Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
2. Material and Methods
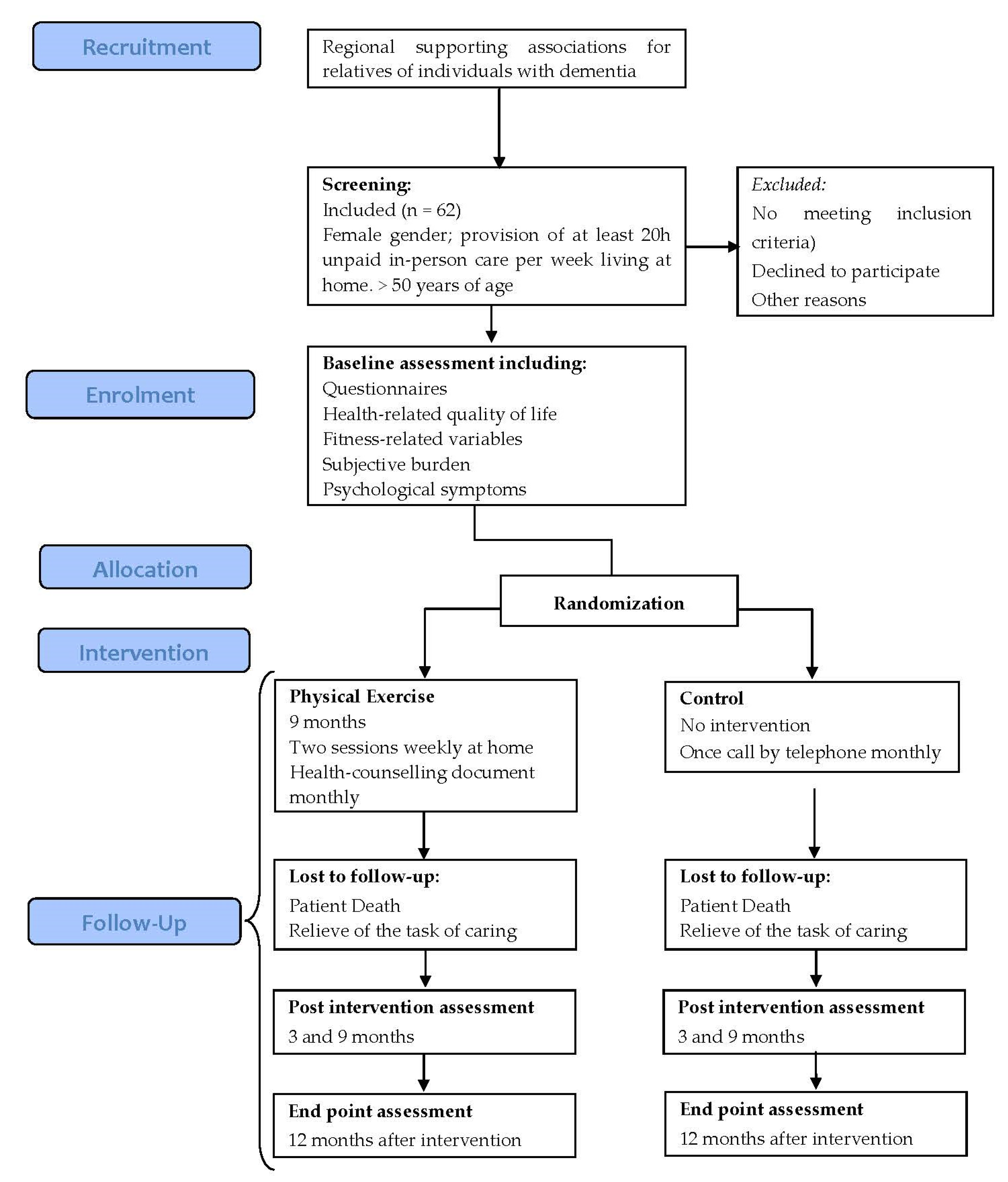
2.1. Design
2.2. Participants
2.2.1. Sample Size Calculation
2.2.2. Participants’ Randomization and Personal Data Management
2.3. Intervention
2.4. Measures
2.4.1. General Measures
2.4.2. Analysis of the Effects of the Intervention
2.5. Data Statistical Analysis
Cost Utility Analysis
3. Outcomes
3.1. Primary Outcomes
3.1.1. Health-Related Quality of Life and Cost-Utility
Cost-Utility: Cost Units
3.1.2. Subjective Burden
3.1.3. Depression
3.1.4. Psychological Symptomatology
3.1.5. Fitness
- Weight, height, and the circumference of the waist and hip will be assessed according to the recommendations established by the European Council [84] for the calculation of body mass index (BMI) and waist / hip ratio (WHR).
- Handgrip strength will be assessed in both hands using a hand dynamometer (TKK; Tokyo, Japan). The mean value for the two hands will be used as the final outcome.
- Lumbar trunk muscle endurance will be assessed using two tests [85]. To evaluate flexor endurance, the subject will be asked to lie in a supine position and to raise the lower extremities with 90° flexion of the hip and knee joints. To evaluate extensor endurance, the subject will be asked to lie in a prone position while holding the sternum off the floor. During both procedures, the subject will be asked to maintain the original positions for as long as possible but without exceeding a 2-min time limit.
- Flexibility will be measured using the sit-and-reach test [86]. During this trunk flexion exercise, the distance between the tips of the fingers at the start and final positions will be recorded. The best result of three trials will be used as the outcome.
- Postural balance will be evaluated using the blind flamingo test [87,88,89]. Here, the barefoot subject stands on one leg with his/her eyes closed, while the other leg is flexed at the knee and held at the ankle by the hand of the same side. The chronometer is stopped whenever the subject does not comply with the protocol conditions. The number of trials required to remain static in this position for 30 s is measured. The outcome is expressed as the number of trials (=number of falls + 1).
- Lower extremity function will be assessed using the chair-stand test [90]. The subject sits in a standardized chair (0.43 m in height). The subject is asked to stand upright with his/her arms folded across the chest and then sit back down again. This is repeated 10 times, as quickly and as safely as possible. The best result of two trials (expressed in s) separated by 3 min is used as the outcome.
3.2. Secondary Outcomes
3.2.1. Exercise Adherence
3.2.2. Potential Adverse Effects of the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer Disease International. The Global Impact of Dementia. An analysis of Prevalence, Incidence, Cost and Trends; Alzheimer Disease International: London, UK, 2015; p. 88. [Google Scholar]
- Birkenhäger-Gillesse, E.; Kollen, B.; Zuidema, S.; Achterberg, W. The “more at home with dementia” program: A randomized controlled study protocol to determine how caregiver training affects the well-being of patients and caregivers. BMC Geriatr. 2018, 18, 252–259. [Google Scholar] [CrossRef]
- Garcés, M. Study on Neurodegenerative Diseases in Spain and Its Economic and Social Impact [Estudio Sobre Las Enfermedades Neurodegenerativas en España Y SU Impacto Económico Y Social]; Universidad Complutense Madrid, Neuroalianza: Madrid, Spain, 2016; p. 180. (in Spanish) [Google Scholar]
- CEAFA; Sanitas, F. The Caretaker in Spain. Current Context and Future Perspectives. Proposals for Intervention [El Cuidador en EspañA. Contexto Actual Y Perspectivas DE Futuro. Propuestas DE Intervención]; CEAFA and Sanita Foundation: Pamplona—Barcelona, Spain, 2016; p. 265. (in Spanish) [Google Scholar]
- Salazar, A.; Murcia, L.; Solano, J. Evaluation and intervention overload informal caregiver of dependent elderly: Review of articles published between 1997–2014. [Evaluación e intervención de la sobrecarga del cuidador informal de adultos mayores dependientes: Revisión de artículos publicados entre 1997–2014.]. Arch. Med. 2016, 16, 144–154. (in Spanish). [Google Scholar]
- Graff, M.J.; Adang, E.M.; Vernooij-Dassen, M.J.; Dekker, J.; Jonsson, L.; Thijssen, M.; Hoefnagels, W.H.; Rikkert, M.G. Community occupational therapy for older patients with dementia and their care givers: Cost effectiveness study. BMJ 2008, 336, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Erol, R.; Brooker, D.; Peel, E. Women and Dementia: A Global Research Review; Association for Dementia Studies: London, UK, 2015; p. 57. [Google Scholar]
- Badia, X.; Lara, N.; Roset, M. Quality of life, time commiment and burden perceived by the principal informal caregiver of Alzheimer’s patients. Aten. Primaria 2004, 34, 170–177. [Google Scholar] [CrossRef]
- Ploeg, J.; Markle-Reid, M.; Valaitis, R.; McAiney, C.; Duggleby, W.; Bartholomew, A.; Sherifali, D. Web-based interventions to improve mental health, general caregiving outcomes, and general health for informal caregivers of adults with chronic conditions living in the community: Rapid evidence review. J. Med. Internet. Res. 2017, 19, e263. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer Disease International. The Global Impact of Dementia 2013–2050; Alzheimer Disease International: London, UK, 2013; p. 8. [Google Scholar]
- Vandepitte, S.; Van Den Noortgate, N.; Putman, K.; Verhaeghe, S.; Annemans, L. Effectiveness and cost-effectiveness of an in-home respite care program in supporting informal caregivers of people with dementia: Design of a comparative study. BMC Geriatr. 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.G.; Swartz, K. Caregiver care. Am. Fam. Physician 2011, 83, 1309–1317. [Google Scholar] [PubMed]
- Chiao, C.Y.; Wu, H.S.; Hsiao, C.Y. Caregiver burden for informal caregivers of patients with dementia: A systematic review. Int. Nurs. Rev. 2015, 62, 340–350. [Google Scholar] [CrossRef]
- Fredman, L.; Bertrand, R.; Martire, L.; Hochberg, M.; Harris, E. Leisure-time and overall physical activity in older women caregivers and non-caregivers from the Caregiver-SOF study. Prev. Med. 2006, 43, 226–229. [Google Scholar] [CrossRef]
- Bauer, R.; Koepke, F.; Sterzinger, L.; Spiessl, H. Burden, rewards, and coping—The ups and downs of caregivers of people with mental illness. J. Nerv. Ment. Dis. 2012, 200, 928–934. [Google Scholar] [CrossRef]
- Del Rio Lozano, M.; Garcia-Calvente, M.D.M.; Calle-Romero, J.; Machon-Sobrado, M.; Larranaga-Padilla, I. Health-related quality of life in Spanish informal caregivers: Gender differences and support received. Qual. Life Res. 2017, 26, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Jenaro, C.; Moro, L.; Tomsa, R. Health and quality of life of family caregivers and professionals of dependent elderly people: Comparative study. [Salud y calidad de vida de cuidadores familiares y profesionales de personas mayores dependientes: Estudio comparativo.]. Eur. J. Investig. Health Psychol. Educa. 2014, 4, 79–88. [Google Scholar] [CrossRef][Green Version]
- Garzón-Maldonado, F.; Gutiérrez-Bedmar, M.; García-Casares, N.; Pérez-Errázquin, F.; Gallardo-Tur, A.; Martínez-Valle, M. Health-related quality of life in caregivers of patients with Alzheimer’s disease. [Calidad de vida relacionada con la salud en cuidadores de pacientes con enfermedad de Alzheimer.]. Neurología 2016, 32, 508–515. [Google Scholar] [CrossRef]
- Dawood, S. Caregiver burden, quality of life and vulnerability towards psychopathology in caregivers of patients with dementia/Alzheimer’s disease. J. Coll. Physicians Surg. Pak. 2016, 26, 892–895. [Google Scholar] [PubMed]
- Gusi, N.; Prieto, J.; Madruga, M.; Adsuar, J.; González-Guerrero, J.; García -Domínguez, J. Health-related quality of life and fitness differences between family caregivers of patient with dementia and non-caregivers. Med. Sci. Sports Exerc. 2009, 41, 1182–1187. [Google Scholar] [CrossRef]
- Farran, C.J.; Paun, O.; Cothran, F.; Etkin, C.D.; Rajan, K.B.; Eisenstein, A.; Navaie, M. Impact of an individualized physical activity intervention on improving mental health outcomes in family caregivers of persons with dementia: A randomized controlled trial. AIMS Med. Sci. 2016, 3, 15–31. [Google Scholar] [CrossRef]
- Lee, S.; Colditz, G.A.; Berkman, L.F.; Kawachi, I. Caregiving and risk of coronary heart disease in U.S. women: A prospective study. Am. J. Prev. Med. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Farran, C.J.; Etkin, C.D.; Eisenstein, A.; Paun, O.; Rajan, K.B.; Sweet, C.M.C.; McCann, J.J.; Barnes, L.L.; Shah, R.C.; Evans, D.A. Effect of moderate to vigorous physical activity intervention on improving dementia family caregiver physical function: A randomized controlled trial. J. Alzheimers Dis. Parkinsonism 2016, 6. [Google Scholar] [CrossRef]
- Pérez-Fuentes, M.; Gázquez, J.; Ruiz, M.; Molero, M. Inventory of overburden in Alzheimer’s patient family caregiver with no specialized training. Int. J. Clin. Health Psychol. 2017, 17, 56–64. [Google Scholar] [CrossRef]
- Gustaw, K.; Beltowska, K.; Makara-Studzinska, M. Dementive patients’ caregivers—Psychological aspect of their needs. Przegl Lek 2008, 65, 304–307. [Google Scholar]
- Ho, S.C.; Chan, A.; Woo, J.; Chong, P.; Sham, A. Impact of caregiving on health and quality of life: A comparative population-based study of caregivers for elderly persons and noncaregivers. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Sorensen, S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychol. Aging 2003, 18, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Delgado, E.; Suárez, O.; De Dios, R.; Valdespino, I.; Sousa, Y.; Braña, G. Characteristics and factors associated with dementia caregivers burden. [Características y factores relacionados con sobrecarga en una muestra de cuidadores principales de pacientes ancianos con demencia.]. SEMERGEN 2014, 40, 8. (in Spanish). [Google Scholar]
- Haines, K.J.; Denehy, L.; Skinner, E.H.; Warrillow, S.; Berney, S. Psychosocial outcomes in informal caregivers of the critically ill: A systematic review. Crit. Care Med. 2015, 43, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Losada-Baltar, A.; Izal, M.; Montorio, I.; Márquez, M.; Pérez, G. Differential efficacy of two psychoeducational interventions for dementia family caregivers. [Eficacia diferencial de dos intervenciones psicoeducativas para cuidadores de familiares con demencia.]. Rev. Neurologia 2004, 38, 701–708. (in Spanish). [Google Scholar] [CrossRef]
- Ownby, R.; Saeed, M.; Wohlgemuth, W.; Capasso, R.; Acevedo, A.; Peruyera, G.; Sevush, S. Caregiver reports of sleep problems in non-Hispanic white, Hispanic, and African American patients with Alzheimer dementia. J. Clin. Sleep Med. 2010, 15, 281–289. [Google Scholar] [CrossRef]
- Thomas, P.; Hazif-Thomas, C.; Pareault, M.; Vieban, F.; Clément, J.P. Sleep disturbances in home caregivers of persons with dementia. Europe PMC 2010, 36, 159–165. [Google Scholar] [CrossRef]
- Franco, C.; Sola, M.; Justo, E. Reducing psychological discomfort and overload in Alzheimer’s family caregivers through a mindfuness mediation program. Rev. Esp. Geriatr. Gerontol. 2010, 45, 252–258. [Google Scholar] [CrossRef]
- Feast, A.; Orrell, M.; Russell, I.; Charlesworth, G.; Moniz-Cook, E. The contribution of caregiver psychosocial factors to distress associated with behavioural and psychological symptoms in dementia. Int. J. Geriatr. Psychiatry 2017, 32, 76–85. [Google Scholar] [CrossRef]
- Cooper, C.; Selwood, A.; Blanchard, M.; Livingston, G. Abusive behaviour experienced by family carers from people with dementia: The CARD (caring for relatives with dementia) study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 592–596. [Google Scholar] [CrossRef]
- Vandepitte, S.; Van Den Noortgate, N.; Putman, K.; Verhaeghe, S.; Faes, K.; Annemans, L. Effectiveness of supporting informal caregivers of people with dementia: A systematic review of randomized and non-randomized controlled trials. J. Alzheimers Dis. 2016, 52, 929–965. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer Disease International. World Alzheimer Report 2016. Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer Disease International: London, UK, 2016; p. 140. [Google Scholar]
- Gaugler, J.; Roth, D.; Haley, W.; Mittelman, M. Can counseling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer’s disease during the transition to institutionalization? Results from the New York University caregiver intervention study. J. Am. Geriatr. Soc. 2008, 56, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.; Kane, R. A Perfect Storm? The Future of Family Caregiving. Family Caregiving in the New Normal; Elsevier: San Diego, CA, USA, 2015. [Google Scholar]
- Sorensen, S.; Pinquart, M.; Duberstein, P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist 2002, 42, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, L.N.; Marx, K.; Stanley, I.H.; Hodgson, N. Translating evidence-based dementia caregiving interventions into practice: State-of-the-science and next steps. Gerontologist 2015, 55, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.S.; Verreault, P.; Hudon, C. Reducing burden for caregivers of older adults with mild cognitive impairment: A systematic review. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 401–404. [Google Scholar] [CrossRef]
- Orgeta, V.; Miranda-Castillo, C. Does physical activity reduce burden in carers of people with dementia? A literature review. Int. J. Geriatr. Psychiatry 2014, 29, 771–783. [Google Scholar] [CrossRef]
- Jensen, M.; Agbata, I.N.; Canavan, M.; McCarthy, G. Effectiveness of educational interventions for informal caregivers of individuals with dementia residing in the community: Systematic review and meta-analysis of randomised controlled trials. Int. J. Geriatr. Psychiatry 2015, 30, 130–143. [Google Scholar] [CrossRef]
- Boots, L.M.; de Vugt, M.E.; van Knippenberg, R.J.; Kempen, G.I.; Verhey, F.R. A systematic review of Internet-based supportive interventions for caregivers of patients with dementia. Int. J. Geriatr. Psychiatry 2014, 29, 331–344. [Google Scholar] [CrossRef]
- Dam, A.E.; de Vugt, M.E.; Klinkenberg, I.P.; Verhey, F.R.; van Boxtel, M.P. A systematic review of social support interventions for caregivers of people with dementia: Are they doing what they promise? Maturitas 2016, 85, 117–130. [Google Scholar] [CrossRef]
- Mollinedo Cardalda, I.; Lopez, A.; Cancela Carral, J.M. The effects of different types of physical exercise on physical and cognitive function in frail institutionalized older adults with mild to moderate cognitive impairment. A randomized controlled trial. Arch. Gerontol. Geriatr. 2019, 83, 223–230. [Google Scholar] [CrossRef]
- Cuthbert, C.A.; King-Shier, K.M.; Ruether, J.D.; Tapp, D.M.; Wytsma-Fisher, K.; Fung, T.S.; Culos-Reed, S.N. The effects of exercise on physical and psychological outcomes in cancer caregivers: Results from the RECHARGE randomized controlled trial. Ann. Behav. Med. 2018, 52, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Wilcox, S.; O’Sullivan, P.; Baumann, K.; King, A. An exercise program for women who are caring for relatives with dementia. Psychosom. Med. 2002, 64, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Smith, R.; Fearn, M.; Rydberg, M.; Oliphant, R. Physical and psychological outcomes of a supported physical activity program for older carers. J. Aging Phys. Act. 2007, 15, 257–271. [Google Scholar] [CrossRef][Green Version]
- King, A.C.; Baumann, K.; O’Sullivan, P.; Wilcox, S.; Castro, C. Effects of moderate-intensity exercise on physiological, behavioural and emotional responses to family caregiving: A randomized controlled trial. J Gerontol. 2002, 57A, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Suzuki, Y.; Kuzuya, M.; Onishi, J.; Ban, N.; Umegaki, H. Influence of regular exercise on subjective sense of burden and physical symptoms in community-dwelling caregivers of dementia patients: A randomized controlled trial. Arch. Gerontol. Geriatr. 2011, 53, e158–e163. [Google Scholar] [CrossRef] [PubMed]
- Lok, N.; Lok, S.; Canbaz, M. The effect of physical activity on depressive symptoms and quality of life among elderly nursing home residents: Randomized controlled trial. Arch. Gerontol. Geriatr. 2017, 70, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lowery, D.; Cerga-Pashoja, A.; Iliffe, S.; Thune-Boyle, I.; Griffin, M.; Lee, J.; Bailey, A.; Bhattacharya, R.; Warner, J. The effect of exercise on behavioural and psychological symptoms of dementia: The EVIDEM-E randomised controlled clinical trial. Int. J. Geriatr. Psychiatry 2014, 29, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.M.; Dow, B.; Ames, D.; Moore, K.; Hill, K.; Russell, M.; Lautenschlager, N. Physical activity in caregivers: What are the psychological benefits? Arch. Gerontol. Geriatr. 2014, 59, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Waelde, L.C.; Thompson, L.; Gallagher-Thompson, D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J. Clin. Psychol. 2004, 60, 677–687. [Google Scholar] [CrossRef]
- Lavretsky, H.; Epel, E.S.; Siddarth, P.; Nazarian, N.; Cyr, N.S.; Khalsa, D.S.; Lin, J.; Blackburn, E.; Irwin, M.R. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: Effects on mental health, cognition, and telomerase activity. Int. J. Geriatr. Psychiatry 2013, 28, 57–65. [Google Scholar] [CrossRef]
- Chan, W.C.; Lautenschlager, N.; Dow, B.; Ma, S.L.; Wong, C.S.; Lam, L.C. A home-based exercise intervention for caregivers of persons with dementia: Study protocol for a randomised controlled trial. Trials 2016, 17, 460. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.; Browne, D. Supporting carers of people with dementia: What is effective? BJPsych. Adv. 2017, 23, 179–189. [Google Scholar] [CrossRef]
- Lamotte, G.; Shah, R.C.; Lazarov, O.; Corcos, D.M. Exercise training for persons with Alzheime’s disease and caregivers: A review of dyadic exercise interventions. J. Mot. Behav. 2017, 49, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Pinquart, M.; Sorensen, S. Correlates of physical health of informal caregivers: A meta-analysis. J. Geront. Ser. B Psychol. Sci. Soc. Sci. 2007, 62, P126–P137. [Google Scholar] [CrossRef]
- Hopwood, J.; Walker, N.; McDonagh, L.; Rait, G.; Walters, K.; Iliffe, S.; Ross, J.; Davies, N. Internet-based interventions aimed at supporting family caregivers of people with dementia: Systematic review. J. Med. Internet Res. 2018, 20, e216. [Google Scholar] [CrossRef] [PubMed]
- Cerga-Pashoja, A. Evaluation of Exercise on Individuals with Behavioural and Psychological Symptoms of Dementia and Their Carers: A Randomized Controlled Trial. Ph.D. Thesis, University College London, London, UK, 2015. [Google Scholar]
- Steinberg, M.; Leoutsakos, J.M.; Podewils, L.J.; Lyketsos, C.G. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: The maximizing independence in dementia (MIND) study. Int. J. Geriatr. Psychiatry 2009, 24, 680–685. [Google Scholar] [CrossRef]
- Goy, E.; Kansagara, D.; Freeman, M. A Systematic Evidence Review of Interventions for Non-professional Caregivers of Individuals with Dementia; VA-ESP Project: Washington, DC, USA, 2010. [Google Scholar]
- Eysenbach, G. What is e-health? J. Med. Internet. Res. 2001, 3, E20. [Google Scholar] [CrossRef]
- Nickel, F.; Barth, J.; Kolominsky-Rabas, P.L. Health economic evaluations of non-pharmacological interventions for persons with dementia and their informal caregivers: A systematic review. BMC Geriatr. 2018, 18, 69. [Google Scholar] [CrossRef]
- Zijlstra, T.R.; Braakman-Jansen, L.M.; Taal, E.; Rasker, J.J.; van de Laar, M.A. Cost-effectiveness of Spa treatment for fibromyalgia: General health improvement is not for free. Rheumatology 2007, 46, 1454–1459. [Google Scholar] [CrossRef][Green Version]
- Sacristan, J.; Oliva, J.; Del Llano, J.; Prieto, L.; Pinto, J. What is an efficient health technology in Spain? Gac. Sanit. 2002, 16, 334–343. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Morris, S. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Hunter, J.; Schmidt, F. Method of Meta-Analysis: Correcting Error and Bias in Research Findings, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2004. [Google Scholar]
- Fenwick, E.; Byford, S. A guide to cost-effectiveness acceptability curves. Br. J. Psychiatry 2005, 187, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Willan, A.R. On the probability of cost-effectiveness using data from randomized clinical trials. BMC Medical Res. Methodol. 2001, 1, 8. [Google Scholar] [CrossRef]
- Sevick, M.A.; Dunn, A.L.; Morrow, M.S.; Marcus, B.H.; Chen, G.J.; Blair, S.N. Cost-effectiveness of lifestyle and structured exercise interventions in sedentary adults: Results of project ACTIVE. Am. J. Prev. Med. 2000, 19, 1–8. [Google Scholar] [CrossRef]
- Herdman, M.; Badia, X.; Berra, S. EuroQol-5D: A simple alternative for measuring health-related quality of life in primary care. Aten. Primaria 2001, 28, 425–430. [Google Scholar] [CrossRef]
- Badia, X.; Roset, M.; Monserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: Description and uses. Med. Clin. (Barc.) 1999, 112, 79–85. [Google Scholar]
- Manca, A.; Hawkins, N.; Sculpher, M.J. Estimating mean QALYs in trial-based cost-effectiveness analysis: The importance of controlling for baseline utility. Health Econ. 2005, 14, 487–496. [Google Scholar] [CrossRef]
- Vademecum. Available online: http://www.vademecum.es (accessed on 4 January 2020).
- Zarit, S.H.; Reever, K.E.; Bach-Peterson, J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Yesavage, J.; Brink, T.L.; Rose, T.L.; Lum, O. Development and validation of a geriatric depression scale: A preliminary report. J. Psychiatry Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Shua-Haim, J.R.; Haim, T.; Shi, Y.; Kuo, Y.H.; Smith, J.M. Depression among Alzheimer’s caregivers: Identifying risk factors. Am. J. Alzheimers Dis. Other Dement. 2001, 16, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Derogatis, L. SCL-90-R, Administration, Scoring and Procedures Manual I for the Revised Version of the SCL-90; Johns Hopkins University Press: Baltimore, MD, USA, 1977. [Google Scholar]
- Oja, P.; Tuxworth, B. Eurofit for Adults, Assessment of Health- Related Fitness; UKK Institute for Health Promotion Research: Tampere, Finland, 1995; p. 2. [Google Scholar]
- Ito, T.; Shirado, O.; Suzuki, H.; Takahashi, M.; Kaneda, K.; Strax, T.E. Lumbar trunk muscle endurance testing: An inexpensive alternative to a machine for evaluation. Arch. Phys. Med. Rehabil. 1996, 77, 75–79. [Google Scholar] [CrossRef]
- Wells, K.; Dillon, E. The sit and reach—A test of back and leg flexibility. Res. Quart. 1952, 23, 115–118. [Google Scholar] [CrossRef]
- Gusi, N.; Raimundo, A.; Leal, A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC. Musculoskelet. Disord. 2006, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Valenzuela, A.; Gusi, N.; Nacher, S.; Gallardo, I. Evaluation of the health-related fitness in adults (II): Reliability, feasibility and reference norms by means of the AFISAL-INEFC. Apunts Educación Física y Deportes. 1998, 54, 54–65. [Google Scholar]
- Rodríguez, F.; Gusi, N.; Valenzuela, A.; Nácher, S.; Nogués, J.; Marina, M. Evaluation of health-related fitness in adults (I): Background and protocols of the AFISAL-INEFC battery. Apunts Educación Física y Deportes. 1998, 52, 54–75. [Google Scholar]
- Csuka, M.; McCarty, D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Madruga, M.; Gozalo, M.; Prieto, J.; Adsuar, J.C.; Gusi, N. Psychological symptomatology in informal caregivers of persons with dementia: Influences on health-related quality of life. Inter. J. Env. Res. Pub. Heal. 2020, 17, 1078. [Google Scholar] [CrossRef]
- Blom, M.M.; Zarit, S.H.; Groot Zwaaftink, R.B.; Cuijpers, P.; Pot, A.M. Effectiveness of an Internet intervention for family caregivers of people with dementia: Results of a randomized controlled trial. PLoS ONE 2015, 10, e0116622. [Google Scholar] [CrossRef]
- Marim, C.M.; Silva, V.; Taminato, M.; Barbosa, D.A. Effectiveness of educational programs on reducing the burden of caregivers of elderly individuals with dementia: A systematic review. Rev. Lat.-Am. Enferm. 2013, 21, 267–275. [Google Scholar] [CrossRef]
- Torrance, G.W. Preferences for health outcomes and cost-utility analysis. Am. J. Manag. Care 1997, 3, S8–S20. [Google Scholar] [PubMed]
- Regional Government of Extremadura. Decree 21/2009, of February 13 (Diario Oficial de Extremadura, 19-02-2009, No. 34, pp 4466–90): 2009. Available online: http://doe.juntaex.es/pdfs/doe/2009/340O/09040022.pdf (accessed on 26 January 2019).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madruga, M.; Prieto, J.; Rohlfs, P.; Gusi, N. Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial. Healthcare 2020, 8, 54. https://doi.org/10.3390/healthcare8010054
Madruga M, Prieto J, Rohlfs P, Gusi N. Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial. Healthcare. 2020; 8(1):54. https://doi.org/10.3390/healthcare8010054
Chicago/Turabian StyleMadruga, Miguel, Josué Prieto, Paloma Rohlfs, and Narcís Gusi. 2020. "Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial" Healthcare 8, no. 1: 54. https://doi.org/10.3390/healthcare8010054
APA StyleMadruga, M., Prieto, J., Rohlfs, P., & Gusi, N. (2020). Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial. Healthcare, 8(1), 54. https://doi.org/10.3390/healthcare8010054






