Adverse Event Circumstances and the Case of Drug Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Datasets
2.2. Experiments
2.2.1. Preventable and Avoidable Errors
2.2.2. Individual Patient Molecular Models for Optimizing Therapeutic Use of Combinatorial Treatment Regiments
3. Results
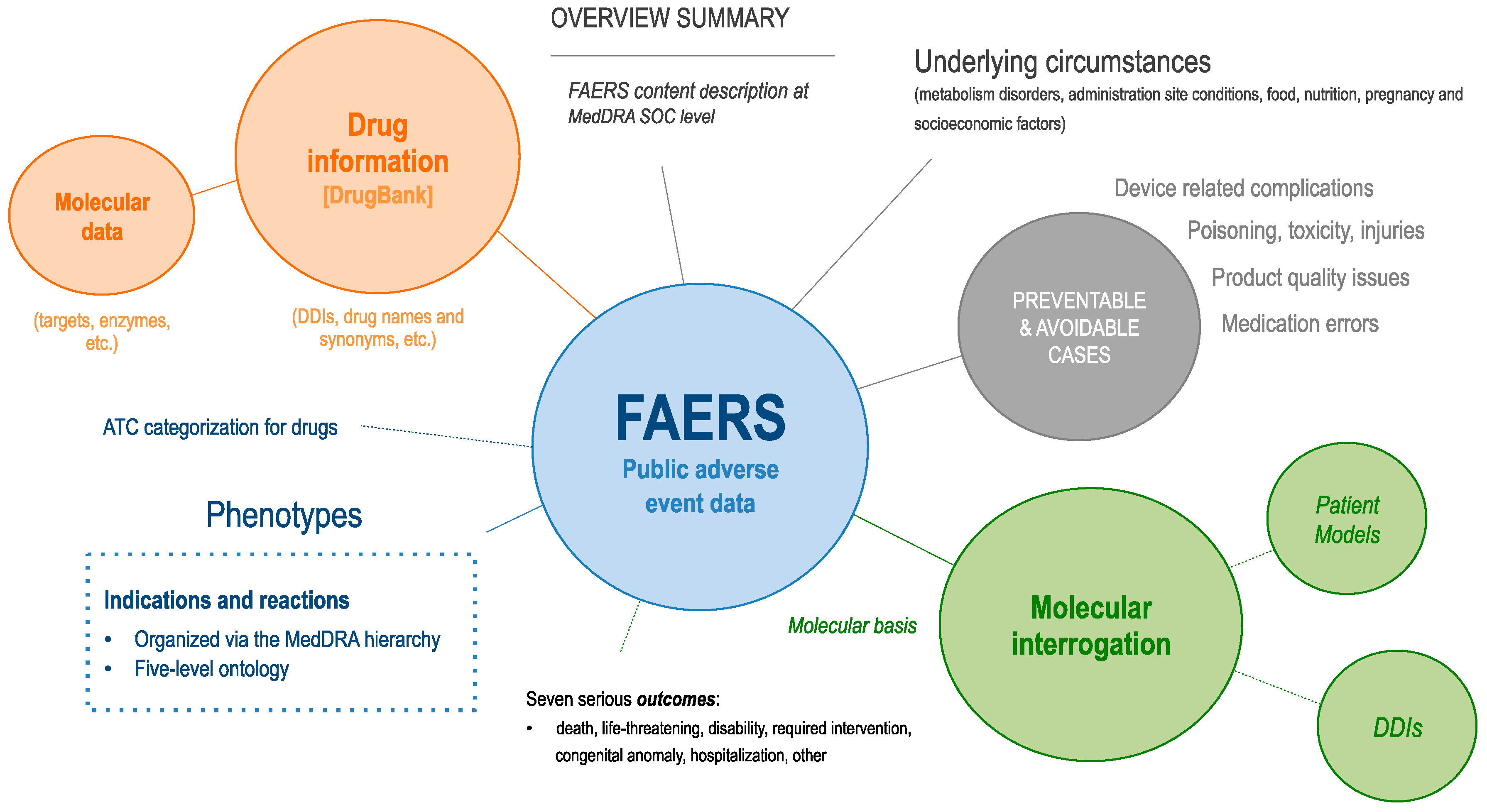
3.1. FAERS Overview
3.1.1. How May AEs Be Explained?
3.1.2. Preventable and Avoidable Errors
- Patient factors: 3.43% FAERS reported accidents, 2.62% FAERS reported off-label use or self-medication, 1.99% FAERS reported substance related disorders (drug abuse, alcoholism); 1.89% FAERS reported multiple allergic conditions and 1.25% allergies to foods, food additives, drugs, and other chemicals (e.g., drug hypersensitivity); 0.75% FAERS reported drug withdrawal and rebound effects; and 0.14% FAERS reported accidental exposures (e.g., intake by child, via breast milk).
- Iatrogenic, therapeutic, and procedural: 4.76% FAERS reported maladministration errors (wrong technique or drug, inappropriate site, incorrect duration or dose); 1.48% FAERS reported different types of overdoses (intentional, accidental, due to multiple drugs); 1.12% FAERS reported different forms of poisoning or toxicity; 1.03% FAERS reported procedural complications or iatrogenic injury; 0.98% reported interactions (drug-drug, alcohol); 0.87% FAERS reported medication errors (prescribing, dispensing, interception); and 0.05% FAERS reported product label issues (confusion, lot number, expiration date).
- Device and product issues: 1.91% FAERS reported product quality issues (storage, formulation); 0.88% FAERS reported device related complications (infection, misuse, accident); 0.59% FAERS reported device malfunctions (dislocation, occlusion, delivery system); 0.28% FAERS reported device component findings (breakage, failure, leakage); 0.14%; and 0.09% FAERS reported product physical and packaging issues (abnormal color, size, odor, solubility, container, quantity).
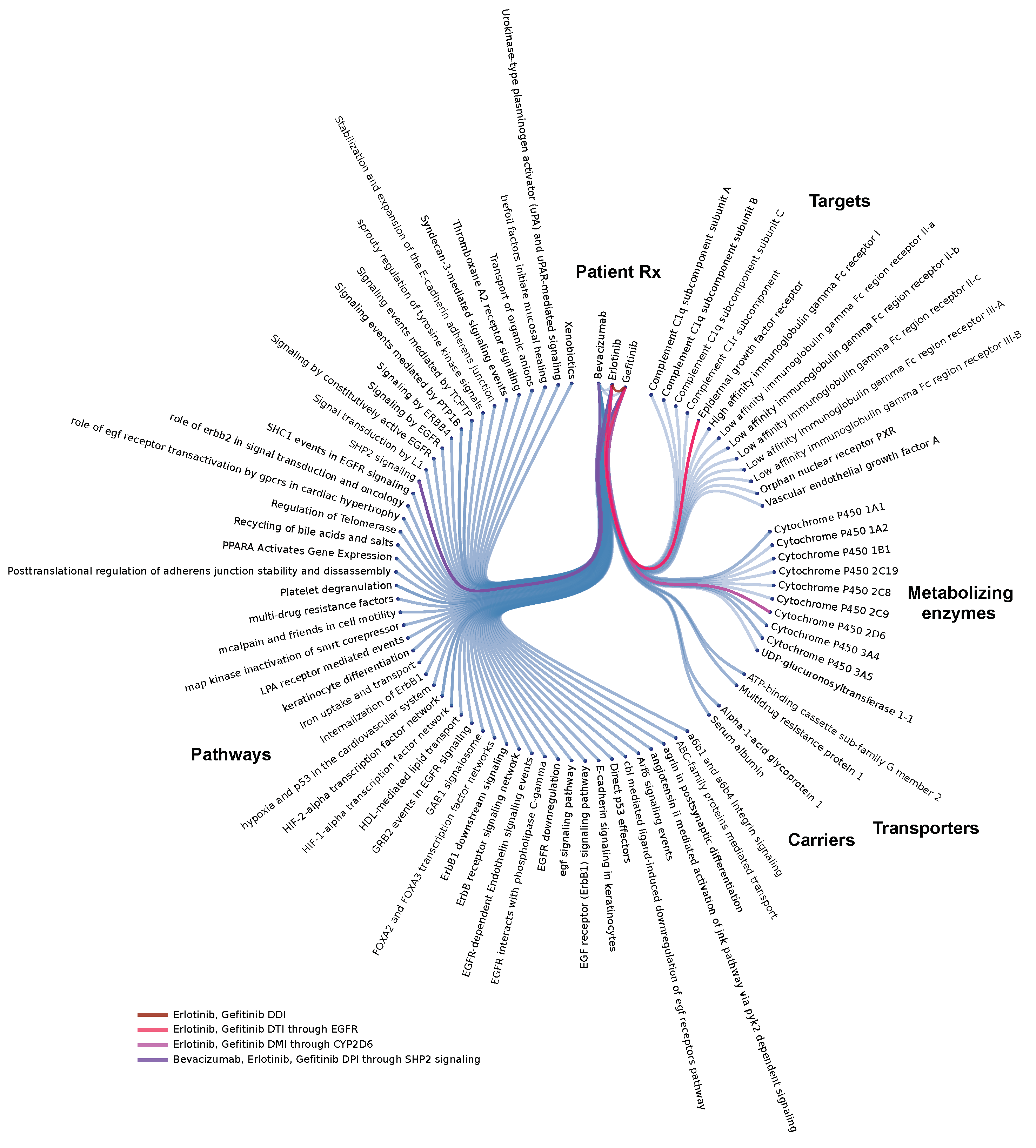
3.2. Molecular Level Interrogation at the Patient Level
3.2.1. Polypharmacy in Cancer
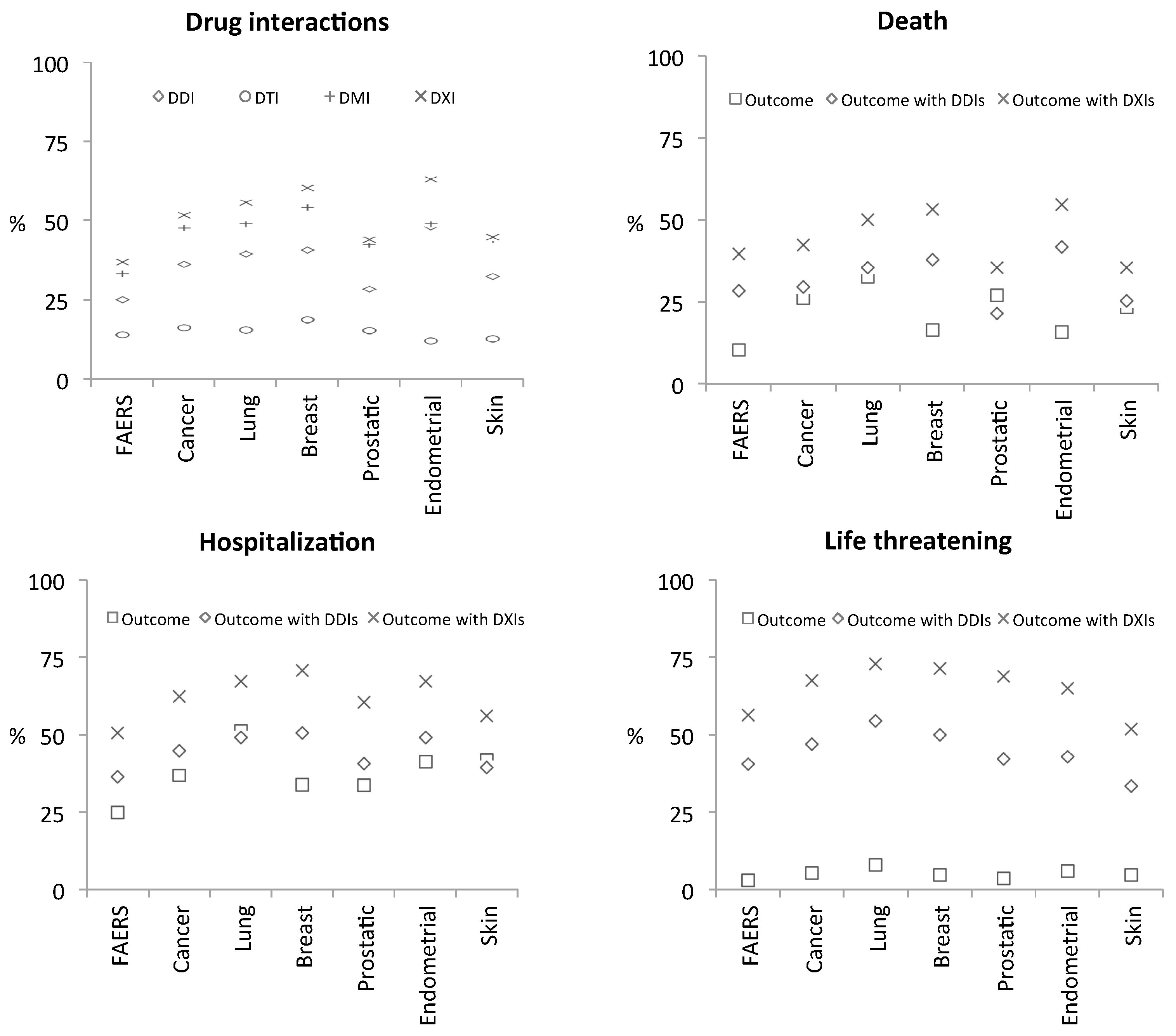
3.2.2. Drug interaction Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bates, D.W.; Spell, N.; Cullen, D.J.; Burdick, E.; Laird, N.; Petersen, L.A.; Small, S.D.; Sweitzer, B.J.; Leape, L.L. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997, 277, 307–311. [Google Scholar] [CrossRef]
- Classen, D.C.; Pestotnik, S.L.; Evans, R.S.; Lloyd, J.F.; Burke, J.P. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997, 277, 301–306. [Google Scholar] [CrossRef]
- Personalised Medicine | Health—Research and Innovation—European Commission. Available online: https://ec.europa.eu/research/health/index.cfm?pg=policy&policyname=personalised (accessed on 22 October 2018).
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of Adverse Drug Reactions in Europe: A Review of Recent Observational Studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.F.; Dembe, A.E.; Seiber, E.E.; Lu, B. Incidence of adverse events in an integrated US healthcare system: A retrospective observational study of 82,784 surgical hospitalizations. Patient Saf. Surg. 2014, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical error—The third leading cause of death in the US. BMJ 2016, 353, i2139. [Google Scholar] [CrossRef]
- Strandell, J.; Bate, A.; Lindquist, M.; Edwards, I.R.; Swedish, Finnish, Interaction X-Referencing Drug-Drug Interaction Database (SFINX Group). Drug-drug interactions—A preventable patient safety issue? Br. J. Clin. Pharmacol. 2008, 65, 144–146. [Google Scholar] [CrossRef]
- Makiani, M.J.; Nasiripour, S.; Hosseini, M.; Mahbubi, A. Drug-drug Interactions: The Importance of Medication Reconciliation. J. Res. Pharm. Pract. 2017, 6, 61–62. [Google Scholar]
- Assiri, G.A.; Shebl, N.A.; Mahmoud, M.A.; Aloudah, N.; Grant, E.; Aljadhey, H.; Sheikh, A. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open 2018, 8, e019101. [Google Scholar] [CrossRef]
- openFDA Datasets FAERS. Available online: https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/defa (accessed on 2 April 2018).
- Soldatos, T.G.; Taglang, G.; Jackson, D.B. In Silico Profiling of Clinical Phenotypes for Human Targets Using Adverse Event Data. High-Throughput 2018, 7, 37. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- MedDRA. Available online: http://meddra.org/ (accessed on 21 March 2017).
- Soldatos, T.G.; Perdigão, N.; Brown, N.P.; Sabir, K.S.; O’Donoghue, S.I. How to learn about gene function: Text-mining or ontologies? Methods 2015, 74, 3–15. [Google Scholar] [CrossRef] [PubMed]
- WHOCC—Structure and Principles. Available online: https://www.whocc.no/atc/structure_and_principles/ (accessed on 21 March 2017).
- Soldatou, V.; Soldatos, A.; Soldatos, T. Examining Socioeconomic and Computational Aspects of Vaccine Pharmacovigilance. BioMed Res. Int. 2019. [Google Scholar] [CrossRef]
- Racz, R.; Soldatos, T.G.; Jackson, D.; Burkhart, K. Association Between Serotonin Syndrome and Second-Generation Antipsychotics via Pharmacological Target-Adverse Event Analysis. Clin. Transl. Sci. 2018. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Dimitrakopoulou-Strauss, A.; Larribere, L.; Hassel, J.C.; Sachpekidis, C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics (Basel) 2018, 8, E76. [Google Scholar] [CrossRef] [PubMed]
- Smithburger, P.L.; Buckley, M.S.; Bejian, S.; Burenheide, K.; Kane-Gill, S.L. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin. Drug Saf. 2011, 10, 871–882. [Google Scholar] [CrossRef]
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; Maciejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef]
- Flockhart TableTM—Drug Interactions. Available online: https://drug-interactions.medicine.iu.edu/main-table.aspx (accessed on 22 October 2018).
- Hachad, H.; Ragueneau-Majlessi, I.; Levy, R.H. A useful tool for drug interaction evaluation: The University of Washington Metabolism and Transport Drug Interaction Database. Hum. Genom. 2010, 5, 61–72. [Google Scholar] [CrossRef]
- European Medicines Agency—Pharmacovigilance—EudraVigilance. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000679.jsp&mid=WC0b01ac05800250b5 (accessed on 2 April 2018).
- UMC | VigiBase. Available online: https://www.who-umc.org/vigibase/vigibase/ (accessed on 2 April 2018).
- Vilar, S.; Lorberbaum, T.; Hripcsak, G.; Tatonetti, N.P. Improving Detection of Arrhythmia Drug-Drug Interactions in Pharmacovigilance Data through the Implementation of Similarity-Based Modeling. PLoS ONE 2015, 10, e0129974. [Google Scholar] [CrossRef]
- Boland, M.R.; Jacunski, A.; Lorberbaum, T.; Romano, J.D.; Moskovitch, R.; Tatonetti, N.P. Systems biology approaches for identifying adverse drug reactions and elucidating their underlying biological mechanisms. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, T.; Nasir, M.; Keiser, M.J.; Vilar, S.; Hripcsak, G.; Tatonetti, N.P. Systems pharmacology augments drug safety surveillance. Clin. Pharmacol. Ther. 2015, 97, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Woodcock, J.; Lesko, L.J. Pharmacological mechanism-based drug safety assessment and prediction. Clin. Pharmacol. Ther. 2011, 89, 793–797. [Google Scholar] [CrossRef]
- Hoffman, K.B.; Dimbil, M.; Kyle, R.F.; Tatonetti, N.P.; Erdman, C.B.; Demakas, A.; Chen, D.; Overstreet, B.M. A Drug Safety Rating System Based on Postmarketing Costs Associated with Adverse Events and Patient Outcomes. J. Manag. Care Spec. Pharm. 2015, 21, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Banda, J.M.; Evans, L.; Vanguri, R.S.; Tatonetti, N.P.; Ryan, P.B.; Shah, N.H. A curated and standardized adverse drug event resource to accelerate drug safety research. Sci. Data 2016, 3, 160026. [Google Scholar] [CrossRef] [PubMed]
| MedDRA SOC Name | Indication AEs * | %Total AEs | Reaction AEs | %Total AEs |
|---|---|---|---|---|
| Blood and lymphatic system disorders | 350,726 | 5.16 | 382,653 | 5.63 |
| Cardiac disorders | 208,679 | 3.07 | 1,075,568 | 15.84 |
| Congenital, familial and genetic disorders | 56,330 | 0.83 | 50,759 | 0.75 |
| Ear and labyrinth disorders | 8,733 | 0.13 | 91,631 | 1.35 |
| Endocrine disorders | 448,743 | 6.61 | 218,981 | 3.22 |
| Eye disorders | 69,279 | 1.02 | 315,958 | 4.65 |
| Gastrointestinal disorders | 452,855 | 6.67 | 1,349,645 | 19.87 |
| General disorders and administration site conditions | 247,082 | 3.64 | 2,712,307 | 39.94 |
| Hepatobiliary disorders | 126,044 | 1.86 | 203,980 | 3.00 |
| Immune system disorders | 799,829 | 11.78 | 477,511 | 7.03 |
| Infections and infestations | 350,316 | 5.16 | 770,049 | 11.34 |
| Injury, poisoning and procedural complications | 58,190 | 0.86 | 1,439,977 | 21.2 |
| Investigations | 242,129 | 3.57 | 954,804 | 14.06 |
| Metabolism and nutrition disorders | 644,521 | 9.49 | 632,797 | 9.32 |
| Musculoskeletal and connective tissue disorders | 922,255 | 13.58 | 930,144 | 13.7 |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 671,890 | 9.89 | 348,083 | 5.13 |
| Nervous system disorders | 784,039 | 11.54 | 1,745,201 | 25.70 |
| Pregnancy, puerperium and perinatal conditions | 17,004 | 0.25 | 118,320 | 1.74 |
| Psychiatric disorders | 543,341 | 8.00 | 1,054,377 | 15.53 |
| Renal and urinary disorders | 179,233 | 2.64 | 433,585 | 6.38 |
| Reproductive system and breast disorders | 246,009 | 3.62 | 296,533 | 4.37 |
| Respiratory, thoracic and mediastinal disorders | 449,304 | 6.62 | 1,077,425 | 15.86 |
| Skin and subcutaneous tissue disorders | 375,556 | 5.53 | 994,511 | 14.64 |
| Social circumstances | 16,268 | 0.24 | 103,877 | 1.53 |
| Surgical and medical procedures | 968,612 | 14.26 | 325,664 | 4.80 |
| Vascular disorders | 500,778 | 7.37 | 1,410,722 | 20.77 |
| Key Utilities Enabled by the Molecular Dissection of Patient Clinical Data |
|---|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatos, T.G.; Jackson, D.B. Adverse Event Circumstances and the Case of Drug Interactions. Healthcare 2019, 7, 45. https://doi.org/10.3390/healthcare7010045
Soldatos TG, Jackson DB. Adverse Event Circumstances and the Case of Drug Interactions. Healthcare. 2019; 7(1):45. https://doi.org/10.3390/healthcare7010045
Chicago/Turabian StyleSoldatos, Theodoros G., and David B. Jackson. 2019. "Adverse Event Circumstances and the Case of Drug Interactions" Healthcare 7, no. 1: 45. https://doi.org/10.3390/healthcare7010045
APA StyleSoldatos, T. G., & Jackson, D. B. (2019). Adverse Event Circumstances and the Case of Drug Interactions. Healthcare, 7(1), 45. https://doi.org/10.3390/healthcare7010045





