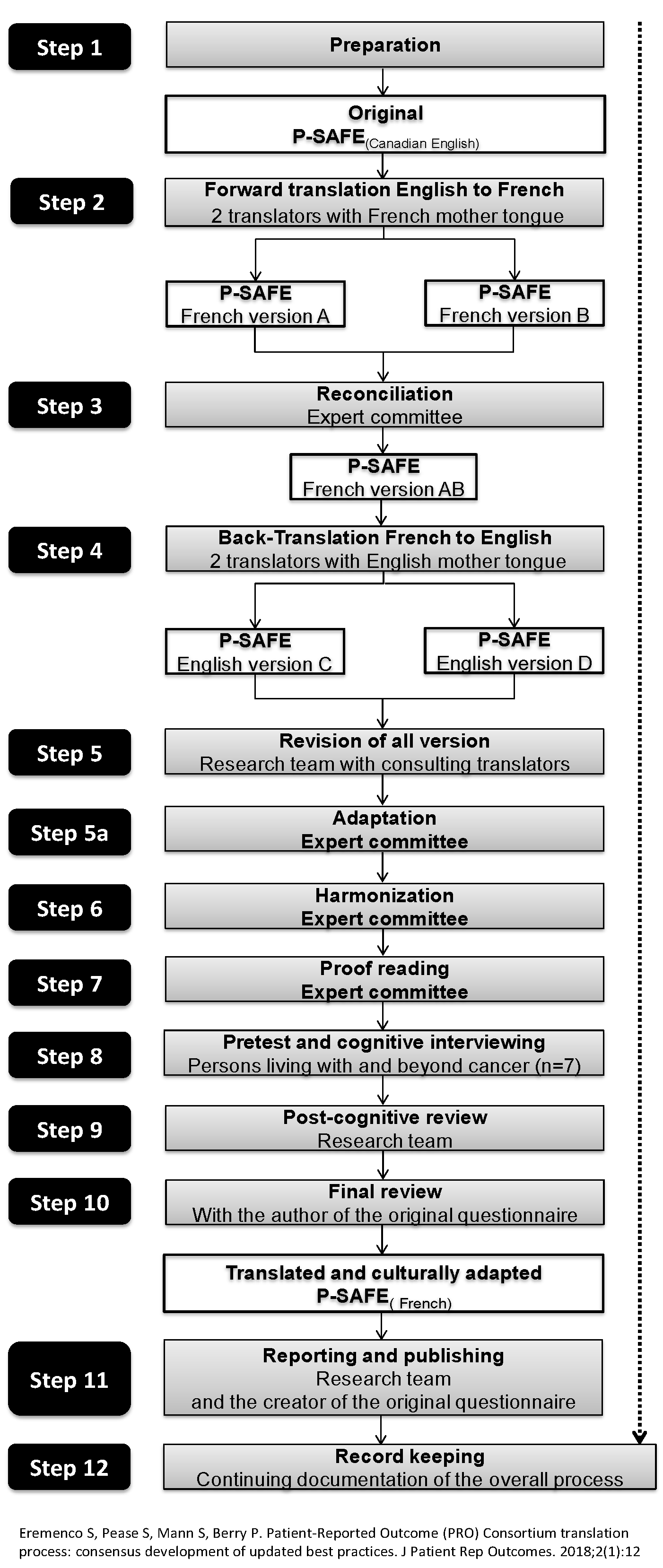
2.1. Steps in the Process of Translation and Cultural Adaptation
2.1.1. Steps 1 to 3: Preparation, Forward Translation and Reconciliation
2.1.2. Step 4: Back-Translation
2.1.3. Steps 5 to 7: Review of All Versions, Adaptation, Harmonization and Proofreading
2.1.4. Steps 8 to 10: Pre-Test, Cognitive Interviewing and Post-Cognitive Interviewing
2.2. Setting, Recruitment and Participants
2.3. Procedure
2.4. Data Analysis
3. Results
Perspective of People Living with and beyond Cancer (PLC)
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical and Research Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Cmaj. 2020, 192, E199–E205. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [PubMed]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2019; p. 95. [Google Scholar]
- Fitch, M.I. Supportive Care Framework: Theoretical Underpinnings. In Supportive Care Framework: A Foundation for Person-Centred Care; Fitch, H.B.M.I., Page, B.D., Eds.; Pappin Communications: Pembroke, ON, Canada, 2008. [Google Scholar]
- Jacobs, A.L.; Shulman, L.N. Follow-up care of cancer survivors: Challenges and solutions. Lancet Oncol. 2017, 18, e19–e29. [Google Scholar] [PubMed]
- Lentz, R.; Benson, A.B., 3rd; Kircher, S. Financial toxicity in cancer care: Prevalence, causes, consequences, and reduction strategies. J. Surg. Oncol. 2019, 120, 85–92. [Google Scholar]
- Pearce, A.; Tomalin, B.; Kaambwa, B.; Horevoorts, N.; Duijts, S.; Mols, F.; van de Poll-Franse, L.; Koczwara, B. Financial toxicity is more than costs of care: The relationship between employment and financial toxicity in long-term cancer survivors. J. Cancer Surviv. 2019, 13, 10–20. [Google Scholar]
- O’Connor, J.M.; Kircher, S.M.; de Souza, J.A. Financial toxicity in cancer care. J. Community Support. Oncol. 2016, 14, 101–106. [Google Scholar]
- Bilodeau, K.; Tremblay, D.; Durand, M.-J. Exploration of return-to-work interventions for breast cancer patients: A scoping review. Support. Care Cancer 2017, 25, 1993–2007. [Google Scholar]
- Longo, C.; Fitch, M.; Grignon, M.; McAndrew, A. Understanding the full breadth of cancer-related patient costs in Ontario: A qualitative exploration. Support. Care Cancer 2016, 24, 4541–4548. [Google Scholar]
- Canadian Cancer Society. Five-Year Action Plan to Address the Financial Hardship of Cancer in Canada: A Call for Action. In Canadian Cancer Action Network and Canadian Cancer Society; Manitoba, D., Ed.; Canadian Cancer Society: Toronto, ON, Canada, 2012. [Google Scholar]
- Zafar, S.Y. Financial toxicity of cancer care: It’s time to intervene. J. Natl. Cancer Inst. 2016, 108, djv370. [Google Scholar]
- De Souza, J.A.; Wong, Y.N. Financial distress in cancer patients. J. Med. Person 2013, 11, 73–77. [Google Scholar]
- Ver Hoeve, E.S.; Ali-Akbarian, L.; Price, S.N.; Lothfi, N.M.; Hamann, H.A. Patient-reported financial toxicity, quality of life, and health behaviors in insured US cancer survivors. Support. Care Cancer 2020. [Google Scholar] [CrossRef]
- Azzani, M.; Roslani, A.C.; Su, T.T. The perceived cancer-related financial hardship among patients and their families: A systematic review. Support. Care Cancer 2015, 23, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Merollini, K.M.; Lowe, A.; Chan, R.J. A systematic review of financial toxicity among cancer survivors: We can’t pay the co-pay. Patient 2017, 10, 295–309. [Google Scholar] [CrossRef]
- Chen, J.; Ou, L.; Hollis, S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 2013, 13, 211. [Google Scholar] [CrossRef]
- Perrone, F.; Jommi, C.; Di Maio, M.; Gimigliano, A.; Gridelli, C.; Pignata, S.; Ciardiello, F.; Nuzzo, F.; de Matteis, A.; Del Mastro, L.; et al. The association of financial difficulties with clinical outcomes in cancer patients: Secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann. Oncol. 2016, 27, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Bhoo-Pathy, N.; Ng, C.W.; Lim, G.C.; Tamin, N.S.I.; Sullivan, R.; Bhoo-Pathy, N.T.; Abdullah, M.M.; Kimman, M.; Subramaniam, S.; Saad, M.; et al. Financial toxicity after cancer in a setting with universal health coverage: A call for urgent action. J. Oncol. Pract. 2019, 15, e537–e546. [Google Scholar] [CrossRef]
- Newton, J.C.; Johnson, C.E.; Hohnen, H.; Bulsara, M.; Ives, A.; McKiernan, S.; Platt, V.; McConigley, R.; Slavova-Azmanova, N.S.; Saunders, C. Out-of-pocket expenses experienced by rural Western Australians diagnosed with cancer. Support. Care Cancer 2018, 26, 3543–3552. [Google Scholar] [CrossRef]
- Altice, C.K.; Banegas, M.P.; Tucker-Seeley, R.D.; Yabroff, K.R. Financial hardships experienced by cancer survivors: A systematic review. J. Natl. Cancer Inst. 2017, 109, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef]
- De Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araujo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Zhao, J.; Han, X.; Zheng, Z. Prevalence and Correlates of Medical Financial Hardship in the USA. J. Gen. Int. Med. 2019, 34, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.M.; Hoerger, M.; Seibert, K.; Gerhart, J.I.; O’Mahony, S.; Duberstein, P.R. Financial strain and physical and emotional quality of life in breast cancer. J. Pain Symptom Manag. 2019, 58, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Miller-Sonet, E.; Gardner, D.; Epstein, I. Exploring the role of psychosocial care in value-based oncology: Results from a survey of 3000 cancer patients and survivors. J. Psychosoc. Oncol. 2019, 37, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Ezeife, D.A.; Morganstein, B.J.; Lau, S.; Law, J.H.; Le, L.W.; Bredle, J.; Cell, D.; Doherty, M.K.; Bradbury, P.; Liu, G.; et al. Financial burden among patients with lung cancer in a publically funded health care system. Clin. Lung Cancer 2019, 20, 231–236. [Google Scholar] [CrossRef]
- Nipp, R.D.; Zullig, L.L.; Samsa, G.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H., Jr.; Abernethy, A.P.; Zafar, S.Y. Identifying cancer patients who alter care or lifestyle due to treatment-related financial distress. Psychooncology 2016, 25, 719–725. [Google Scholar] [CrossRef]
- Schroder, S.L.; Schumann, N.; Fink, A.; Richter, M. Coping mechanisms for financial toxicity: A qualitative study of cancer patients’ experiences in Germany. Support. Care Cancer 2020, 28, 1131–1139. [Google Scholar] [CrossRef]
- Honda, K.; Gyawali, B.; Ando, M.; Kumanishi, R.; Kato, K.; Sugiyama, K.; Mitani, S.; Masuishi, T.; Narita, Y.; Bando, H.; et al. Prospective survey of financial toxicity measured by the comprehensive score for financial toxicity in japanese patients with cancer. J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Longo, C.J. Patient Self-Administered Financial Effects Study. 2019. Available online: https://psafe.mcmaster.ca/ (accessed on 23 September 2020).
- Longo, C.J.; Deber, R.; Fitch, M.; Williams, A.P.; D’souza, D. An examination of cancer patients’ monthly ‘out-of-pocket’costs in Ontario, Canada. Eur. J. Cancer Care 2007, 16, 500–507. [Google Scholar]
- Statistics Canada. Census in Brief: English, French and Official Language Minorities in Canada. 2017. Available online: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/98-200-x/2016011/98-200-x2016011-eng.cfm (accessed on 4 August 2020).
- Banegas, M.P.; Newton, J.C.; Hohnen, H.; Johnson, C.E.; Saunders, C. Out of pocket, out of sight? An unmeasured component of the burden of cancer. JNCI 2013, 105, 252–253. [Google Scholar] [CrossRef][Green Version]
- Fitch, M.I.; Longo, C.J.; Chan, R.J. Cancer patients’ perspectives on financial burden in a universal healthcare system: Analysis of qualitative data from participants from 20 provincial cancer centers in Canada. Patient Educ. Couns. 2020, in press. [Google Scholar]
- Slavova-Azmanova, N.; Newton, J.C.; Hohnen, H.; Johnson, C.E.; Saunders, C. How communication between cancer patients and their specialists affect the quality and cost of cancer care. Support. Care Cancer 2019, 27, 4575–4585. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, R.J. Vers une méthodologie de validation transculturelle de questionnaires psychologiques: Implications pour la recherche en langue française. Psychol. Can. 1989, 30, 662–689. [Google Scholar] [CrossRef]
- Eremenco, S.; Pease, S.; Mann, S.; Berry, P. Patient-reported outcome (PRO) consortium translation process: Consensus development of updated best practices. J. Patient Rep. Outcomes 2018, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Brislin, R.W. Research instruments. In Field Methods in Cross-Cultural Research; Lonner, W.J., Berry, J.W., Eds.; SAGE Publications: Beverly Hills, CA, USA, 1986; pp. 159–162. [Google Scholar]
- Tremblay, D.; Bilodeau, K.; Durand, M.-J.; Coutu, M.-F. Translation and perceptions of the French version of the Cancer Survivor Profile-Breast Cancer (CSPro-BC): A tool to identify and manage unmet needs. J. Cancer Surviv. 2019, 13, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Poder, T.G.; Carrier, N.; Mead, H.; Stevens, K.J. Canadian French translation and linguistic validation of the child health utility 9D (CHU9D). Health Qual. Life Outcomes 2018, 16, 168. [Google Scholar] [CrossRef]
- Vasiliadis, H.M.; Dezetter, A.; Latimer, E.; Drapeau, M.; Lesage, A. Assessing the costs and benefits of insuring psychological services as part of Medicare for depression in Canada. Psychiatr. Serv. 2017, 68, 899–906. [Google Scholar] [CrossRef]
- Tremblay, D.; Touati, N.; Poder, T.; Vasiliadis, H.-M.; Bilodeau, K.; Berbiche, D.; Denis, J.-L.; Pomey, M.-P.; Hébert, J.; Roch, G.; et al. Collaborative governance in the Quebec Cancer Network: A realist evaluation of emerging mechanisms of institutionalization, multi-level governance, and value creation using a longitudinal multiple case study design. BMC Health Serv. Res. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Jääskeläinen, R. Think-aloud protocol. Handb. Transl. Stud. 2010, 1, 371–374. [Google Scholar]
- Epstein, J.; Santo, R.M.; Guillemin, F. A review of guidelines for cross-cultural adaptation of questionnaires could not bring out a consensus. J. Clin. Epidemiol. 2015, 68, 435–441. [Google Scholar] [CrossRef]
- McCabe, M.S.; Partridge, A.H.; Grunfeld, E.; Hudson, M.M. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin. Oncol. 2013, 40, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, A.; Lee, S.S.; Hilton, K.; Ganster, B.; Trupp, R.; McCullough, C.; Mott, E.; Feuerstein, M. Development of the Cancer Survivor Profile-Breast Cancer (CSPro-BC) app: Patient and nurse perspectives on a new navigation tool. J. Cancer Surviv. 2018, 12, 291–305. [Google Scholar] [CrossRef] [PubMed]
| Question Number | Comment | Issue | Decision |
|---|---|---|---|
| All | Expression: “Your cancer” | Appropriateness for people living with and beyond cancer | Replace “your cancer” by “a cancer” or just “cancer” |
| All | Terminology specific to the clinical context | Semantic equivalence | Verify the accuracy and adapt where needed |
| All | Passages using different phrasing to communicate the same meaning | Conceptual equivalence | Reconcile by retaining phrasings closer to those used in the original version |
| Q1 | Terminology specific to insurance | Item equivalence | Verify the accuracy and adapt as needed |
| Q2 | Terminology specific to insurance for life-threatening disease | Item equivalence | Verify the accuracy and adapt as needed |
| Q4 | Uneven phrasing in subquestions 4a to 4j | Semantic equivalence and uniformity | Uniformize the phrasing |
| Q9 | Negative phrasing: “lesser value housing”/“maison de valeur moindre” | Appropriateness for persons living with and beyond cancer | Replace by a positive phrasing: “more affordable housing”/“maison plus abordable” |
| Q12 and Q13 | Designation of the participant in a column of the table: “patient” | Appropriateness for persons living with and beyond cancer | Replace “patient” by “you” |
| Q15 | Choice of income categories | Comparability with national survey instruments | Harmonize with Statistics Canada categories |
| N.A. | Maladapted coping strategies remain unaddressed | Clinical relevance | Add a question: Have you or any of your caregivers taken any of the following actions for financial reasons?
|
| Characteristics | n | (%) |
|---|---|---|
| Gender | ||
| Men | 4 | 0.57 |
| Women | 3 | 0.43 |
| Education level | ||
| University | 2 | 0.29 |
| College | 4 | 0.57 |
| High school | 1 | 0.14 |
| Tumour site | ||
| Colorectal | 3 | 0.43 |
| Lung | 2 | 0.29 |
| Breast | 1 | 0.14 |
| Prostate | 1 | 0.14 |
| With metastases | 2 * | 0.29 |
| Age group | ||
| 50–59 | 4 | 0.57 |
| 60–69 | 2 | 0.29 |
| 70 and above | 1 | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay, D.; Poder, T.G.; Vasiliadis, H.-M.; Touati, N.; Fortin, B.; Lévesque, L.; Longo, C. Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients. Healthcare 2020, 8, 366. https://doi.org/10.3390/healthcare8040366
Tremblay D, Poder TG, Vasiliadis H-M, Touati N, Fortin B, Lévesque L, Longo C. Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients. Healthcare. 2020; 8(4):366. https://doi.org/10.3390/healthcare8040366
Chicago/Turabian StyleTremblay, Dominique, Thomas G. Poder, Helen-Maria Vasiliadis, Nassera Touati, Béatrice Fortin, Lise Lévesque, and Christopher Longo. 2020. "Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients" Healthcare 8, no. 4: 366. https://doi.org/10.3390/healthcare8040366
APA StyleTremblay, D., Poder, T. G., Vasiliadis, H.-M., Touati, N., Fortin, B., Lévesque, L., & Longo, C. (2020). Translation and Cultural Adaptation of the Patient Self-Administered Financial Effects (P-SAFE) Questionnaire to Assess the Financial Burden of Cancer in French-Speaking Patients. Healthcare, 8(4), 366. https://doi.org/10.3390/healthcare8040366








