A Systematic Review of Healthcare-Associated Infectious Organisms in Medical Radiation Science Departments
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Selection Criteria
2.4. Definitions
- Medical radiation scientists were defined as healthcare professionals that perform diagnostic imaging studies or plan and deliver radiation treatments to patients. Australian titles for these professionals include diagnostic radiographers, nuclear medicine scientists, and radiation therapists.
- Healthcare-associated infections (HAIs) comprise of infections acquired by persons in a healthcare setting [4]. These include bloodstream infections and organism-specific infections (such as methicillin-resistant Staphylococcus aureus and Clostridium difficile) that were defined as being associated with healthcare in the literature [4].
- Occupational-associated infections (OAIs) refer to infections that healthcare professionals acquire or are at risk of contracting from occupational exposure to infectious organisms within their departments.
2.5. Study Selection
2.6. Data Extraction
2.7. Risk of Bias
2.8. Data Analysis
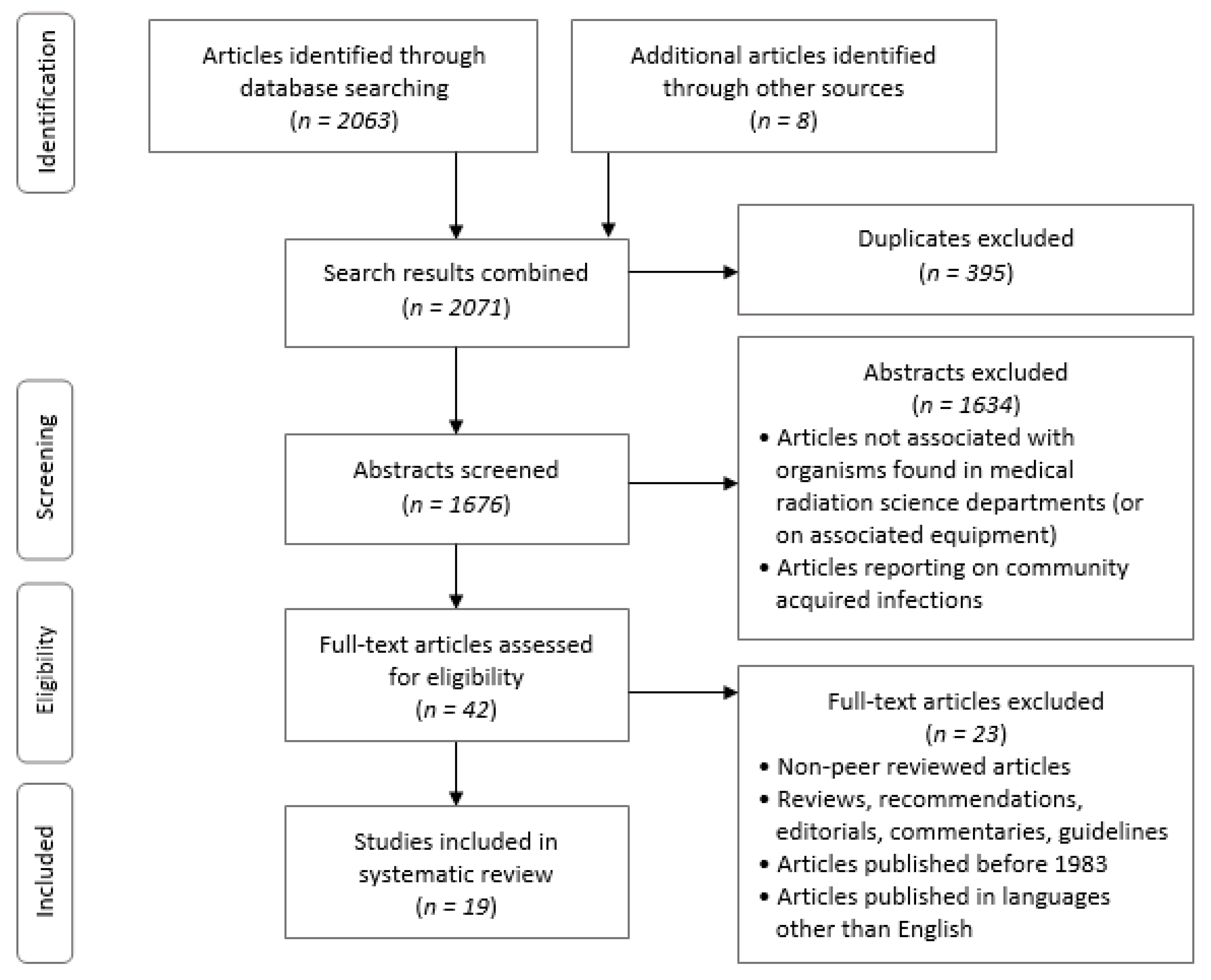
3. Results
3.1. Overview
3.2. Characteristics of Included Studies
3.3. Infectious Organisms
3.4. Contamination of Equipment
3.5. Methodological Quality
4. Discussion
4.1. Infectious Organisms
4.2. Healthcare Equipment as a Reservoir for Microorganisms
4.3. Transmission of Occupational-Associated Infections
4.4. Limitations
4.5. Research Gaps and Avenues for Further Research
- No studies have been published on infectious organisms within nuclear medicine departments, thus the risks of exposure for nuclear medicine staff and the potential of nuclear medicine equipment to be vectors for OAIs remains unknown. Further research needs to be conducted to address this gap and assess the correlation to other MRS professions.
- No studies were undertaken and published from Australia or any South American countries. Further research needs to be undertaken to determine whether the infectious organisms identified within this study or different organisms are present in Australia and South America.
- The reservoirs of infectious microorganisms identified in the SR are the basis for experimental studies to assess the risk and burden of OAI exposure specifically in nuclear medicine departments and among MRS staff and in MRS equipment.
- The literature has not identified if there is common OAI exposure risks when comparing MRS staff and other allied health professionals OAI risk. Further empirical research needs to be undertaken within diagnostic radiography, nuclear medicine, and radiation therapy departments and to be compared to differing allied health professions.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organisation: Geneva, Switzerland, 2011; pp. 3–24.
- Microbial Evolution and Co-Adaptation: A Tribute to the Life and Scientific Legacies of Joshua Lederberg: Workshop Summary. In Proceedings of the Institute of Medicine (US) Forum on Microbial Threats, Washington, DC, USA, 20–21 May 2008.
- Bolyard, E.; Tablan, O.; Williams, W.; Pearson, M.; Shapiro, C.; Deitchman, S. Guideline for infection control in health care personnel. Am. J. Infect. Control 1998, 26, 289–327. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. (Eds.) Bacterial Classification, Structure, and Replication. In Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–118.e102. [Google Scholar] [CrossRef]
- Giacometti, M.; Gualano, M.R.; Bert, F.; Minniti, D.; Bistrot, F.; Grosso, M.; Siliquini, R. Microbiological contamination of radiological equipment. Acta Radiol. 2014, 55, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.G. Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008; Volume 2, p. 1403.
- Infection Prevention and Control Policy; NSW Health: Sydney, Australia, 2017.
- Nienhaus, A.; Kesavachandran, C.; Wendeler, D.; Haamann, F.; Dulon, M. Infectious diseases in healthcare workers—An analysis of the standardised data set of a German compensation board. J. Occup. Med. Toxicol. 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.G.; Gardner, A.; Stone, P.W.; Hall, L.; Pogorzelska-Maziarz, M. Hospital Staffing and Health Care-Associated Infections: A Systematic Review of the Literature. JT. Comm. J. Qual. Patient Saf. 2018, 44, 613–622. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Soule, B. The APIC Curriculum for Infection Control Practice; Kendall-Hunt: Dubuque, IA, USA, 1983. [Google Scholar]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataria, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk; The Joanna Briggs Institute: Adelaide, Austrilia, 2017. [Google Scholar]
- Akpochafor, M.O.; Eze, C.U.; Adeneye, S.O.; Ajekigbe, A.T. Assessment of ultrasound equipment as a possible source of nosocomial infection in Lagos state hospitals and radio-diagnostic centres. Radiography 2015, 21, 154–159. [Google Scholar] [CrossRef]
- Arora, U.; Devi, P.; Chadha, A.; Malhotra, S. Cellphones a modern stayhouse for bacterial pathogens. JK Sci. 2009, 11, 127–129. [Google Scholar]
- Bhat, S.; Sundeep Hegde, K.; Salian, S. Potential of mobile phones to serve as a reservoir in spread of nosocomial pathogens. Online J. Health Allied Sci. 2011, 10, 14. [Google Scholar]
- Boyle, H.; Strudwick, R.M. Do lead rubber aprons pose an infection risk? Radiography 2010, 16, 297–303. [Google Scholar] [CrossRef]
- Brewer, P.S.; Ravine, T.J.; Bru, S.E. Risk of Patient Infection From Heating Appliances Used to Produce Thermoplastic Immobilization Devices. Radiat. Ther. 2014, 23, 125–135. [Google Scholar]
- Denis, M.-A.; Ecochard, R.; Bernadet, A.; Forissier, M.F.; Porst, J.M.; Robert, O.; Volckmann, C.; Bergeret, A. Risk of occupational blood exposure in a cohort of 24,000 hospital healthcare workers: Position and environment analysis over three years. J. Occup. Environ. Med. 2003, 45, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Harvey, J.M. An investigation of infection control for X-ray cassettes in a diagnostic imaging department. Radiography 2008, 14, 306–311. [Google Scholar] [CrossRef]
- Hodges, A. Radiographic markers: Friend or fomite? Radiol. Technol. 2001, 73, 183–185. [Google Scholar] [PubMed]
- Kelly, J.; Trundle, C. Scissors: Are they an infection control risk? Prof. Nurse 2000, 16, 830–833. [Google Scholar] [PubMed]
- Kiran, H.; Aral, M.; Kıran, G.; Serin, S.; Arıkan, D.C.; Erkayıran, U.; Ekerbiçer, H.C. Bacterial contamination of ultrasound probes and coupling gels in a university hospital in Turkey. Eur. Res. J. 2018, 2149–3189. [Google Scholar] [CrossRef][Green Version]
- LaBan, M.M.; Singh, J.; Moll, V.; Zervos, M.J. Pertinacious habit on a rehabilitation unit: Repetitive finger licking while paging through the clinical chart. Am. J. Phys. Med. Rehabil. 2004, 83, 75–78. [Google Scholar] [CrossRef]
- Lawson, S.R.; Sauer, R.; Loritsch, M.B. Bacterial survival on radiographic cassettes. Radiol. Technol. 2002, 73, 507–510. [Google Scholar]
- Ochie, K.; Ohagwu, C. Contamination of X-Ray Equipment and Accessories with Nosocomial Bacteria and the Effectiveness of Common Disinfecting Agents. Afr. J. Basic Appl. Sci. 2009, 1, 31–35. [Google Scholar]
- Ohara, T.; Itoh, Y.; Itoh, K. Ultrasound instruments as possible vectors of staphylococcal infection. J. Hosp. Infect. 1998, 40, 73–77. [Google Scholar] [CrossRef]
- Ota, K.; Profiti, R.; Smaill, F.; Matlow, A.G.; Smieja, M. Identification badges: A potential fomite? Can. J. Infect. Control 2007, 22, 165–166. [Google Scholar]
- Ravine, T.J.; Brewer, P.; Bru, S.E.; Tyler, S. Attachment potential and survival of bacterial pathogens on radiation therapy thermoplastic immobilization forms. Radiat. Ther. 2017, 26, 127–139. [Google Scholar]
- Ridge, C. Sonographers and the fight against nosocomial infections: How are we doing? J. Diagn. Med. Sonogr. 2005, 21, 7–11. [Google Scholar] [CrossRef]
- Tugwell, J.; Maddison, A. Radiographic markers—A reservoir for bacteria? Radiography 2011, 17, 115–120. [Google Scholar] [CrossRef]
- Schabrun, S.; Chipchase, L. Healthcare equipment as a source of nosocomial infection: A systematic review. J. Hosp. Infect. 2006, 63, 239–245. [Google Scholar] [CrossRef]
- Petrisor, B.; Bhandari, M. The hierarchy of evidence: Levels and grades of recommendation. Indian J. Orthop. 2007, 41, 11–15. [Google Scholar] [CrossRef]
- Maczulak, A. Encyclopedia of Microbiology; Facts on File: New York, NY, USA, 2011; p. 858. [Google Scholar]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Schlosser, R.W.; Wendt, O.; Sigafoos, J. Not all systematic reviews are created equal: Considerations for appraisal. Evid. Based Commun. Assess. Interv. 2007, 1, 138–150. [Google Scholar] [CrossRef]
- Collins, J.A.; Fauser, B.C. Balancing the strengths of systematic and narrative reviews. Hum. Reprod. Update 2005, 11, 103–104. [Google Scholar] [CrossRef]
- Egger, M.; Dickersin, K.; Smith, G.D. Problems and limitations in conducting systematic reviews. In Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed.; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Publishing Group: London, UK, 2001; pp. 43–68. [Google Scholar]
- Concato, J. Observational versus experimental studies: What’s the evidence for a hierarchy? NeuroRx 2004, 1, 341–347. [Google Scholar] [CrossRef]
- Paul, M.; Leibovici, L. Observational studies examining patient management in infectious diseases. Clin. Microbiol. Infect. 2017, 23, 127–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
| # | Searches |
| 1 | healthcare associated infection/ |
| 2 | (“hospital acquired infection *” or “healthcare associated infection*” or hospital pathogens or “nosocomial”).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] |
| 3 | 1 or 2 |
| 4 | exp Allied Health Occupations/ or exp Allied Health Personnel/ |
| 5 | (“radiation therap *” or “radiotherap*” or “allied health” or “nuclear medicine” or “molecular imag*”).mp. or radiography/ or “medical imaging”.mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] |
| 6 | 4 or 5 |
| 7 | 3 and 6 |
| 8 | limit 7 to English language |
| 9 | Limit 8 to year = ”1983–2018” |
| Reference | Design | Sample | Setting | Equipment Examined | Infectious Organisms Identified (n) |
|---|---|---|---|---|---|
| Akpochafor et al. (2015) [14] | CO | 36 | DR (US) | Ultrasound probes, couches, coupling gel | P = 3 N = 3 F = 2 |
| Arora et al. (2009) [15] | CS | 160 | NS | Mobile phones | P = 6 N = 4 |
| Bhat et al. (2011) [16] | CS | 204 | NS | Mobile phones | P = 5 N = 7 |
| Boyle and Strudwick (2010) [17] | CS | 15 | DR | Lead rubber aprons | P = 4 |
| Brewer et al. (2014) [18] | CS | 24 | RT | Heating appliances | P = 3 |
| Denis et al. (2003) [19] | CO | 814 | DR | n/a | n/a |
| Fox and Harvey (2008) [20] | CS | 40 | DR | Radiographic cassettes | P = 4 |
| Giacometti et al. (2004) [6] | CS | 144 | DR | X-ray tubes, control panels, radiographic cassettes, imaging plates | n/a |
| Hodges (2001) [21] | CO | 10 | DR | Radiographic markers | P = 6 N = 4 NS = 1 |
| Kelly and Trundle (2014) [22] | CS | 100 | Nursing | Pocket scissors | P = 5 N = 1 |
| Kiran et al. (2018) [23] | CO | 98 | DR (US) | Ultrasound probes, coupling gel | P = 3 |
| LaBan et al. (2004) [24] | CS | 14 | RU | Patient charts | P = 1 |
| Lawson et al. (2002) [25] | QE | 3 | DR | Imaging cassettes | P = 2 N = 1 |
| Ochie and Ohagwu et al. (2009) [26] | CO | 301 | DR | X-ray couches, chest stands, radiographic cassettes, handles of x-ray tube heads, control panels, exposure buttons, patient x-ray gowns | P = 2 N = 2 |
| Ohara et al. (1998) [27] | QE | 9 | DR (US) | Ultrasound probes | P = 2 N = 2 |
| Ota et al. (2007) [28] | CS | 118 | NS | Identification badges | P = 6 N = 5 F = 1 |
| Ravine et al. (2017) [29] | QE | 4 | RT | Thermoplastic immobilization masks | P = 2 N = 2 |
| Ridge (2005) [30] | CS | 28 | DR (US) | Ultrasound probes, gel bottle tips, pressure cuffs | P = 4 N = 1 F = 1 |
| Tugwell and Maddison (2011) [31] | CS | 50 | DR | Radiographic markers | P = 4 |
| Gram-Positive (n = 7) | T | Gram-Negative (n = 10) | T | Fungi (n = 2) | T |
|---|---|---|---|---|---|
| Staphylococcus | C | Acinetobacter | C | Candida | B/C |
| Micrococcus | C | Pseudomonas | C | Cladosporium | C |
| Kocuria | B | Escherichia | C | ||
| Bacillus | A/C | Klebsiella | C | ||
| Streptococcus | A/C | Enterobacter | C | ||
| Enterococcus | C | Citrobacter | C | ||
| Corynebacterium | A/C | Proteus | C | ||
| Neisseria | A/C | ||||
| Moraxella | C | ||||
| Vibrio | C |
| Outcome Measure | Pooled Range | Pooled Mean |
|---|---|---|
| Percentage of contamination of equipment | 13.6–100 | 62.5 |
| Number of colony-forming units present on sampled equipment | 0–1000 | 82.6 |
| Number of different genera on sampled equipment | 1–10 | 5 |
| Study | Critical Appraisal Checklist Item Number | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Akpochafor et al. (2015) [14] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Arora et al. (2009) [15] | Y | Y | Y | Y | U | U | Y | Y | - | - | - | Y |
| Bhat et al. (2011) [16] | Y | Y | Y | Y | Y | Y | Y | Y | - | - | - | Y |
| Boyle and Strudwick (2010) [17] | Y | Y | N | Y | Y | Y | U | U | - | - | - | Y |
| Brewer et al. (2014) [18] | Y | Y | Y | Y | Y | Y | U | Y | - | - | - | Y |
| Denis et al. (2003) [19] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Fox and Harvey (2008) [20] | Y | Y | Y | Y | Y | Y | U | Y | - | - | - | Y |
| Giacometti et al. (2004) [6] | Y | Y | Y | Y | Y | Y | Y | Y | - | - | - | Y |
| Hodges (2001) [21] | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Kelly and Trundle (2014) [22] | Y | Y | Y | Y | Y | Y | Y | Y | - | - | - | Y |
| Kiran et al. (2018) [23] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| LaBan et al. (2004) [24] | Y | Y | U | Y | U | U | U | Y | - | - | - | U |
| Lawson et al. (2002) [25] | Y | Y | Y | Y | Y | Y | Y | U | Y | - | - | Y |
| Ochie and Ohagwu et al. (2009) [26] | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y |
| Ohara et al. (1998) [27] | Y | Y | Y | Y | Y | Y | Y | U | Y | - | - | Y |
| Ota et al. (2007) [28] | Y | Y | Y | Y | Y | Y | Y | Y | - | - | - | Y |
| Ravine et al. (2017) [29] | Y | Y | Y | Y | Y | Y | Y | U | Y | - | - | Y |
| Ridge (2005) [30] | Y | Y | Y | Y | Y | Y | Y | Y | - | - | - | Y |
| Tugwell and Maddison (2011) [31] | Y | Y | Y | Y | Y | Y | U | Y | - | - | - | Y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picton-Barnes, D.; Pillay, M.; Lyall, D. A Systematic Review of Healthcare-Associated Infectious Organisms in Medical Radiation Science Departments. Healthcare 2020, 8, 80. https://doi.org/10.3390/healthcare8020080
Picton-Barnes D, Pillay M, Lyall D. A Systematic Review of Healthcare-Associated Infectious Organisms in Medical Radiation Science Departments. Healthcare. 2020; 8(2):80. https://doi.org/10.3390/healthcare8020080
Chicago/Turabian StylePicton-Barnes, D’arcy, Manikam Pillay, and David Lyall. 2020. "A Systematic Review of Healthcare-Associated Infectious Organisms in Medical Radiation Science Departments" Healthcare 8, no. 2: 80. https://doi.org/10.3390/healthcare8020080
APA StylePicton-Barnes, D., Pillay, M., & Lyall, D. (2020). A Systematic Review of Healthcare-Associated Infectious Organisms in Medical Radiation Science Departments. Healthcare, 8(2), 80. https://doi.org/10.3390/healthcare8020080






