Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada
Abstract
1. Introduction
2. Materials and Methods
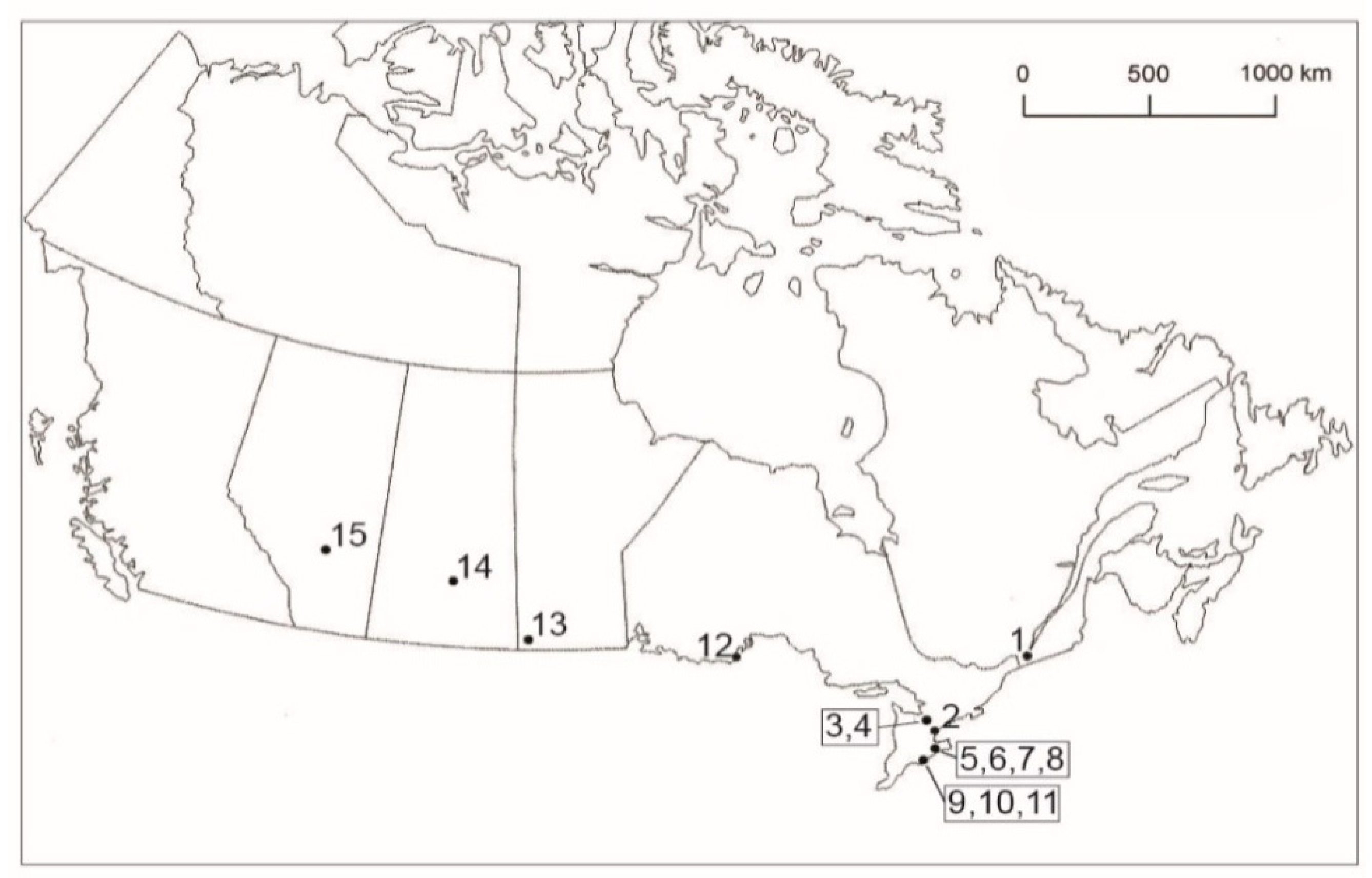
2.1. Tick Collection
2.2. Bacteria and Piroplasm Detection
2.3. DNA Sequence Analysis
3. Results
3.1. Tick Collection
3.2. Pathogen Detection
3.2.1. Detection in Bird-derived Ticks
3.2.2. Detection in Mammal-related Ticks
3.2.3. Detection in Questing Ticks
4. Discussion
4.1. Babesia Species in Ticks
4.1.1. Ticks Collected from Songbirds
4.1.2. Ticks Derived from Mammals
4.1.3. Questing Ticks
4.2. Borrelia burgdorferi Sensu Lato in Ticks
4.2.1. Ticks on Wild-caught Birds
4.2.2. Ticks on Terrestrial Mammals
4.2.3. Questing Ticks
4.3. Babesia and Borrelia burgdorferi Sensu Lato Co-infections in Ticks
4.3.1. Co-infected Ticks on Birds
4.3.2. Co-infected Ticks on Terrestrial Mammals
4.3.3. Co-infected Questing Ticks
4.4. Impact of Babesia and Bbsl on Humans
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the mask of average treatment effects in chronic Lyme disease research using Big Data and subgroup analysis. Healthcare 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.A.; Sonenshine, D.E.; Noden, B.H. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press/Elsevier: London, UK, 2019; pp. 603–672. ISBN 978-0-12-814043-7. [Google Scholar]
- Davidsson, M. The financial implications of a well-hidden and ignored chronic Lyme disease pandemic. Healthcare 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Benach, J.L.; Coleman, J.L.; Habicht, G.S.; MacDonald, A.; Grunwaldt, E.; Giron, J.A. Serological evidence for simultaneous occurrences of Lyme disease and babesiosis. J. Infect. Dis. 1985, 152, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Babes, V. Sur l’hemoglobinurie bactérienne du boeuf. C. R. Acad. Sci. Ser. III Sci. Vie 1888, 107, 692–694. [Google Scholar]
- Škrabalo, Z.; Deanović, Z. Piroplasmosis in man: Report on a case. Doc. Med. Geogr. Trop. 1957, 9, 11–16. [Google Scholar]
- Akel, T.; Mobarakai, N. Hematologic manifestations of babesiosis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 6. [Google Scholar] [CrossRef]
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease—A tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef]
- Miklossy, J.; Kasas, S.; Zurn, A.D.; McCall, S.; Yu, S.; McGeer, P.L. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreiosis. J. Neuroinflamm. 2008, 5, 40. [Google Scholar] [CrossRef]
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Tardo, A.C.; Doyle-Myers, L.A.; Philipp, M.T.; Hodzic, E. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS ONE 2017, 12, e0189071. [Google Scholar] [CrossRef]
- Middelveen, M.J.; Sapi, E.; Burke, J.; Filush, K.R.; Franco, A.; Fesler, M.C.; Stricker, R.B. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare 2018, 6, 33. [Google Scholar] [CrossRef]
- Sapi, E.; Kasliwala, R.S.; Ismail, H.; Torres, J.P.; Oldakowski, M.; Markland, S.; Gaur, G.; Melillo, A.; Eisendle, K.; Liegner, K.B.; et al. The long-term persistence of Borrelia burgdorferi antigens and DNA in the tissues of a patient with Lyme disease. Antibiotics 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Fesler, M.C. Chronic Lyme disease: A working case definition. Am. J. Infect. Dis. 2018, 14, 44. [Google Scholar] [CrossRef]
- Anderson, J.F.; Magnarelli, L.A. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 1984, 57, 627–641. [Google Scholar] [PubMed]
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, migration and emerging zoonoses: West Nile virus, Lyme disease, influenza A and enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Beati, L.; Mazerolle, D.F.; Geddes, G.; Durden, L.A. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 2005, 91, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Bierman, B.C.; Durden, L.A. Far-reaching dispersal of Borrelia burgdorferi sensu lato-infected blacklegged ticks by migratory songbirds in Canada. Healthcare 2018, 6, 89. [Google Scholar] [CrossRef]
- Scott, J.D.; Fernando, K.; Banerjee, S.N.; Durden, L.A.; Byrne, S.K.; Banerjee, M.; Mann, R.B.; Morshed, M.G. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol. 2001, 38, 493–500. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. First isolation of Lyme disease spirochete, Borrelia burgdorferi, from ticks collected from songbirds in Ontario, Canada. N. Am. Bird Bander 2009, 34, 97–101. [Google Scholar]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Durden, L.A.; Jorgensen, D.R.; Mak, S.; Morshed, M.G. Detection of Lyme disease spirochete, Borrelia burgdorferi sensu lato, including three novel genotypes in ticks (Acari: Ixodidae) collected from songbirds (Passeriformes) across Canada. J. Vector Ecol. 2010, 35, 124–139. [Google Scholar] [CrossRef]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Bierman, B.C.; Durden, L.A. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare 2018, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Brewer, D.A.; Diamond, A.; Woodsworth, E.J.; Collins, B.T.; Dunn, E.H. Doves, cuckoos, and hummingbirds through passerines. In Canadian Atlas of Bird Banding, 1921–1995; Canadian Wildlife Service, Environment Canada: Hull, UK, 2000; Volume 1, pp. 1–395. ISBN 0-662-28946-3. [Google Scholar]
- DeLuca, W.V.; Woodworth, B.K.; Rimmer, C.C.; Marra, P.P.; Taylor, P.D.; McFarland, K.P.; Mackenzie, S.A.; Norris, D.R. Transoceanic migration by a 12 g songbird. Biol. Lett. 2015, 11, 20141045. [Google Scholar] [CrossRef] [PubMed]
- Dunson, W. The Incredible Flight of a Willow Flycatcher. 2014. Available online: http://lemonbayconservancy.org/incredible-flight-willow-flycatcher/ (accessed on 5 November 2018).
- Stutchbury, B.J.M.; Tarof, S.A.; Done, T.; Gow, E.; Kramer, P.M.; Tautin, J.; Fox, J.W.; Afanasyev, V. Tracking long-distance songbird migration by using geolocators. Science 2009, 323, 896. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of I. scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. New records of the Lyme disease bacterium in ticks collected from songbirds in central and eastern Canada. Int. J. Acarol. 2015, 41, 241–249. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. Amblyomma dissimile Koch (Acari: Ixodidae) parasitizes bird captured in Canada. Syst. Appl. Acarol. 2015, 20, 854–860. [Google Scholar]
- Scott, J.D.; Durden, L.A. First record of Amblyomma rotundatum tick (Acari: Ixodidae) parasitizing a bird collected in Canada. Syst. Appl. Acarol. 2015, 20, 155–161. [Google Scholar]
- Scott, J.D. Birds widely disperse pathogen-infected ticks. In Seabirds and Songbirds: Habitat Preferences, Conservation, Migratory Behavior; Mahala, G., Ed.; Nova Publishers, Inc.: New York, NY, USA, 2015; pp. 1–22. ISBN 978-1-63463-496-0. [Google Scholar]
- Scott, J.D.; Scott, C.M.; Anderson, J.F. The establishment of a blacklegged tick population by migratory songbirds in Ontario, Canada. J. Vet. Sci. Med. 2014, 2, 5. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Geddes, G.; McNabb, A.; Mak, S.; Durden, L.A. Distribution and characterization of Borrelia burdgorferi isolates from Ixodes scapularis and presence in mammalian hosts in Ontario, Canada. J. Med. Entomol. 2006, 43, 762–773. [Google Scholar] [CrossRef]
- Scott, J.D.; Fernando, K.; Durden, L.A.; Morshed, M.G. Lyme disease spirochete, Borrelia burgdorferi, endemic in epicenter at Turkey Point, Ontario. J. Med. Entomol. 2004, 41, 226–230. [Google Scholar] [CrossRef]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Jorgensen, D.R.; Durden, L.A.; Morshed, M.G. Rapid introduction of Lyme disease spirochete, Borrelia burgdorferi sensu stricto, in Ixodes scapularis (Acari: Ixodidae) established at Turkey Point Provincial Park, Ontario, Canada. J. Vector Ecol. 2008, 33, 64–69. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Banerjee, S.N.; Fernando, K.; Mann, R.; Isaac-Renton, J. First isolation of Lyme disease spirochete, Borrelia burgdorferi from blacklegged tick, Ixodes scapularis, collected at Rondeau Provincial Park, Ontario. Can. Com. Dis. Rep. 2000, 26, 42–44. [Google Scholar]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Mann, R.B.; Durden, L.A. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J. Med. Entomol. 2003, 40, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L.A.; Smith, M.L.; Manord, J.M.; Clark, K.L. Prevalence of the Lyme disease spirochete, Borrelia burgdorferi, in blacklegged ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario. Int. J. Med. Sci. 2016, 13, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Presence of Babesia odocoilei and Borrelia burgdorferi sensu stricto in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Milnes, E.L.; Thornton, G.; Léveillé, A.N.; Delinatte, P.; Barta, J.R.; Smith, D.A.; Nemeth, N. Babesia odocoilei and zoonotic pathogens identified from Ixodes scapularis ticks in southern Ontario, Canada. Ticks Tick-Borne Dis. 2019, 10, 670–676. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Abley, M.J.; Sullivan, B.; Grindle, N.; Clay, K.; Fuqua, C. Detection of Anaplasma phagocytophilum and Babesia odocoilei DNA in Ixodes scapularis (Acari: Ixodidae) collected in Indiana. J. Med. Entomol. 2006, 43, 437–442. [Google Scholar] [CrossRef][Green Version]
- Shock, B.C.; Moncayo, A.; Cohen, S.; Mitchell, E.A.; Williamson, P.C.; Lopez, G.; Garrison, L.E.; Yabsley, M.J. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick-Borne Dis. 2014, 5, 373–380. [Google Scholar] [CrossRef]
- Available online: https://flap.org (accessed on 18 November 2019).
- Keirans, J.E.; Durden, L.A. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998, 35, 489–495. [Google Scholar] [CrossRef]
- Guzmán–Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel–Parra, G.; Pérez, M. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa 2011, 2998, 16–38. [Google Scholar]
- Jones, E.K.; Clifford, C.M.; Keirans, J.E.; Kohls, G.M. Ticks of Venezuela (Acarina: Ixodoidea) with a Key to the Species of Amblyomma in the Western Hemisphere; Biological Series; Brigham Young University Science Bulletin: Provo, Utah, 1972; Volume VXII, pp. 1–40. [Google Scholar]
- Clifford, C.M.; Anastos, G.; Elbl, A. The larval ixodid ticks of the eastern United States. Misc. Publ. Entomol. Soc. Am. 1961, 2, 213–237. [Google Scholar]
- Durden, L.A.; Keirans, J.E. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Monographs; Thomas Say Publications in Entomology, Entomological Society of America: Lanham, MD, USA, 1996; p. 95. ISBN 0-938522-57. [Google Scholar]
- Keirans, J.E.; Clifford, C.M. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J. Med. Entomol. 1978, 15 (Suppl. S2), 1–38. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Hendricks, A.; Burge, D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 2005, 71, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.-C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2016, 13, 65–70. [Google Scholar]
- McCombie, W.R.; Heiner, C.; Kelly, J.M.; Fitzgerald, M.G.; Gocayne, J.D. Rapid and reliable fluorescent cycle sequencing of double stranded templates. DNA Seq. 1992, 2, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX–Windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tools. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Caporale, D.A.; Johnson, C.M.; Millard, B.J. Presence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in southern Kettle Moraine State Forest, Wisconsin, and characterization of strain W97F51. J. Med. Entomol. 2005, 42, 457–472. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Shein, E. The piroplasms: Life cycle and sexual stages. Adv. Parasitol. 1984, 23, 37–103. [Google Scholar]
- Telford, S.R.; Gorenflot, A.; Brasseur, P.; Spielman, A. Babesial infections in humans and wildlife. In Parasitic Protozoa; Kresier, J.P., Ed.; Academic Press: San Diego, CA, USA, 1993; Volume 5, pp. 1–47. [Google Scholar]
- Holman, P.J.; Madeley, J.; Craig, T.M.; Allsopp, B.A.; Allsopp, M.T.; Petrini, K.R.; Waghela, S.D.; Wagner, G.G. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J. Wildl. Dis. 2000, 36, 518–530. [Google Scholar] [CrossRef]
- Gorenflot, A.; Moubri, K.; Percigout, E.; Carey, B.; Schetters, T.P. Human babesiois. Ann. Trop. Med. Parasitol. 1998, 92, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L.; de Bruyn, G.; Pieniazek, N.J.; Homer, M.; Lofy, K.H.; Shemenda, S.B.; Fritsche, T.R.; Persing, D.H.; Limaye, A.P. Babesia divergens-like infection, Washington state. Emerg. Infect. Dis. 2004, 10, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kjemtrup, A.M.; Conrad, P.A. Human babesiosis: An emerging tick-borne disease. Int. J. Parasitol. 2000, 30, 1323–1337. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Cacciò, S.; Gherlinzoni, F.; Aspöck, H.; Siemenda, S.B.; Piccaluga, P.; Martinelli, G.; Edelhofer, R.; Hollenstein, U.; Poletti, G.; et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003, 9, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Pattullo, K.M.; Wobeser, G.; Lockerbie, B.P.; Burgess, H.J. Babesia odocoilei infection in a Sakatchewan elk (Cervus elaphus canadensis) herd. J. Vet. Diagn. Investig 2013, 25, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 1988, 539, 180–191. [Google Scholar] [CrossRef]
- Richter, D.; Spielman, A.; Komar, N.; Matuschka, F.-R. Competence of American Robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000, 6, 133–138. [Google Scholar] [CrossRef]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick-Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Mannelli, A.; Kitron, U.; Jones, C.J.; Slajchert, T.L. Influence of season and habitat on Ixodes scapularis infestation on white-footed mice in northeastern Illinois. J. Parasitol. 1994, 80, 1038–1043. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Carey, D.; Hoodless, A.N.; Nuttall, P.A.; Randolph, S.E. Competence of pheasants as reservoirs for Lyme disease spirochetes. J. Med. Entomol. 1998, 35, 77–81. [Google Scholar] [CrossRef]
- Littman, M.P.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM small animal consensus statement on Lyme disease in dogs: Diagnosis, treatment, and prevention. J. Vet. Intern. Med. 2006, 20, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [PubMed]
- Divers, T.J.; Gardner, R.B.; Madigan, J.E.; Witonsky, S.G.; Bertone, J.J.; Swinebroad, E.L.; Schutzer, S.E.; Johnson, A.L. Borrelia burgdorferi infection and Lyme disease in North American horses: A consensus statement. J. Vet. Intern. Med. 2018, 32, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.C.; Gendron-Fitpatrick, A.; Mattison, M. Foal mortality associated with natural infection of pregnant mares with Borrelia burgdorferi. In Proceedings of the Fifth International Conference of Equine Infectious Diseases; Press of Kentucky: Lexington, KY, USA, 1988; pp. 217–220. [Google Scholar]
- Scott, J.D.; Clark, K.L.; Anderson, J.F.; Foley, J.E.; Young, M.R.; Durden, L.A. Lyme disease bacterium, Borrelia burgdorferi sensu lato, detected in multiple tick species at Kenora, Ontario, Canada. J. Bacteriol. Parasitol. 2017, 8, 304. [Google Scholar] [CrossRef]
- Heisig, M.; Abraham, N.M.; Liu, L.; Neelakanta, G.; Mattessich, S.; Sultana, H.; Shang, Z.; Anari, J.M.; Killiam, C.; Walker, W.; et al. Anti-virulence properties of an antifreeze protein. Cell Rep. 2014, 9, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Scott, C.M. Lyme disease propelled by Borrelia burgdorferi-infected blacklegged ticks, wild birds and public awareness—Not climate change. J. Vet. Sci. Med. 2018, 6, 8. [Google Scholar]
- Scott, J.D.; Schillberg, E.; Lunny, D.; Lindsay, L.; Nelder, M.; Russell, C.; Mackie, M.; Coats, D.; Berry, A.; Young Hoon, K. Distribution of Ixodes scapularis in Northwestern Ontario: Results from active and passive activities in the Northwestern Health Unit catchment area. Int. J. Environ. Res. Public Health 2019, 16, 1939. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Cooksey, L.M. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi). J. Wildl. Dis. 1993, 29, 527–532. [Google Scholar] [CrossRef]
- Holman, P.J.; Waldrup, K.A.; Wagner, G.G. In vitro cultivation of a Babesia isolated from a white–tailed deer (Odocoileus virginianus). J. Parasitol. 1988, 74, 111–115. [Google Scholar] [CrossRef]
- Hersh, M.H.; Osfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-infestation of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Hall, J.E.; Amrine, J.W., Jr.; Gais, R.D.; Kolanko, V.P.; Hagenbuch, B.E.; Gerencser, V.F.; Clark, S.M. Parasitization of humans in West Virginia by Ixodes cookei (Acari: Ixodidae), a potential vector of Lyme borreliosis. J. Med. Entomol. 1991, 28, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.A.; Durden, L.A. A state-by-state survey of ticks recorded from humans in the United States. J. Vect. Ecol. 2000, 25, 102–113. [Google Scholar]
- Scott, J.D.; Foley, J.E.; Anderson, J.F.; Clark, K.L.; Durden, L.A. Detection of Lyme disease bacterium, Borrelia burgdorferi sensu lato, in blacklegged ticks collected in the Grand River Valley, Ontario, Canada. Int. J. Med. Sci. 2017, 14, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, K.; Chandrashekar, R.; Beall, M.; Leutenegger, C.; Lappin, M.R. Evidence for clinical anaplasmosis and borreliosis in cats in Maine. Top. Companion Anim. Med. 2018, 33, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Savick, K.; Butler, J. Babesia microti in rodents and raccoons from northeast Florida. J. Parasitol. 2012, 98, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Mintz, E.D.; Gadbaw, J.J.; Magnarelli, L.A. Babesia microti, human babesiosis, and Borrelia burgdorferi in Connecticut. J. Clin. Microbiol. 1991, 29, 2779–2783. [Google Scholar]
- Anderson, J.F.; Armstrong, P.M. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am. J. Trop. Med. Hyg. 2012, 87, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R., III; Mather, T.N.; Moore, S.I.; Wilson, M.L.; Spielman, A. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 1988, 39, 105–109. [Google Scholar] [CrossRef]
- Brown, J.H. The rabbit tick Haemaphysalis leporispalustris (Pack.) as an ectoparasite on man. Can. Entomol. 1945, 77, 176. [Google Scholar] [CrossRef]
- Banerjee, S.N.; Banerjee, M.; Fernando, K.; Dong, M.Y.; Smith, J.A.; Cook, D. Isolation of Borrelia burgdorferi, the Lyme disease spirochete from rabbit ticks, Haemaphysalis leporispalustris from Alberta. J. Spir. Tick-Borne Dis. 1995, 2, 23–24. [Google Scholar]
- Herc, E.; Pritt, B.; Huizenga, T.; Douce, R.; Hysell, M.; Newton, D.; Sidge, J.; Losman, E.; Sherbeck, J.; Kaul, D.R. Probable locally acquired Babesia divergens-like infection in woman, Michigan, USA. Emerg. Infect. Dis. 2018, 24, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Goethert, H.K.; Telford, S.R. Enzootic transmission of Babesia divergens among cottontail rabbits on Nantucket Island, Massachusetts. Am. J. Trop. Med. Hyg. 2003, 69, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Clark, K.L.; Foley, J.E.; Anderson, J.F.; Durden, L.A.; Manord, J.M.; Smith, M.L. Detection of Borrelia genomospecies 2 in Ixodes spinipalpis ticks collected from a rabbit in Canada. J. Parasitol. 2017, 103, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, S.L.; Naik, M.; Abdel-Hamid, H. Child Neurology: Tick paralysis: A diagnosis not to miss. Neurology 2014, 82, e91–e93. [Google Scholar] [CrossRef]
- Scott, J.D. First record of locally acquired human babesiosis in Canada caused by Babesia duncani: A case report. SAGE Open Med. Case Rep. 2017, 5, 2050313X17725645. [Google Scholar]
- Benach, J.L.; Habicht, G.S. Clinical characteristics of human babesiosis. J. Infect. Dis. 1981, 144, 481. [Google Scholar] [CrossRef]
- Homer, M.J.; Aquilar-Delfin, I.; Telford, S.R., III; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef]
- Ruebush, T.K.; Cassaday, P.B.; Marsh, H.J.; Lisker, S.A.; Voorhees, D.B.; Mahoney, E.B.; Healy, G.R. Human babesiosis on Nantucket Island. Ann. Intern. Med. 1977, 86, 6–9. [Google Scholar] [CrossRef]
- Mayne, P.J. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int. J. Gen. Med. 2014, 8, 15–26. [Google Scholar] [CrossRef]
- Hatcher, J.C.; Greenberg, P.D.; Antique, J.; Jimenez-Lucho, V.E. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin. Infect. Dis. 2001, 32, 1117. [Google Scholar] [CrossRef]
- Marcus, L.C.; Steere, A.C.; Duray, P.H.; Anderson, A.E.; Mahoney, E.B. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis: Demonstration of spirochete in myocardium. Ann. Intern. Med. 1985, 103, 374–376. [Google Scholar] [CrossRef] [PubMed]
- LeBel, D.P.; Moritz, E.D.; O’Brien, J.J.; Lazarchick, J.; Tormos, L.M.; Duong, A.; Fontaine, M.J.; Squires, J.E.; Stramer, S.L. Cases of transfusion-transmitted babesiosis occurring in nonendemic areas: A diagnostic dilemma. Transfusion 2017, 57, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Levin, A.E.; Williamson, P.C.; Cyrus, S.; Shaz, B.H.; Kessler, D.; Gorlin, J.; Bruhn, R.; Lee, T.-H.; Montalvo, L.; et al. A prospective evaluation of chronic Babesia microti infection in seroreactive blood donors. Transfusion 2016, 56, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, T.; Karp, J.K. Transfusion-transmitted babesiosis. Arch. Pathol. Lab. Med. 2019, 143, 130–134. [Google Scholar] [CrossRef]
- Klevens, R.M.; Cumming, M.A.; Caten, E.; Stramer, S.L.; Townsend, R.L.; Tonnetti, L.; Rios, J.; Young, C.T.; Soliva, S.; DeMaria, A., Jr. Transfusion-transmitted babesiosis: One state’s experience. Transfusion 2018, 58, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; Winger, S.; Ahmed, A.; Arnold, A.; Chou, J.; Rhein, L.; Levy, O. Neonatal babesiosis: Case report and review of the literature. Pediatr. Infect. Dis. J. 2006, 25, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cornett, J.K.; Malhotra, A.; Hart, D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect. Dis. Clin. Pract. 2012, 20, 408–410. [Google Scholar] [CrossRef]
- Iyer, S.; Goodman, K. Congenital babesiosis from maternal exposure: A case report. J. Emerg. Med. 2009, 56, e39–e41. [Google Scholar] [CrossRef]
- Khangura, R.K.; Williams, N.; Cooper, S.; Prabulos, A.M. Babesiosis in pregnancy: An imitator of HELLP syndrome. AJP Rep. 2019, 9, e147–e152. [Google Scholar] [CrossRef]
- Krause, P.J.; Spielman, A.; Telford, S.R., III; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef]
- Abraham, A.; Brasov, I.; Thekkiniath, J.; Kilian, N.; Lawres, L.; Gao, R.; DeBus, K.; He, L.; Yu, X.; Zhu, G.; et al. Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J. Biol. Chem. 2018, 293, 19974–19981. [Google Scholar] [CrossRef] [PubMed]
- Liegner, K.B. Disulfiram (tetraethylthiuram disulfide) in the treatment of Lyme disease and babesiosis: Report of experience in three cases. Healthcare 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Concurrent neotropical borreliosis and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1988, 539, 468–470. [Google Scholar] [CrossRef]
- Sapi, E.; MacDonald, A. Biofilms of Borrelia burgdorferi in chronic cutaneous borreliosis. Am. J. Clin. Pathol. 2008, 129, 988–989. [Google Scholar]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef]
- Oksi, J.; Mertsola, J.; Reunanen, M.; Marjamäki, M.; Viljanen, M.K. Subacute multiple-site osteomyelitis caused by Borrelia burgdorferi. Clin. Infect. Dis. 1994, 19, 891–896. [Google Scholar] [CrossRef]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamäki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Inflammatory brain changes in Lyme borreliosis: A report on three patients and review of literature. Brain 1996, 119, 2143–2154. [Google Scholar] [CrossRef]
- MacDonald, A.B. Alzheimer’s neuroborreliosis and trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med. Hypotheses 2007, 68, 822–825. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease—A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 2011, 8, 90. [Google Scholar] [CrossRef]
- Preac-Mursic, V.; Pfister, H.W.; Spiegel, H.; Burk, R.; Wilske, B.; Reinhardt, S.; Böhmer, R. First isolation of Borreia burgdorferi from an iris biopsy. J. Clin. Neuroophthalmol. 1993, 13, 155–161. [Google Scholar]
- Frey, M.; Jaulhac, B.; Piemont, Y.; Marcellin, L.; Boohs, P.M.; Vautravers, P.; Jesel, M.; Kuntz, J.L.; Monteil, H.; Sibilia, J. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am. J. Med. 1998, 104, 591–594. [Google Scholar] [CrossRef]
- Häupl, T.; Hahn, G.; Rittig, M.; Krause, A.; Schoerner, C.; Schönherr, U.; Kalden, J.R.; Burmester, G.R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993, 36, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.E. Damage of collagen and elastic fibres by Borrelia burgdorferi—Known and new clinical histopathogical aspects. Open Neurol. J 2012, 6 (Suppl. S1-M11), S179–S186. [Google Scholar]
- Livengood, J.A.; Gilmore, R.D., Jr. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Borda, J.T.; Dufour, J.; Kaushal, D.; Ramamoorthy, R.; Lackner, A.A.; Philipp, M.T. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol. 2008, 173, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Santana-Gould, L.; Inglis, F.M.; England, J.D.; Philipp, M.T. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J. Neuroinflamm. 2013, 10, 88. [Google Scholar] [CrossRef]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef]
- Stricker, R.B. Counterpoint: Long-term antibiotic therapy improves persistent symptoms associated with Lyme disease. Clin. Infect. Dis. 2007, 45, 149–157. [Google Scholar] [CrossRef]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Bransfield, R.C. Suicide and Lyme and associated diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C. Aggressiveness, violence, homocidality, homicide, and Lyme disease. Neuropsychiatr. Dis. Treat. 2018, 14, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C.; Cook, M.J.; Bransfield, D.R. Proposed Lyme disease guidelines and psychiatric illnesses. Healthcare 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Liegner, K.B.; Ziska, M.; Agricola, M.D.; Hubbard, J.D.; Klempner, M.S.; Coyle, P.K.; Bayer, M.E.; Duray, P.H. Fatal chronic meningoencephalomyelitis with massive hydocephalus, in a New York state patient with evidence of Borrelia burgdorferi (Bb) exposure. In Proceedings of the Sixth International Conference on Lyme Borreliosis, Bologna, Italy, 19–22 June 1994. Abstract P041T. [Google Scholar]
- Liegner, K.B.; Duray, P.; Agricola, M.; Rosenkilde, C.; Yannuzzi, L.A.; Ziska, M.; Tilton, R.C.; Hulinska, D.; Hubbard, J.; Fallon, B.A. Lyme disease and the clinical spectrum of antibiotic responsive chronic meningoencephalomyelitides. J. Spir. Tick-Borne Dis. 1997, 4, 61–73. [Google Scholar]
- Liegner, K.B.; Jones, C.R. Fatal progressive encephalitis following an untreated deer tick attachment in a 7 year-old Fairfield County, Connecticut child. In Proceedings of the VIII International Conference on Lyme Borreliosis, Abstract P380, Munich, Germany, 20–24 June 1999. [Google Scholar]
- Fallon, B.A.; Pavlicova, M.; Coffino, S.W.; Brenner, C. A comparison of Lyme disease serologic test results from four laboratories in patients with persistent symptoms after antibiotic treatment. Clin. Infect. Dis. 2014, 59, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Paparone, P.W.; Paparone, P.A. Lyme disease in the elderly. J. Spir. Tick-Borne Dis. 1995, 2, 14–18. [Google Scholar]
- Schutzer, S.E.; Coyle, P.K.; Reid, P.; Holland, B. Borrelia burgdorferi-specific immune complexes in acute Lyme disease. JAMA 1999, 282, 1942–1945. [Google Scholar] [CrossRef]
- Stricker, R.B.; Johnson, L. Lyme disease diagnosis and treatment: Lessions from the AIDS epidemic. Minerva Med. 2010, 101, 419–425. [Google Scholar]
- Berndtson, K. Review of evidence from immune evasion and persistent infection in Lyme disease. Int. J. Gen. Med. 2013, 6, 291–306. [Google Scholar] [CrossRef]
- Feder, H. Borrelia infections: Lyme disease. In Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant, 8th ed.; Wilson, C.B., Nizet, V., Maldonado, Y., Remington, J.S., Klein, J.O., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 544–557. ISBN 9780321472. [Google Scholar]
- MacDonald, A.B. Gestational Lyme borreliosis: Implications for the fetus. In Rheumatic Disease Clinics of North America; Johnson, R.C., Ed.; Saunders: Philadelphia, PA, USA, 1989; Volume 15, pp. 657–677. Available online: http://www.molecularalzheimer.org/files/Gestational_Lyme_Borreliosis-Annotated_1989.pdf (accessed on 14 October 2017).
- Horowitz, R.I. Lyme disease and pregnancy: Implications of chronic infection, PCR testing, and prenatal treatment. In Proceedings of the 16th International Scientific Conference on Lyme Disease & Other Tick-Borne Disorders, Hartford, CT, USA, 7–8 June 2003. [Google Scholar]
- Trevisan, G.; Stinco, G.; Cinco, M. Neonatal skin lesions due to spirochetal infection: A case of congenital Lyme bororeliosis? Int. J. Dermatol. 1997, 36, 677–680. [Google Scholar]
- Lavoie, P.E.; Lattner, B.P.; Duray, P.H.; Barbour, A.G.; Johnson, P.C. Culture positive seronegative transplacental Lyme borreliosis infant mortality. Arthritis Rheum. 1987, 30, S50. [Google Scholar]
- Burrascano, J.J. Transmission of Borrelia burgdorferi by blood transfusion. In Proceedings of the V International Conference on Lyme Borreliosis, Biology of Borrelia Burgdorferi II, Abstract 265A, Arlington, VA, USA, 30 May–2 June 1992. [Google Scholar]
- Sapi, E.; Pabbati, N.; Datar, A.; Davies, E.M.; Kuo, B.A. Improved culture conditions for the growth and detection of Borrelia from human serum. Int. J. Med. Sci 2013, 10, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, F.R.; Hoffmann, E. VorläuFigureer Bericht über das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen. Arb. Kais. Gesundh. 1905, 22, 527–534. [Google Scholar]
- Fesler, M.C.; Middelveen, M.J.; Burke, J.M.; Stricker, R.B. Erosive vulvovaginitis associated with Borrelia burgdorferi infection. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619842901. [Google Scholar] [CrossRef]





| No. of Ticks Collected from Hosts and No. of Ticks Infected | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of | I. scapularis | No. of | Pathogens Detected | ||||||||||
| Hosts | Hosts | Ain | Da | Dv | Hlp | Ic | Imu | L | N | F | Ticks | Bbsl | Bab |
| Birds | |||||||||||||
| House Wren, Troglodytes aedon (Vieillot) | 4 | 0 | 0 | 0 | 0 | 0 | 1L | 0 | 3 | 0 | 4 | 0 | 0 |
| Ovenbird, Seiurus aurocapilla (L.) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Common Yellowthroat, Geothlypis trichas (L.) | 20 | 0 | 0 | 0 | 0 | 0 | 2L*, 1N | 7 | 21 ***** | 0 | 31 | 6 | 0 |
| White-throated Sparrow, Zonotrichia albicollis (Gmelin) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Nashville Warbler, Oreothlypis ruficapilla (Wilson) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Northern Waterthrush, Parkesia noveboracensis (Gmelin) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Red-breasted Grosbeak, Pheucticus ludovicianus (L.) | 2 | 0 | 0 | 0 | 0 | 0 | 1N * | 0 | 0 | 0 | 1 | 1 | 0 |
| Veery, Catharus fuscescens (Stephens) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 1 |
| Gray Catbird, Dumetella carolinensis (L.) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 ** | 0 | 3 | 0 | 2 |
| Lincoln’s Sparrow, Melospiza lincolnii (Audubon) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 * | 0 | 2 | 0 | 1 |
| Baltimore Oriole, Icterus galbula (L.) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Song Sparrow, Melospiza melodia (Wilson) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 5 | 0 | 0 |
| Swainson’s Thrush, Catharus ustulatus (Nuttall) | 4 | 0 | 0 | 0 | 13L, 4N | 0 | 4L, 1N | 0 | 2 | 0 | 24 | 0 | 0 |
| Magnolia Warbler, Setophaga magnolia (Wilson) | 1 | 0 | 0 | 0 | 0 | 0 | 4L*, 2N | 0 | 0 | 0 | 6 | 1 | 0 |
| Hermit Thrush, Catharus guttatus (Pallas) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Canada Warbler, Cardellina canadensis (L.) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 |
| Mammals ⊗ | |||||||||||||
| Domestic dog, Canis lupus familiaris L. | 7 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 6 ** | 7 | 2 | 0 |
| Domestic cat, Felis catus (L.) | 4 | 0 | 0 | 0 | 0 | 1N | 0 | 0 | 0 | 3 | 4 | 1 | 1 |
| Horse, Equus ferus caballus L. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 * | 1 | 1 | 0 |
| Moose, Alces alces Gray | 1 | 0 | 11M, 5F* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 1 | 0 |
| Snowshoe hare, Lepus americanus Erxleben | 1 | 0 | 0 | 0 | 1M | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cottontail rabbit, Sylvilagus floridanus (J.A. Allen) | 3 | 0 | 0 | 0 | 3N,4M,7F | 0 | 0 | 0 | 0 | 0 | 14 | 1 | 1 |
| Human, Homo sapiens L. | 3 | 0 | 0 | 3M, 4F | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 |
| Source | Province, | Tick Species, | 18S rRNA GenBank | Co-infection |
|---|---|---|---|---|
| Site * | Life Stage | Accession Numbers | Yes/No | |
| House Wren | ON, 5 | I. scapularis, nymph | MN058030 | No |
| Vegetation | ON, 10 | I. scapularis, male | MK986467 | No |
| Vegetation | ON, 9 | I. scapularis, female | MK986468 | Yes ‡1 |
| Vegetation | ON, 9 | I. scapularis, female | MK986469 | No |
| Vegetation | ON, 6 | I. scapularis, male | MK986470 | Yes ‡2 |
| Gray Catbird | ON, 5 | I. scapularis, nymph | MK986471 | No |
| Gray Catbird | ON, 5 | I. scapularis, nymph | MK986472 | No |
| Eastern cottontail rabbit | MB, 13 | H. leporispalustris, female | MK986487 | Yes ‡3 |
| Domestic cat | ON, 3 | I. cookei, nymph | MK986488 | Yes ‡4 |
| Veery | ON, 5 | I. scapularis, nymph | MK628544§ | Yes ‡5 |
| Lincoln’s Sparrow | ON, 11 | I. scapularis, nymph | MK986473 | No |
| Source | Province, | Tick Species, | flaB Gene GenBank | Co-infection |
|---|---|---|---|---|
| Site * | Life Stage | Accession Numbers | Yes/No | |
| House Wren ♦ | QC,1 | I. muris, larva | MH290738 † | No |
| Domestic cat | ON, 3 | I. cookei, nymph | MN073831 | Yes ‡4 |
| Common Yellowthroat | ON, 5 | I. muris, larva | MN073832 | No |
| Magnolia Warbler | QC, 1 | I. muris, larva | MN073833 | No |
| Vegetation | ON, 9 | I. scapularis, female | MN073834 | Yes ‡1 |
| Common Yellowthroat ⸿ | QC, 1 | I. scapularis, nymph | MN080502 | No |
| Common Yellowthroat ⸿ | QC, 1 | I. scapularis, nymph | MN080503 | No |
| Vegetation | ON, 6 | I. scapularis, female | MN080504 | Yes ‡2 |
| Horse | ON, 4 | I. scapularis, female | MN086887 | No |
| Vegetation | ON, 6 | I. scapularis, male | MN086888 | No |
| Eastern cottontail rabbit | MB, 13 | H. leporispalustris, female | MN086889 | Yes ‡3 |
| Veery | ON, 5 | I. scapularis, nymph | MK620851 § | Yes ‡5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.D.; Clark, K.L.; Coble, N.M.; Ballantyne, T.R. Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada. Healthcare 2019, 7, 155. https://doi.org/10.3390/healthcare7040155
Scott JD, Clark KL, Coble NM, Ballantyne TR. Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada. Healthcare. 2019; 7(4):155. https://doi.org/10.3390/healthcare7040155
Chicago/Turabian StyleScott, John D., Kerry L. Clark, Nikki M. Coble, and Taylor R. Ballantyne. 2019. "Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada" Healthcare 7, no. 4: 155. https://doi.org/10.3390/healthcare7040155
APA StyleScott, J. D., Clark, K. L., Coble, N. M., & Ballantyne, T. R. (2019). Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada. Healthcare, 7(4), 155. https://doi.org/10.3390/healthcare7040155





