Learning from a Feasibility Trial of a Simple Intervention: Is Research a Barrier to Service Delivery, or is Service Delivery a Barrier to Research?
Abstract
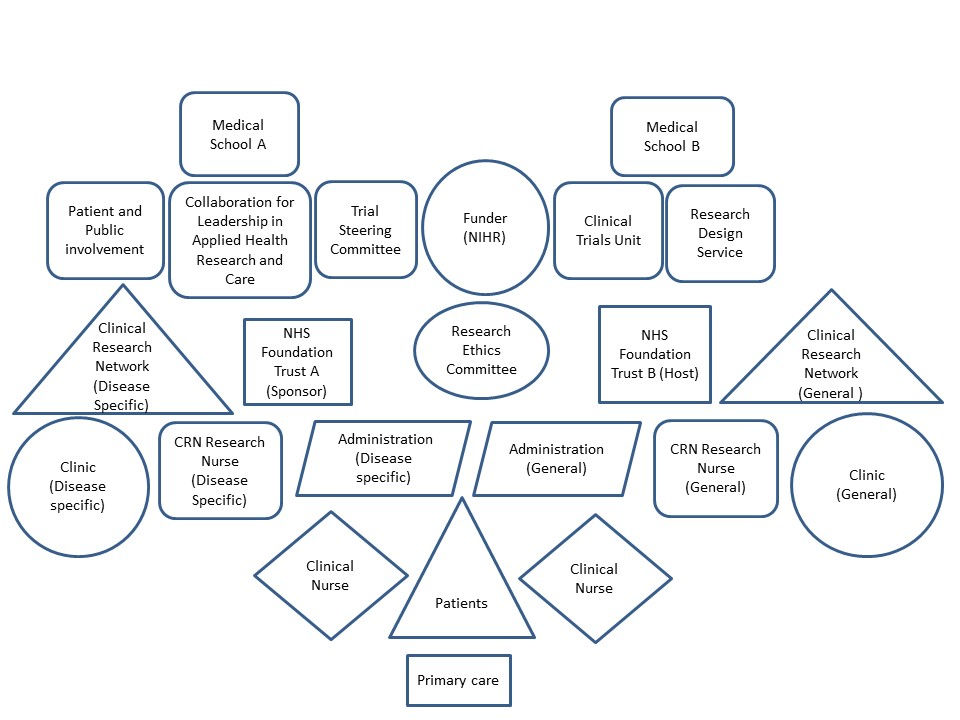
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Physicians’ Reactions
“[Dr Abbot] suggested that participation rates are likely to be high and estimated an attrition rate of 50% for the 6-month follow up….”(Minutes of TMG Meeting, 2 years pre-trial)
“I was quite disappointed that we didn’t get the recruitment as we needed, because I think initially I thought that oh, this would be plain sailing, and so many obstacles came around… it’s a learning experience.”Dr Abbott
“So I think there would be more interest in doing this in people at or soon after diagnosis… that would mean a primary care study… if you could do it outside the consultation room it’s probably, yeah, maybe it could be simplified.”Dr Blake
“My initial perception was that I like to think that I conduct … consultations vaguely in that manner anyway…the times where I say ‘what do you want to talk about’, tend to be those that I know the consultation will last for an hour if I don’t limit it to that.”Dr Cross
“One of the reasons I was interested in the study was ‘cause I am concerned, so—my background to this is I watch my friends who’ve all become GPs, get trained in consultation skills and how to set up consultations… and I’m very conscious that we, as hospital physicians, have actually no such training… So being involved with something that was actually looking at the consultation itself, trying to structure it and measuring effect and seeing whether the outcome was more satisfactory for patients was appealing.”Dr Dicker
“As in anything there are more doctors that are more research-friendly than others. So um, [Physician] is fantastic because he’s research based, he deals with a lot of research and, you know, he understands the process of research and maybe not all of the physicians are as keyed up in research as [Physician]…”Nurse Elion
3.2. Nursing Staff Reactions
“[Department manager] at the time approached me and asked if I would mind taking part in the study, because I used to do the [condition] clinics a lot and also because I was quite good at IT and I didn’t have any other roles within the department at the time so… She told me it would be on an iPad and you just had to press a couple of buttons and that was it….”Nurse Fleming
“I thought ‘Ooh, what have I got myself into!’, um, not because of the study itself but basically some of the skills needed for the study. And I was thinking ‘Oh heck! I’m terrible with computers’ etc. etc. but in the end I just took it in my stride and thought ‘Well, yes, I can do this!’”Nurse Gatson
“Purely because in their opinion I was a [clinical nurse], so, bottom of the picking litter [sic]. And actually if they want jobs done, I’m the one that, they want me to go and do it and couldn’t appreciate – it was almost like it would have run better if I was in a different hospital, if I was suddenly not around my colleagues, ‘cause they weren’t accommodating, they didn’t help. I was seen as actually just sitting in a room, ‘Oh why do you get to do that, why can’t we do that?’ Almost like school children being jealous that I’m getting almost a day off, when I wasn’t getting a day off, I was just helping take part in something else that they’d already said they didn’t want to do or they weren’t suitable to help with.”Nurse Fleming
“I think the main problem was getting the clinic dates and information from [Departmental manager]…she’s not clinically-based or, you know, she wasn’t clinical, she was an admin person. I don’t think she understood research… as with any research that we take part in, we have to ring the patient, get the verbal consent and then send them the information within a certain length of time so then they can decide whether they want to take part in the study or not. And then, for us, we would do that, and then they would contact [Hospital] and then [CTU] had to get more information out to the patient and then get it back… All they have to do is, you know, that day you just book an extra room, and I don’t understand why that was a problem. When I went we went in the room upstairs.”Nurse Elion
“I’d look at the list and I’d think ‘I know them… they’ll do it.’ So that was quite useful… And I think if you recognize patients’ names and patients have taken part in other research studies and have said they’re happy to take part in research. Then I think it’s only right to include those patients, ‘cause you think ‘Oh yeah, they will take part’”Nurse Elion
“Um [the screening team] made one phone call and if they didn’t get that person that seemed to be enough work done, um, with regard to that patient, whereas I thought we needed to be chasing them up and one phone call just wasn’t enough… I’d already done a study similar to this, so I already know that you can’t ring someone up and expect an answer there and then, you have to keep chasing… I just thought one phone call and a tick in a box to say ‘I’ve done that job’, wasn’t good enough….”Nurse Hackett
“You had some of the public members in… I think the people that you had in were eager, and I think that’s that side of the spectrum, but there wasn’t anybody, even if it was like an actor, with a negative, um, spectrum, do you understand what I mean?... people that were ringing patients asking them if they’d like to take part… maybe, they wouldn’t have known what to have said to somebody who was like ‘No, why? duh!’”Nurse Elion
3.3. Network Managers’ Reactions
“I was approached by one of the SRN nurses to look at an early form of protocol, so I kind of saw it in its very early stages... And then when it finally came on to the books it was probably about a year later, and we were involved, not so much in the feasibility but once it had been accepted at the site and they realized that they needed some involvement from the research team, so slightly the wrong way round.”Nurse Inglis
“[CRN]: Negative feedback received; the SRN indicated at their recent meeting that their primary interest is in projects with potential for income generation. [researcher] has responded to SRN email re areas of clarification but has not had a response yet.”(Minutes of TMG Meeting, 1 year pre-trial)
“[Patient] reported that the [CRN] lay panel had met and requested that the [Trial name] study will be a rolling agenda item. [Patient] will inform the panel that we will be happy to talk to them once the study has progressed.”(Minutes of TMG Meeting, 1 year pre-trial)
“Um, it was easy to identify the patients, but there weren’t enough of them in the clinics that were identified, but then it involved actually speaking to them… so it meant doing some evening calls, which is not unusual practice, but we felt that that was necessary in order to get hold of people. Um, and then we had to talk to them about it, send out information, they had to post back to [CTU], they then posted stuff out to them, and this all had to happen in quite a quick, tight, timeframe, which often meant that patients weren’t then being seen in the clinic as research patients, they kind of missed the timings and missed the opportunity.”Nurse Inglis
“The problem’s with [Department]! I would say I don’t just get this with this study, I get it with a lot of studies, it’s like: actually just do it, it’s part of NHS core business doing research and it’s actually very ‘them and us’, and that’s the thing throughout the - not throughout the whole of the NHS, but we get that quite a lot in studies ‘Oh that’s research, that’s not us’, but it is actually you, because we are the NHS and we are research as a core business, etc.…And it’s just one of those things, until there’s a change of culture within the NHS, it’s not going to change.”Nurse Joshi
3.4. Patients’ Reactions
“Some of the people that had [condition] for a long time ‘It’s a bit bloody late for that’ or ‘If I don’t know what I’m doing by now …’ type thing”Nurse Elion
“One guy I had to read it for him because he couldn’t read, so he was just discussing the whole thing with me.”Nurse Fleming
- P:
- First [questionnaire is] obviously a bit daunting, ‘cause it’s, you see all these boxes, you think ‘Oh my God all these pages!’ But once you get into it, and I suppose ‘cause I’m used to doing them anyway…I would say, about an hour over all… ‘cause I took me time on it, I didn’t …
- I:
- Did the intervention in any way help [you]?
- P:
- I think [questionnaires] helped me more… Completing those give a chance to actually say what I thought of it, where I was going.
Mr Smith
3.5. Resource Constraints
“I think when the study arose it was a simple question and, I think, but when people talked about it and developed into a study, I think it became more complex, it was no longer a simple question. I think the question was still simple, but the process was [laughs] pretty long and complex… I thought that the patient would just come to the clinic, will [engage with the intervention] and will go home, but I think they have lots to do at home …in the process of the study”Dr Abbott
“[Intervention] made the consultations longer …and bearing in mind we are very pressurized now, and we’re constantly pressurized that we’ve got to see more patients, and this economic clime that we are currently living in– I like motivational interviewing…[but] my consultations often go over and then I keep patients waiting and then I get management on my back…”Dr Lang
- I:
- OK. Um, what, if any, impact do you think [intervention] had…
- P:
- Possibly speeded it up…Because there was a focus… prioritizing from the patient’s perspective, um, that we could then, you know, set to on what they really wanted to talk about.
Dr Ross
“It’s a research grant and so I think if you were going to put this into practice, I think, if you were going to make it viable, then there may have to be a change… that could make it more efficient and maybe that could be, um, telephone, you could actually do it by FaceTime, there may be other ways of doing it.”Dr Lang
“The fact that we missed our [recruitment] target had implications for funding … which was really disappointing…so I think that felt really unfair, um, knowing how hard we’d worked here, you know, with [Nurse Elion] and the other [nurses], getting them on board and trained and research ready. And the amount of things we’d done kind of above and beyond, you know, the evening phone calling, setting up the pre-screening, um, logs so that we could then go back and cross-reference. I think that felt a bit sort of unfair, somehow.”Nurse Inglis.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selby, P. The impact of the process of clinical research on health service outcomes. Ann. Oncol. 2011, 22, vii2–vii4. [Google Scholar] [CrossRef] [PubMed]
- Barratt, H.; Shaw, J.; Simpson, L.; Bhatia, S.; Fulop, N. Health services research: Building capacity to meet the needs of the health care system. J. Health Serv. Res. Policy 2017, 22, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. The NHS Constitution; Department of Health Publications: London, UK, 2015. [Google Scholar]
- Department of Health. A Short Guide to NHS Foundation Trusts; Department of Health Publications: London, UK, 2005. [Google Scholar]
- National Health Service Health Research Authority. UK Policy Framework for Health and Social Care Research; HRA: London, UK, 2017. [Google Scholar]
- Department of Health. The Government’s Revised Mandate to NHS England for 2018–19; Department of Health Publications: London, UK, 2019. [Google Scholar]
- KPMG. NIHR Clinical Research Network: Impact and Value Assessment; KPMG: Amstelveen, The Netherlands, 2016. [Google Scholar]
- Denis, J.-L.; Lehoux, P.; Hivon, F.; Champagne, F. Creating a new articulation between research and practice through policy? The views and experiences of researchers and practitioners. J. Health Serv. Res. Policy 2003, 8, S244–S250. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, S.; Ball, S.; Harshfield, A.; Dimova, S.; Prideaux, R.; Carpenter, A.; Punch, D.; Simmons, R. Involving NHS staff in research. In The Healthcare Improvement Studies Institute; University of Cambridge: Cambridge, UK, 2019. [Google Scholar]
- General Medical Council. Tomorrow’s Doctors; GMC: London, UK, 2002. [Google Scholar]
- Murdoch-Eaton, D.; Drewery, S.; Elton, S.; Emmerson, C.; Marshall, M.; Smith, J.; Stark, P.; Whittle, S. What Do Medical Students Understand By Research And Research Skills? Identifying Research Opportunities Within Undergraduate Projects. Med. Teach. 2010, 32, e152–e160. [Google Scholar] [CrossRef] [PubMed]
- Soper, B.; Yaqub, O.; Hinrichs, S.; Marjanovich, S.; Drabble, S.; Hanney, S.; Nolte, E. CLAHRCS in practice: Combined knowledge transfer and exchange strategies. J. Health Serv. Res. Policy 2013, 18, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institute of Health Research. Evaluation of the Strategy for Patient Patient-Oriented Research; Canadian Institute of Health Research: Ottawa, ON, Canada, 2016. [Google Scholar]
- Selby, J.; Lipstein, S.H. PCORI at 3 years -progress, lessons and plans. N. Engl. J. Med. 2014, 370, 592–595. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Clinical Research Collaboration. The 2019–2024 UKCRC Registered CTU Network Strategy; Leeds Institute of Clinical Trials Research: Leeds, UK, 2019. [Google Scholar]
- Croghan, I.T.; Viker, S.D.; Limper, A.H.; Evans, T.K.; Cornell, A.R.; Ebbert, J.O.; Gertz, M.A. Developing a clinical trial unit to advance research in an academic institution. Contemp. Clin. Trials 2015, 45, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Hind, D.; Reeves, B.; Bathers, S.; Bray, C.; Corkhill, A.; Hayward, C.; Harper, L.; Napp, V.; Norrie, J.; Speed, C.; et al. Comparative costs and activity from a sample of UK clinical trials units. Trials 2017, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Addicott, R.; McGivern, G.; Ferlie, E. Networks, Organizational learning and knowledge management: NHS Cancer Networks. Public Policy Manag. 2006, 26, 87–94. [Google Scholar] [CrossRef]
- Snooks, H.; Hutchings, H.; Seagrove, A.; Stewart-Brown, S.; Williams, J.; Russell, I. Bureaucracy stifles medical research in Britain: A tale of three trials. BMC Med. Res. Methodol. 2012, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Angell, E.; Bryman, A.; Ashcroft, R.; Dixon-Woods, M. An analysis of decision letters by research ethics committees: The ethics/scientific quality boundary examined. Qual. Saf. Health Care 2008, 17, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Reed, K. Bureaucracy and beyond: The impact of ethics and governance procedures on health research in the social sciences. Sociol. Res. Online 2007, 12, 80–84. [Google Scholar] [CrossRef]
- NVivo Qualitative Data Analysis Software, 11th ed.; QSR International Pty Ltd.: Doncaster, Australia, 2015.
- Phillips, N.; Hardy, C. Discourse Analysis: Investigating Processes of Social Construction; Sage: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Willig, C. Discourse and discourse analysis. In The SAGE Handbook of Qualitative Data Analysis; Flick, E., Ed.; Sage: London, UK, 2013. [Google Scholar]
- Langley, C.; Gray, S.; Selley, S.; Bowie, C.; Price, C. Clinicians’ attitudes to recruitment to randomised trials in cancer care: A qualitative study. J. Health Serv. Res. Policy 2000, 5, 164–169. [Google Scholar] [CrossRef] [PubMed]
- French, C.; Stavropoulou, C. Specialist nurses’ perceptions of inviting patients to participate in clinical research studies: A qualitative descriptive study of barriers and facilitators. BMC Med. Res. Methodol. 2016, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Dimova, S.; Prideaux, R.; Ball, S.; Harshfrild, A.; Carpenter, A.; Marjovic, S. Enabling NHS Staff to Contribute to Research: Reflecting on Current Practice and Informing Future Opportunities; Rand Corporation: Cambridge, UK, 2018. [Google Scholar]
- Boaz, A.; Hanney, S.; Jones, T.; Soper, B. Does the engagement of clinicians and organisations in research improve healthcare performance: A three-stage review. BMJ Open 2015, 5, e009415. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.; Ignacio Ricci-Cabello, I.; Parker, A.; Hirst, J.; Chant, A.; Petit-Zeman, S.; Evans, D.; Rees, S. Impact of patient and public involvement on enrolment and retention in clinical trials: Systematic review and meta-analysis. Br. Med. J. 2018, 363, k4738. [Google Scholar] [CrossRef] [PubMed]
- Kislov, R.; Wilson, P.; Knowles, S.; Boaden, R. Learning from the emergence of NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRCs):A systematic review of evaluations. Implement. Sci. 2018, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Ziebland, S.; Featherstone, K.; Snowdon, C.; Barker, K.; Frost, H.; Fairbank, J. Does it matter if clinicians recruiting for a trial don’t understand what the trial is really about? Qualitative study of surgeons’ experiences of participating in a pragmatic multi-centred RCT. Trials 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Pelone, F.; Harrison, R.; Goldman, J.; Zwarenstein, M.; Reeves, S.; Pelone, F.; Harrison, R.; Goldman, J.; Zwarenstein, M. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Best Research for Best Health: A New National Health Research Strategy; Department of Health Publications: London, UK, 2006. [Google Scholar]
| Stakeholders | Assumptions Underpinning Discourse | Discursive Resources | Potential Consequences | What May Be Gained or Lost |
|---|---|---|---|---|
| Physicians | Co-applicants thought that recruitment would be easy. Participating physicians engaged with the research on their own terms. | Research processes disrupted research ethos. The research disrupted clinical practice, or physicians disrupted the research. | Perception of the trialization of something simple. Buy in: More holistic practice. Business as usual Subversion of trial protocol. | Disappointement and possible aversion to future research. Opportunity to empower individual patients. Lack of evidence to support the conduct of a full trial. |
| Nurses | Clinical nurses lacked agency. Research nurses were frustrated by boundary work. | New role was challenging. Extended role reinforced assumptions about research/practice gap. | Felt unsupported. Research/practice gap further substantiated. | Negative experience of research. Further evidence to support notion of research/ practice gap. |
| Network managers | Experiental knowledge of tensions between research and clinical practice | ‘They’ (researchers and/or practioners) don’t know what they are doing. | Managers’ expertise is not recognised. Sense that no-one consults them or listens to their opinion. | Missed opportunities for shared learning or strengthening collaboration. |
| Patients | Like physicians, patients engaged with the research on their own terms. | Already have enough knowledge. Lack of understanding of the research. | Need for the intervention not well established. Patients confused by the trial processes. | Intervention may have been targeted in wrong population. Recruitment over complicated. |
| Shared perceptions about resources | Study perceived as overcomplicated. | Misperception that consultations took longer than usual. | Perception that intervention too difficult to implement in clinical practice. | Perception that research diffcult to conduct/ replicate in ‘real world’. CRN financially penalised. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frost, J.; Britten, N. Learning from a Feasibility Trial of a Simple Intervention: Is Research a Barrier to Service Delivery, or is Service Delivery a Barrier to Research? Healthcare 2020, 8, 53. https://doi.org/10.3390/healthcare8010053
Frost J, Britten N. Learning from a Feasibility Trial of a Simple Intervention: Is Research a Barrier to Service Delivery, or is Service Delivery a Barrier to Research? Healthcare. 2020; 8(1):53. https://doi.org/10.3390/healthcare8010053
Chicago/Turabian StyleFrost, Julia, and Nicky Britten. 2020. "Learning from a Feasibility Trial of a Simple Intervention: Is Research a Barrier to Service Delivery, or is Service Delivery a Barrier to Research?" Healthcare 8, no. 1: 53. https://doi.org/10.3390/healthcare8010053
APA StyleFrost, J., & Britten, N. (2020). Learning from a Feasibility Trial of a Simple Intervention: Is Research a Barrier to Service Delivery, or is Service Delivery a Barrier to Research? Healthcare, 8(1), 53. https://doi.org/10.3390/healthcare8010053





