Effect of Decontamination Treatments on Micro-Shear Bond Strength between Blood–Saliva-Contaminated Post-Etched Dentin Substrate and Composite Resin
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Morley, J.; Eubank, J. Macroesthetic elements of smile design. J. Am. Dent. Assoc. 2001, 132, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, M.G.; Matsui, A.; Gwinnett, A.J. Penetration of resin dental material into enamel surfaces with reference to bonding. Arch. Oral. Biol. 1968, 13, 61–70. [Google Scholar] [CrossRef]
- Yoshida, Y.; van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Van Schalkwyk, J.H.; Botha, F.S.; van der Vyver, P.J.; de Wet, F.A.; Botha, S.J. Effect of biological contamination on dentine bond strength of adhesive resins. SADJ 2003, 58, 143–147. [Google Scholar] [PubMed]
- Sattabanasuk, V.; Shimada, Y.; Tagami, J. Effects of saliva contamination on dentin bond strength using all-in-one adhesives. J. Adhes. Dent. 2006, 8, 311–318. [Google Scholar]
- De Carvalho, M.E.C.; Vieira, S.N.; Kawaguchi, F.A.; Powers, J.; Matos, A.B. Influence of blood contamination on bond strength of a selfetching system. Eur. J. Dent. 2010, 4, 280–286. [Google Scholar]
- Damé, J.L.D.; Torriani, D.D.; Demarco, F.F. Effect of blood contamination and decontamination procedures on marginal adaptation and bond strength of composite restorations. Rev. Odonto Ciência 2009, 24, 283–289. [Google Scholar]
- Elkassas, D.; Arafa, A. Assessment of post-contamination treatments affecting different bonding stages to dentin. Eur. J. Dent. 2016, 10, 327–332. [Google Scholar] [CrossRef]
- Juneja, R.; Duhan, J.; Tewari, S.; Sangwan, P.; Bhatnagar, N. Effect of blood contamination and decontamination protocols on acetone-based and ethanol-based total etch adhesive systems. J. Esthet. Restor. Dent. 2014, 26, 403–416. [Google Scholar] [CrossRef]
- Faltermeier, A.; Behr, M.; Rosentritt, M.; Reicheneder, C.; Mussig, D. An in vitro comparative assessment of different enamel contaminants during bracket bonding. Eur. J. Orthod. 2007, 29, 559–563. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, K.C. The influence of salivary contamination on shear bond strength of dentin adhesive systems. Oper. Dent. 2004, 29, 437–442. [Google Scholar] [PubMed]
- Lee, S.B.; Gonzalez-Cabezas, C.; Kim, K.M.; Kim, K.N.; Kuroda, K. Catechol-Functionalized Synthetic Polymer as a Dental Adhesive to Contaminated Dentin Surface for a Composite Restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.M.; Pereira, P.N. Effect of blood contamination with 1-step selfetching adhesives on microtensile bond strength to dentin. Oper. Dent. 2006, 31, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Preis, V.; Rosentritt, M. Effect of Decontamination and Cleaning on the Shear Bond Strength of High Translucency Zirconia. Dent. J. 2017, 5, 32. [Google Scholar] [CrossRef]
- Prasad, M.; Mohamed, S.; Nayak, K.; Shetty, S.K.; Talapaneni, A.K. Effect of moisture, saliva, and blood contamination on the shear bond strength of brackets bonded with a conventional bonding system and self-etched bonding system. J. Nat. Sci. Biol. Med. 2014, 5, 123–129. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G * Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Dietrich, T.; Kraemer, M.L.; Roulet, J.F. Blood contamination and dentin bonding-effect of anticoagulant in laboratory studies. Dent. Mater. 2002, 18, 159–162. [Google Scholar] [CrossRef]
- Torres, C.P.; Corona, S.A.; Ramos, R.P.; Palma-Dibb, R.G.; Borsatto, M.C. Bond strength of self-etching primer and total-etch adhesive systems to primary dentin. J. Dent. Child 2004, 71, 131–134. [Google Scholar]
- Dos Santos, R.A.; de Lima, E.A.; Pontes, M.M.d.A.; do Nascimento, A.B.L.; Montes, M.A.J.R.; Braz, R. Bond strength to dentin of total-etch and self-etch adhesive systems. RGO Rev. Gaúcha Odontol. 2014, 62, 365–370. [Google Scholar] [CrossRef][Green Version]
- Albaladejo, A.; Osorio, R.; Toledano, M.; Ferrari, M. Hybrid layers of etch-and-rinse versus self-etching adhesive systems. Med. Oral. Patol. Oral. Cir. Bucal. 2010, 15, e112–e118. [Google Scholar] [CrossRef]
- Agostini, F.G.; Kaaden, C.; Powers, J.M. Bond strength of self-etching primers to enamel and dentin of primary teeth. Pediatr. Dent. 2001, 23, 481–486. [Google Scholar] [PubMed]
- Van Meerbeek, B.; de Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 3, 215–235. [Google Scholar]
- Rajagopal, R.; Padmanabhan, S.; Gnanamani, J. A comparison of shear bond strength and debonding characteristics of conventional, moistureinsensitive, and self-etching primers in vitro. Angle Orthod. 2004, 74, 264.e8. [Google Scholar] [PubMed]
- El-Kalla, I.H.; García-Godoy, F. Saliva contamination and bond strength of single-bottle adhesives to enamel and dentin. Am. J. Dent. 1997, 10, 83–87. [Google Scholar]
- Kaneshima, T.; Yatani, H.; Kasai, T.; Watanabe, E.K.; Yamashita, A. The influence of blood contamination on bond strengths between dentin and an adhesive resin cement. Oper. Dent. 2000, 25, 195–201. [Google Scholar]
- Sayinsu, K.; Isik, F.; Sezen, S.; Aydemir, B. Effect of blood and saliva contamination on bond strength of brackets bonded with a protective liquid polish and a light-cured adhesive. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 391–394. [Google Scholar] [CrossRef]
- Oonsombat, C.; Bishara, S.E.; Ajlouni, R. The effect of blood contamination on the shear bond strength of orthodontic brackets with the use of a new self-etch primer. Am. J. Orthod. Dentofac. Orthop. 2003, 123, 547–550. [Google Scholar] [CrossRef]
- Öztoprak, M.O.; Isik, F.; Sayınsu, K.; Arun, T.; Aydemir, B. Effect of blood and saliva contamination on shear bond strength of brackets bonded with 4 adhesives. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 238–242. [Google Scholar] [CrossRef]
- Kermanshah, H.; Ghabraei, S.; Bitaraf, T. Effect of salivary contamination during different bonding stages on shear dentin bond strength of one-step self-etch and total etch adhesive. J. Dent. 2010, 7, 132–138. [Google Scholar]
- Townsend, R.D.; Dunn, W.J. The effect of saliva contamination on enamel and dentin using a self etching adhesive. J. Am. Dent. Assoc. 2004, 135, 895–901. [Google Scholar] [CrossRef]
- Soares, C.J.; Branco, C.A.; Soares, P.B.F.; Fonseca, R.B.; Carlo, H.L.; Neto, A.J.F. Effect of blood contamination during adhesive restorative procedures on dentin-resin cement shear bond strength. Braz. J. Oral. Sci. 2007, 6, 1320–1325. [Google Scholar]
- Raffaini, M.S.; Gomes-Silva, J.M.; Torres-Mantovani, C.P.; Palma-Dibb, R.G.; Borsatto, M.C. Effect of blood contamination on the shear bond strength at resin/dentin interface in primary teeth. Am. J. Dent. 2008, 21, 159–162. [Google Scholar] [PubMed]
- Chang, S.W.; Cho, B.-H.; Lim, R.Y.; Kyung, S.H.; Park, D.S.; Oh, T.S.; Yoo, H.M. Effects of blood contamination on microtensile bond strength to dentin of three self-etch adhesives. Oper. Dent. 2010, 35, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Mutluay, M.; Seseogullari-Dirihan, R.; Agee, K.A.; Key, W.O.; Scheffel, D.L.; Breschi, L.; Mazzoni, A.; Tjäderhane, L.; Nishitani, Y.; et al. Effect of phosphoric acid on the degradation of human dentin matrix. J. Dent. Res. 2013, 92, 87–91. [Google Scholar] [CrossRef]
- Furuse, A.Y.; da Cunha, L.F.; Benetti, A.R.; Mondelli, J. Bond strength of resin-resin interfaces contaminated with saliva and submitted to different surface treatments. J. Appl. Oral. Sci. 2007, 15, 501–505. [Google Scholar] [CrossRef]
- Vargas, M.A.; Cobb, D.S.; Armstrong, S.R. Resin-dentin shear bond strength and interfacial ultrastructure with and without a hybrid layer. Oper. Dent. 1997, 22, 159–166. [Google Scholar]
- Fawzy, A.S.; Amer, M.A.; El-Askary, F.S. Sodium hypochlorite as dentin pretreatment for etch-and-rinse single-bottle and two-step self-etching adhesives: Atomic force microscope and tensile bond strength evaluation. J. Adhes Dent. 2008, 10, 135–144. [Google Scholar]
- Morris, M.D.; Lee, K.-W.; Agee, K.A.; Bouillaguet, S.; Pashley, D.H. Effects of sodium hypochlorite and RC-prep on bond strengths of resin cement to endodontic surfaces. J. Endod. 2001, 27, 753–757. [Google Scholar] [CrossRef]
- Misra, D.N. Interaction of chlorhexidine digluconate with and adsorption of chlorhexidine on hydroxyapatite. J. Biomed. Mater. Res. 1994, 28, 1375–1381. [Google Scholar] [CrossRef]
- Di Hipolito, V.; Rodrigues, F.P.; Piveta, F.B.; Azevedo Lda, C.; Alonso, R.C.B.; Silikas, N.; Carvalho, R.M.; de Goes, M.F.; D’Alpino, P.H.P. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dent. Mater. 2012, 28, 495–501. [Google Scholar] [CrossRef]
- Hassan, A.M.; Goda, A.A.; Baroudi, K. The effect of different disinfecting agents on bond strength of resin composites. Int. J. Dent. 2014, 2014, 231235. [Google Scholar]
- De Castro, F.L.A.; De Andrade, M.F.; Júnior, S.L.L.D.; Vaz, L.G.; Ahid, F.J.M. Effect of 2% chlorhexidine on microtensile bond strength of composite to dentin. J. Adhes. Dent. 2003, 5, 129–138. [Google Scholar] [PubMed]
- Yoshikawa, H.; Hirano, A.; Arakawa, T.; Shiraki, K. Mechanistic insights into protein precipitation by alcohol. Int. J. Biol. Macromol. 2012, 50, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Saraç, D.; Bulucu, B.; Saraç, Y.S.; Kulunk, S. The effect of dentin-cleaning agents on resin cement bond strength to dentin. J. Am. Dent. Assoc. 2008, 139, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Al-Twaijri, S.; Viana, G.; Bedran-Russo, A.K. Effect of prophylactic pastes containing active ingredients on the enamel-bracket bond strength of etch-and-rinse and self-etching systems. Angle Orthod. 2011, 81, 788–793. [Google Scholar] [CrossRef] [PubMed]
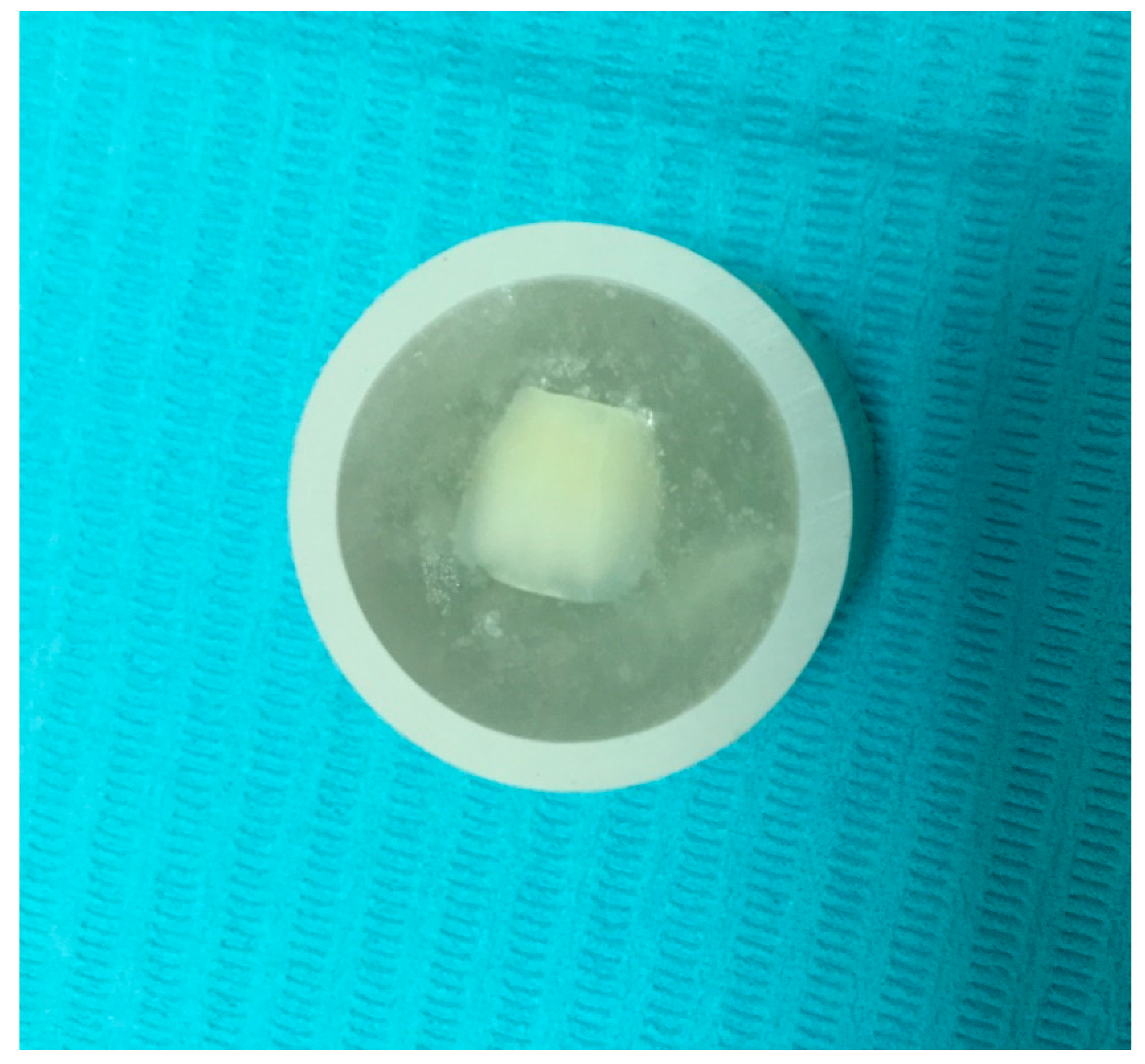
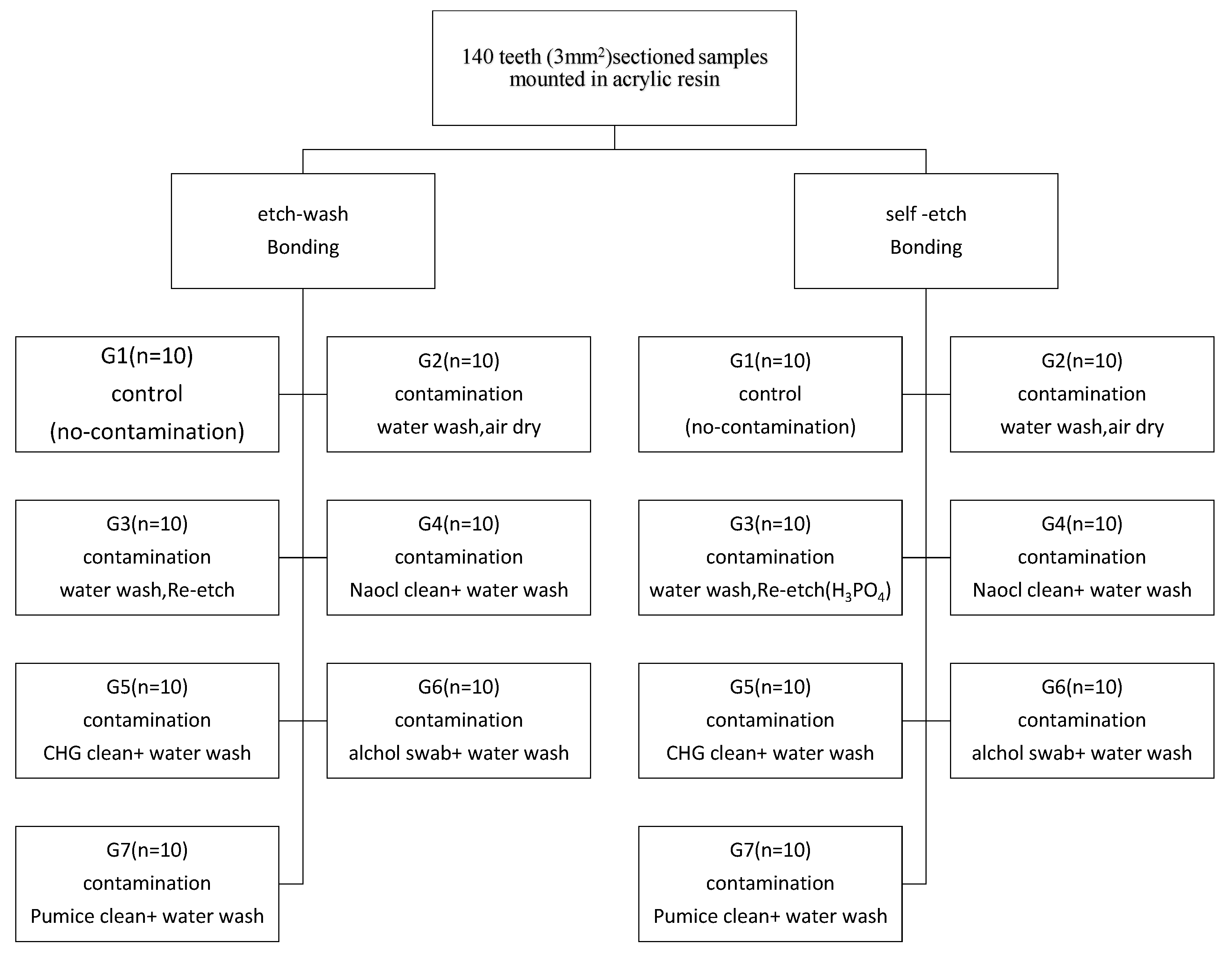
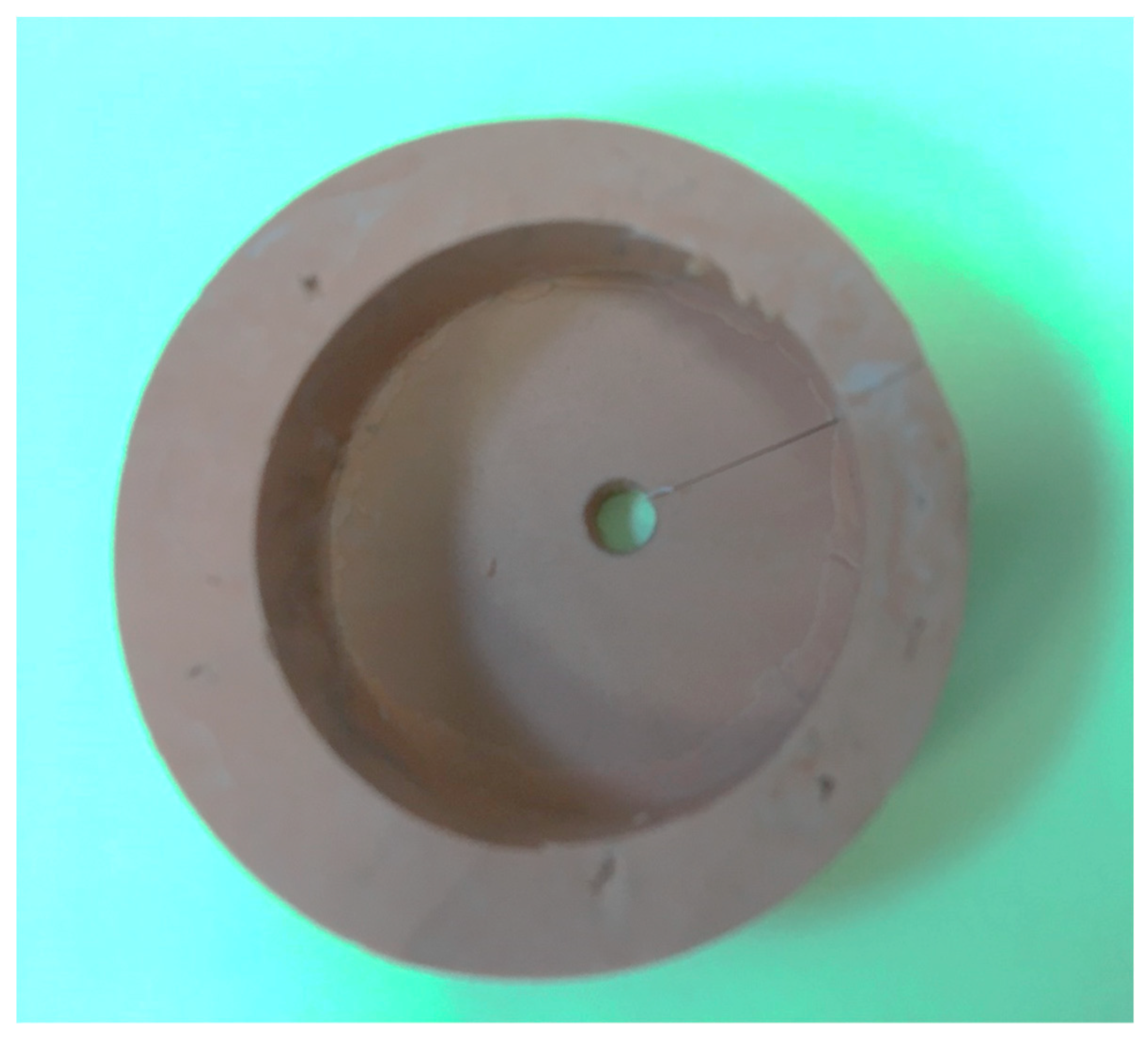

| Group | Total-Etch | Self-Etch |
|---|---|---|
| No contamination | 37.5% phosphoric acid gel (Kerr, Karlsruhe, Germany) 15 s. Water rinse 10 s, gentle air dry. Apply bonding agent (Tetric-N Bond total etch, Ivoclar Vivadent AG, Schaan, Liechtenstein). Brush the material gently into the dentin for 10 s, air dry and light cure 10 s (LED unit (700 mW/cm2) | Applied one layer of one bottle self-etch bonding agent (G-ænial, GC America Inc. St, Alsip, IL, USA) left undisturbed 10 s, dry thoroughly for 5 s, light cure 10 s with LED unit (700 mW/cm2) |
| Contamination water rinse | Contaminated the etched surface with blood/saliva mix for 1 min, water rinsed for 10 s, gently air dried for 5 s, application of bonding agent, composite application | Contaminated the self-etched surface with blood/saliva mix for 1 min, water rinsed for 10 s, Gently air dried for 5 s. Re-application of adhesive |
| Contamination, water wash, Re-etch | Blood/saliva contamination of etched surface, water rinsed, air dried, subsequently re-etched with 37.5% H3PO4 for 10 s, application of bonding agent | Contaminated the self-etched surface with blood/saliva mix, water rinsed, air dried, re-etched with 37.5% H3PO4 for 10 s, application of self-etch bonding agent |
| Contamination Naocl clean, water wash | Blood/saliva contamination of etched surface, 6% sodium hypochlorite (Vita dental products, Racine, WI, USA) applied with micro brush for 15 s, water rinse for 10 s, application of bonding agent | Contaminated the self-etched surface with blood/saliva mix, 6% sodium hypochlorite applied with micro brush for 15 s, water rinse for 10 s, re-apply self-etch bonding |
| Contamination, water wash CHG disinfection | Blood/saliva contamination of etched surface, water rinse for 10 s, 2% chlorhexidine gluconate (Consepsis, Ultradent INC, South Jordan, UT, USA) rub the area for 10 s, air dried and bonding agent application | Contaminated the self-etched surface with blood/saliva mix, water rinse for 10 s, 2% chlorhexidine gluconate (Consepsis, Ultradent INC, South Jordan, UT, USA) rub the area for 10 s, air dried and re-apply self-etch bonding |
| Contamination, water wash, isopropyl alcohol swabs | Blood/saliva contamination of etched surface, water rinse for 10 s, rub the surface with 70% isopropyl alcohol swabs (sterie swab), for 10 s, bonding agent application | Contaminated the self-etched surface with blood/saliva mix, water rinse for 10 s, rub the surface with isopropyl alcohol swabs for 10 s, re-apply self–etch bonding |
| Contamination, cleaning with pumice, alcohol swabs | Blood/saliva contamination of etched surface, water rinse, rub the area with un-fluoridated pumice for 15 s at 2000 rpm prophy brush, water rinse, bond agent application | Blood/saliva contamination of etched surface, water rinse, rub the area with un-fluoridated pumice for 15 g, water rinse, re-apply self-etch bonding |
| Group | N | Total-Etch | Self-Etch |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| No Contamination | 10 | 25.93 (0.96) | 10.10 (0.59) |
| Wash | 10 | 20.29 (1.08) | 8.83 (0.81) |
| Re-Etch | 10 | 24.58 (0.94) | 9.23 (0.83) |
| NaOCl | 10 | 23.25 (1.08) | 7.20 (0.56) |
| CHG | 10 | 14.95 (0.80) | 6.58 (0.45) |
| IPA | 10 | 13.14 (0.86) | 6.25 (0.66) |
| Pumice | 10 | 9.73 (0.83) | 7.79 (0.97) |
| Surface Treatment | Total-Etch Bonding | Self-Etch Bonding | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Rank | χ2 | df | p | Mean Rank | χ2 | df | p | |
| No Contamn | 63.50 | 65.768 | 6 | 0.000 | 62.30 | 54.33 | 6 | 0.000 |
| Wash | 35.60 | 48.25 | ||||||
| Re-Etch | 55.95 | 52.30 | ||||||
| NaOCl | 46.95 | 26.25 | ||||||
| CHG | 25.00 | 15.35 | ||||||
| IPA | 16.00 | 11.25 | ||||||
| Pumice | 5.50 | 32.80 | ||||||
| Group | Wash | Re-Etch | NaOCl | CHG | IPA | Pumice |
|---|---|---|---|---|---|---|
| No Contamn | 0.000 | 0.016 | 0.000 | 0.000 | 0.000 | 0.000 |
| Wash | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Re-Etch | 0.006 | 0.000 | 0.000 | 0.000 | ||
| NaOCl | 0.000 | 0.000 | 0.000 | |||
| CHG | 0.001 | 0.000 | ||||
| IPA | 0.000 |
| Group | Wash | Re-Etch | NaOCl | CHG | IPA | Pumice |
|---|---|---|---|---|---|---|
| No Contamn | 0.003 | 0.023 | 0.000 | 0.000 | 0.000 | 0.000 |
| Wash | 0.199 | 0.000 | 0.000 | 0.000 | 0.007 | |
| Re-Etch | 0.000 | 0.000 | 0.000 | 0.010 | ||
| NaOCl | 0.021 | 0.002 | 0.173 | |||
| CHG | 0.273 | 0.007 | ||||
| IPA | 0.004 |
| Group | Total-Etch | One-Step Self-Etch | ||||
|---|---|---|---|---|---|---|
| Adhesive | Cohesive | Mixed | Adhesive | Cohesive | Mixed | |
| No contamination | 0 | 3 | 7 | 1 | 3 | 6 |
| Water Irrigation | 4 | 2 | 4 | 3 | 2 | 5 |
| Re-etch (37% H3PO4) | 1 | 3 | 6 | 2 | 2 | 6 |
| NaOCl | 3 | 1 | 6 | 2 | 1 | 7 |
| CHG | 4 | 1 | 5 | 4 | 2 | 4 |
| IPA | 5 | 1 | 4 | 6 | 0 | 4 |
| Pumice | 6 | 0 | 4 | 2 | 2 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haralur, S.B.; Alharthi, S.M.; Abohasel, S.A.; alqahtani, K.M. Effect of Decontamination Treatments on Micro-Shear Bond Strength between Blood–Saliva-Contaminated Post-Etched Dentin Substrate and Composite Resin. Healthcare 2019, 7, 128. https://doi.org/10.3390/healthcare7040128
Haralur SB, Alharthi SM, Abohasel SA, alqahtani KM. Effect of Decontamination Treatments on Micro-Shear Bond Strength between Blood–Saliva-Contaminated Post-Etched Dentin Substrate and Composite Resin. Healthcare. 2019; 7(4):128. https://doi.org/10.3390/healthcare7040128
Chicago/Turabian StyleHaralur, Satheesh B., Salem Mohammed Alharthi, Saeed Aied Abohasel, and Khalid Mohammed alqahtani. 2019. "Effect of Decontamination Treatments on Micro-Shear Bond Strength between Blood–Saliva-Contaminated Post-Etched Dentin Substrate and Composite Resin" Healthcare 7, no. 4: 128. https://doi.org/10.3390/healthcare7040128
APA StyleHaralur, S. B., Alharthi, S. M., Abohasel, S. A., & alqahtani, K. M. (2019). Effect of Decontamination Treatments on Micro-Shear Bond Strength between Blood–Saliva-Contaminated Post-Etched Dentin Substrate and Composite Resin. Healthcare, 7(4), 128. https://doi.org/10.3390/healthcare7040128





