Developing a Case-Based Blended Learning Ecosystem to Optimize Precision Medicine: Reducing Overdiagnosis and Overtreatment
Abstract
1. Introduction
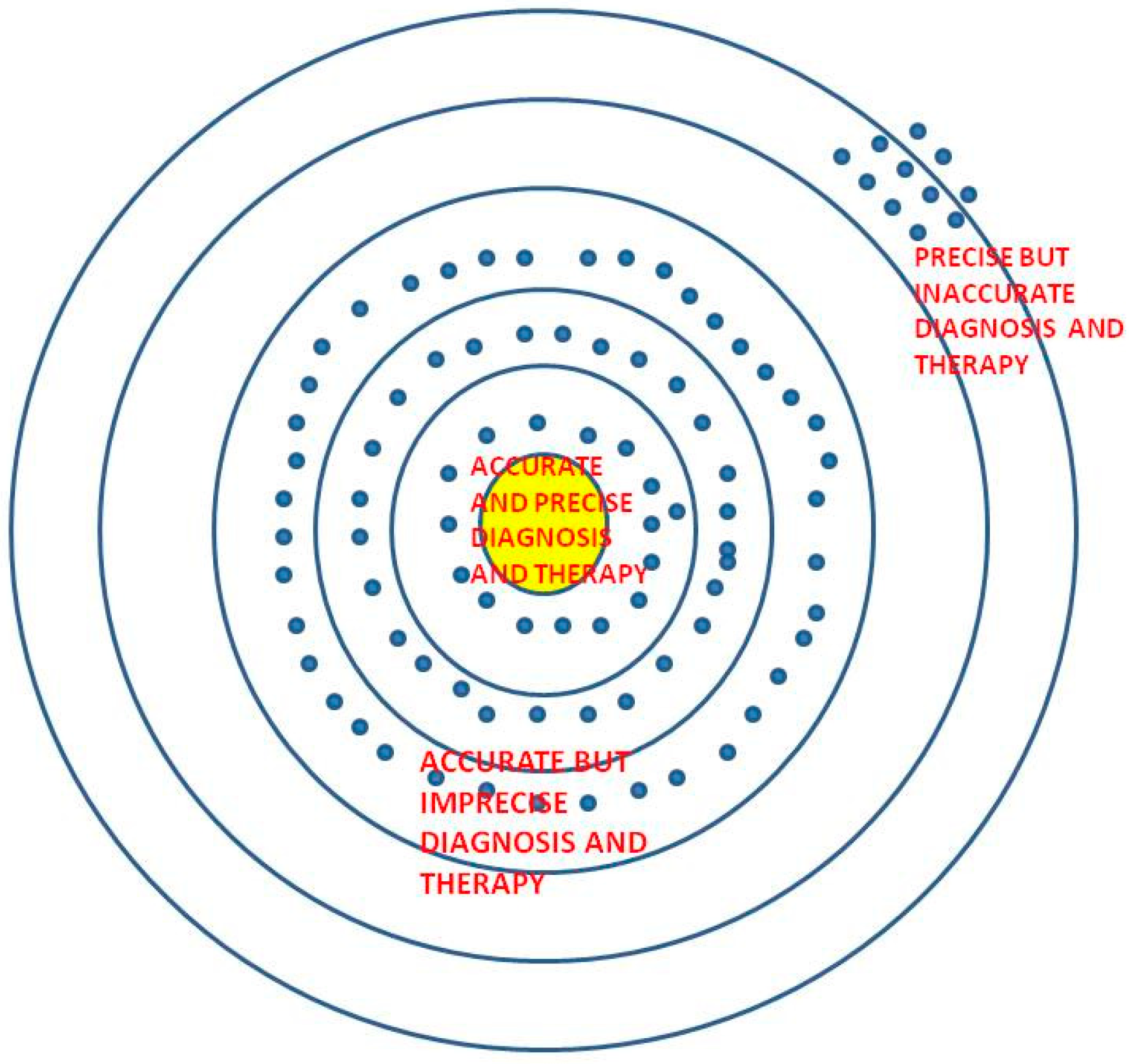
1.1. Precision versus Accuracy
“An elderly patient from a country endemic with tuberculosis presented with a chronic cough and weight loss. A lung pathology was detected on imaging that was not amenable to further biopsy efforts as a result of unavailable resources. He was started on empirical treatment for tuberculosis after sending a sputum for acid-fast bacillus (AFB) and culture.”
- (a)
- The elderly patient’s sputum comes out to be positive and once he is begun on antitubercular therapy, he recovers. His cough subsides and weight improves and his sputum culture report that comes after 6 weeks also turns out to be positive for tuberculosis. This is an example of precise and accurate diagnosis and treatment.
- (b)
- The elderly patient’s sputum turns out to be negative and yet once he is begun on antitubercular therapy, he recovers. His cough subsides and weight improves and his sputum culture report that comes after 6 weeks turns out to be positive for tuberculosis although his initial sputum smear was negative. This would be an example of initially imprecise, but finally accurate outcomes.
- (c)
- The elderly patient’s sputum turns out to be negative and a cartridge-based nucleic acid amplification assay test (CBNAAT) sent at the same time also turns out to be negative for tuberculosis and once he is begun on antitubercular therapy, he does not appear to recover at all. His cough worsens along with his appetite and his weight loss increases. A bronchoscopy with bronchoalveolar lavage is performed and sent again for malignant cytology, AFB, CBNAAT, and culture. His sputum culture report that comes after 6 weeks turns out to be positive for drug-resistant tuberculosis. Although, he receives second-line therapy for his drug-resistant tuberculosis, his condition worsens, and he dies.
- (d)
- The elderly patient’s sputum turns out to be negative for tuberculosis and no further tests are done due to lack of resources. He is put on empirical antitubercular therapy, but he does not appear to recover at all. His cough worsens along with his appetite and his weight loss increases. One day he has a sudden shortness of breath and dies. An autopsy reveals bronchogenic carcinoma and pulmonary embolism.
1.2. Case-based Blended Learning Ecosystem (CBBLE): Current Case Studies and Implications for Precision Medicine
2. Materials and Methods
3. Results
3.1. Precision Medicine through a Physician Resident’s Narrative Lens (in Relatively Low-Resource Settings)
After dinner on my night duty, I rushed to casualty as a patient was brought who needed immediate attention. On going to casualty, I saw that a 60-year-old lady was struggling to breathe and appeared tachypneic. Her % oxygen saturation was only 86% at room air. We had to provide her immediate oxygen support which improved her saturation but she was still tachypneic, and opening her mouth to take in the air, basically struggling to take the air in.
After making sure that her saturation was well maintaining I had called the attenders to take a detailed history. Her complaints were not of recent onset. She had complaints since the past 4 months. The lady had been having complaints of burning micturition since 4 months. She had been visiting the hospital often for the above complaints. This time, she had the same complaints of burning micturition, fever, abdominal pain and decreased urine output.
On asking further the attenders related that she hadn’t passed urine since 3–4 days. So I had immediately asked for a Foley’s catheterization. The moment we had inserted the Foley’s the urine was milky white for the initial few minutes. By this time we had got a few of their old prescriptions which showed a urology op card and the urologist made a diagnosis based upon the history of “thin stream of urine” as stricture urethra. Now everything was falling into place. Her persistent complaints from the past few months, her burning micturition, fever.
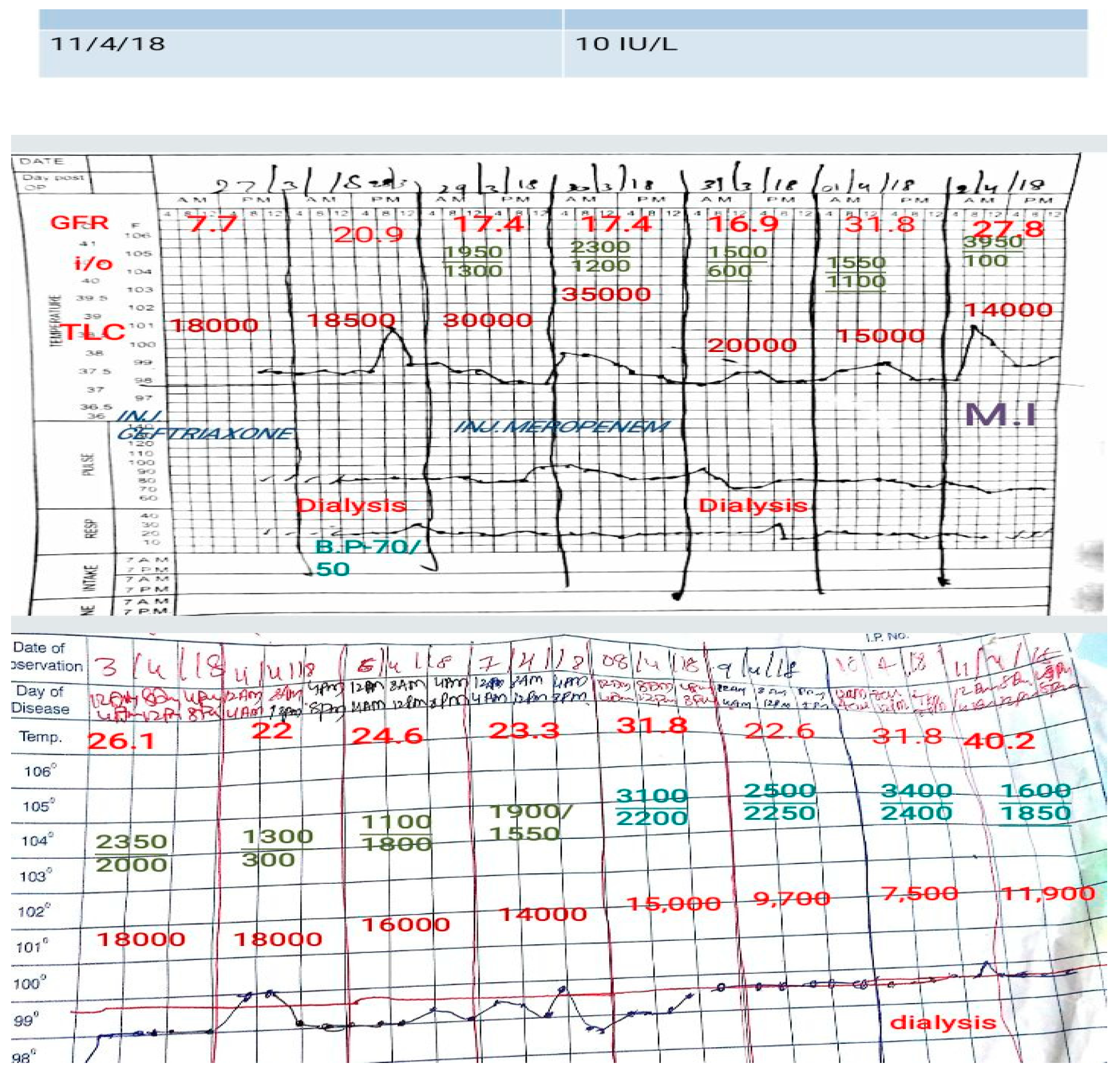
We got the necessary investigations done, firstly sent a blood and urine culture as I could sense that she may deteriorate. Sent immediately blood for an arterial blood gas (ABG) analysis which showed a metabolic acidosis thus ruling out any lung pathology leading to shortness of breath. She needed dialysis. Blood counts showed elevated leukocytes. Her urine routine microscopy showed plenty of pus cells. Her ultrasonogram (USG)of the abdomen revealed pyonephrosis with dilated ureters.
Had got her dialysis done the next day. In her Foley’s interestingly we noted some debris. We had a discussion around it as what it could be. But post dialysis though her acidosis resolved she went into hypotension. We had to start her on inotropic support. Had to monitor her blood pressure (BP) closely to tailor the dose accordingly. We were eagerly waiting for her culture reports as the leucocyte count showed an increasing trend. By this time we had got all her previous outpatient cards which not only revealed her past history and procedures done but were also a representation of the case-based workflow of multiple departments in our rural tertiary medical college hospital.
Her previous outpatient cards revealed that her first visit was in December last year. She had first gone to a gynecologist with complaints of burning micturition, whitish discharge per vaginum and fever. Probably the gynecologist had asked her to void and come as they wanted to examine her per vaginally. But this is where we lost track of the information in her outpatient card progress notes. She then landed up in urology OPD where her op card revealed that she had a thin stream of urine. The diagnosis of stricture urethra was made solely based on this and a dilatation procedure was done, she was catheterized with Foley’s and urine culture sensitivity was sent. She was started on antibiotic and asked to turn up after 3 days which she did. She got her Foley’s removed and was prescribed the 1st line antibiotic for urinary tract infection (UTI). The urine culture report was, unseen by the care provider, and unasked by the patients. She was prescribed the same 1st line antibiotic as in the first visit. She was then asymptomatic for the next 2 months. When she developed her current symptoms she turned up in the casualty this time.
The organism was the same since the first report, but sadly the culture reports being unseen she was getting the antibiotic for which the organism was never sensitive. The organism had been evolving since then into a more virulent one.
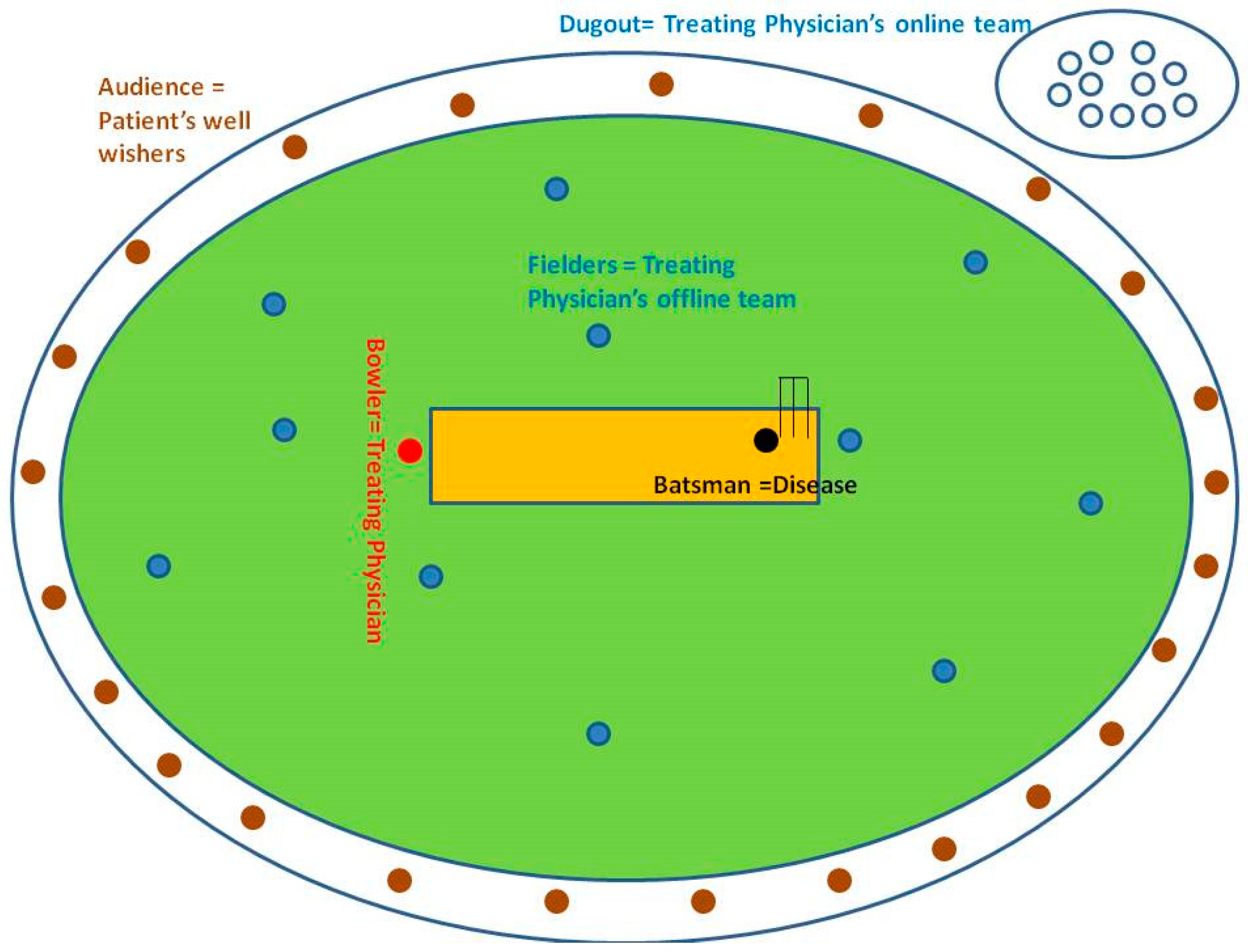
3.1.1. Analogy-Driven Analysis: Retrospective Notes from the Dugout
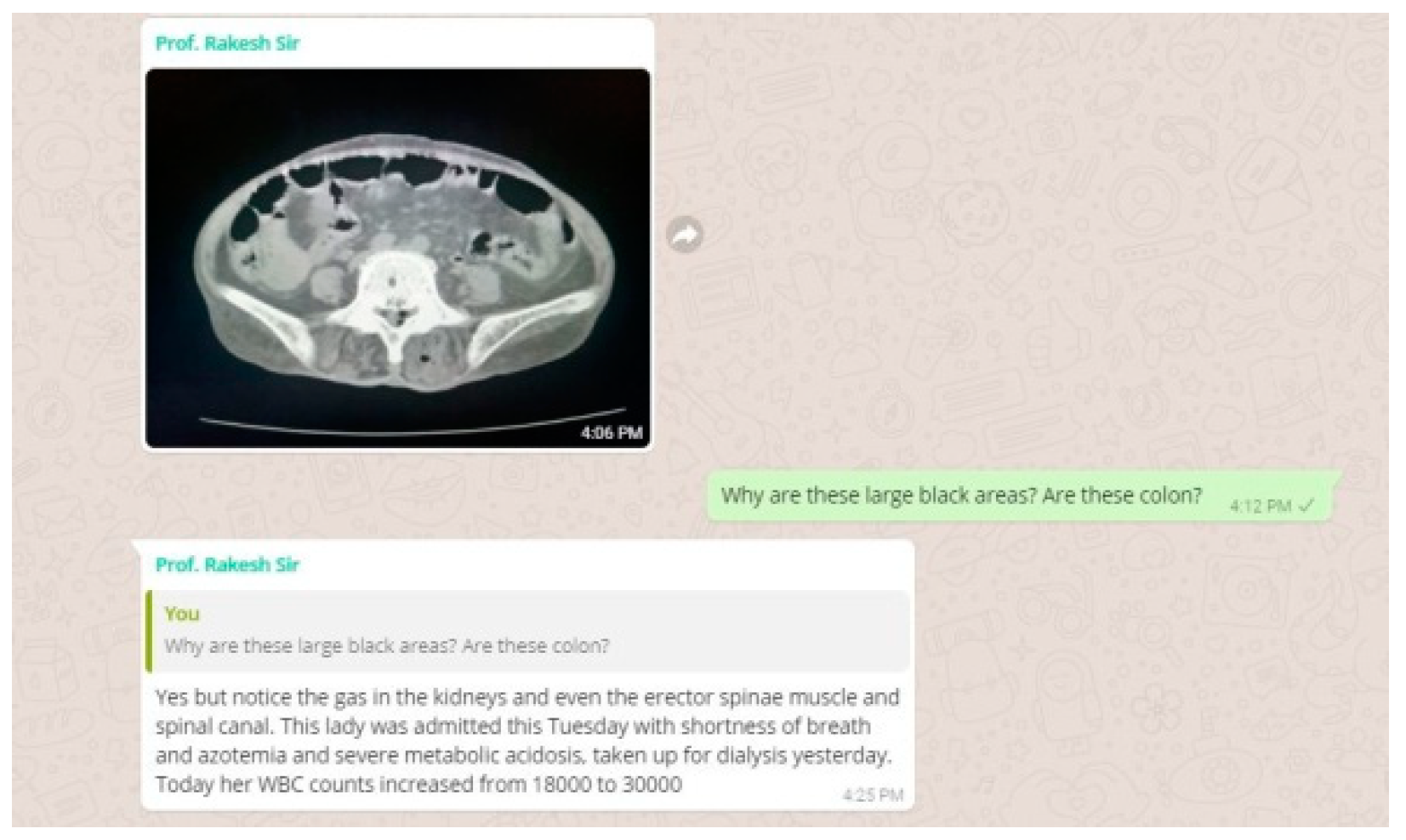
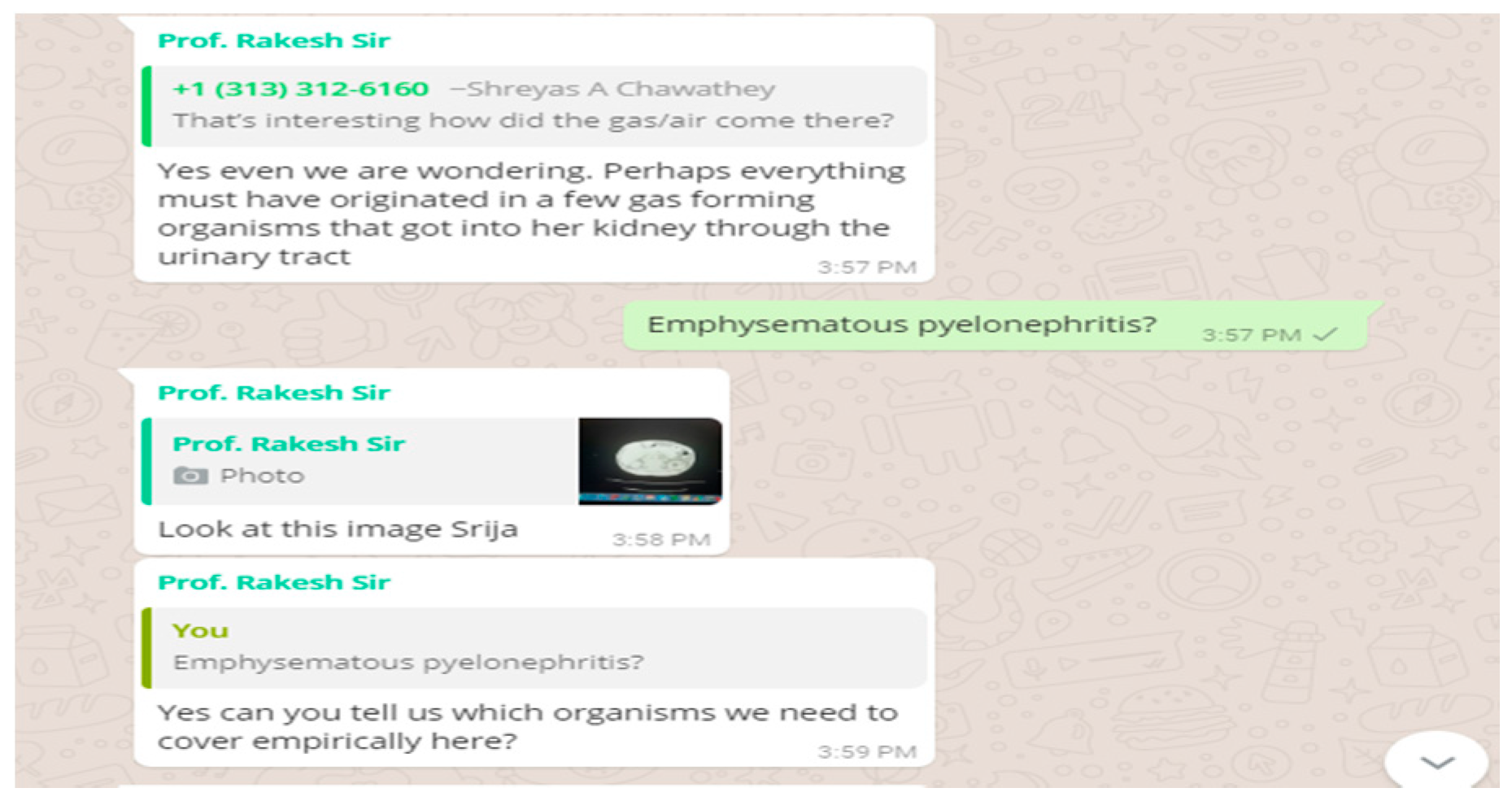
We got a CT abdomen done for her as the USG abdomen revealed pyonephrosis. We had something very unusual and unexpected in store for us in the CT. She had air pockets in the kidney, in the erector spinae muscle and spinal canal. We decided to change the antibiotic. We were still waiting for the culture report and had started her on a higher antibiotic. But to our dismay, our microbiology department didn’t have the sensitivity checking disc for the antibiotic which we had started her on.
3.1.2. Online Work flow in Parallel with the Offline Component of Our CBBLE
3.2. Precision Medicine through a Neurologist’s Narrative Lens (in Relatively High-Resource Settings)
A 29-year-old male, presented to our Neurology Clinic with his first known episode of generalized tonic-clonic seizures. He denies drug or alcohol usage and his growth and developmental history was normal. Prior to this hospital visit, he had not attended any medical consultation. He was not on any medications for any other ailment. His neurological examination was normal. He was then subjected to an electroencephalography (EEG) and magnetic resonance (MR)imaging of the brain. EEG was normal. However, MR imaging (with contrast) revealed a single ring-enhancing lesion in the left temporal lobe with a visible scolex. A diagnosis of Neurocysticercosis was made. The patient was prescribed Albendazole and steroids for the acute problem and then prescribed T. Levetiracetam 500 mg twice a day for the seizures.
Two weeks into treatment he was brought to the emergency room (ER) in an unresponsive state. His parents found him unresponsive and noticed that he had inadvertently passed urine in his clothes. On examination, his vitals were stable. He had a gaze preference to the left, with paucity of movements on the right side. The possibility of an unwitnessed seizure, Todd’s palsy, and postictal confusion were suspected. A repeat MR imaging revealed acute infarct in the left middle cerebral artery (MCA) and left anterior cerebral artery (ACA) territories. MR angiogram revealed a left carotid occlusion. His older MR images were reviewed and showed no evidence of carotid or intracranial vascular disease. He was transferred to the angiography suite and a diagnostic cerebral angiogram was performed. Digital subtraction angiography (DSA) revealed a left carotid T occlusion. A mechanical thrombectomy was successfully performed. However, there was also evidence of left MCA occlusion. Mechanical thrombectomy was repeated, with partial recanalization. Repeat brain imaging revealed significant. Left hemispheric infarct with evidence of evolving cerebral edema. A decompressive craniectomy was done 3 days after the surgery, repeat imaging revealed persistent cerebral edema and hence he was referred for re-exploration. He underwent left frontal and temporal lobectomy. The patient is slowly recovering with occupational and rehabilitative therapies.
A young stroke workup was considered. Routine blood investigations were normal. Autoimmune markers including antinuclear antibody (ANA) and antineutrophil cytoplasmic antibodies (ANCA) were normal. He had normal homocysteine levels and a normal echocardiogram. Holter monitoring was normal. We screened him for genetic thrombophilic conditions. He was found to have a homozygous MTHFR C677T gene mutation. MTHFR mutation has been previously linked to ischemic strokes.
Two hit hypothesis is well discussed in the field of oncology where a reactive genetic mutation can be triggered by an epigenetic event such as an infection. However, if we are to apply the same principle in this case scenario, our patient had a ‘first hit’ with a homozygous MTHFR C677T mutation. The ‘second hit’ may have been the neurocysticercosis infection, followed by the interventions or the resultant systemic distress and functional recovery.
Analogy-Driven Analysis: Retrospective Notes from the Dugout
3.3. Precision Medicine (in Relatively Low-Resource Settings) through the Lens of a Medical Student’s Online Interaction with Our CBBLE
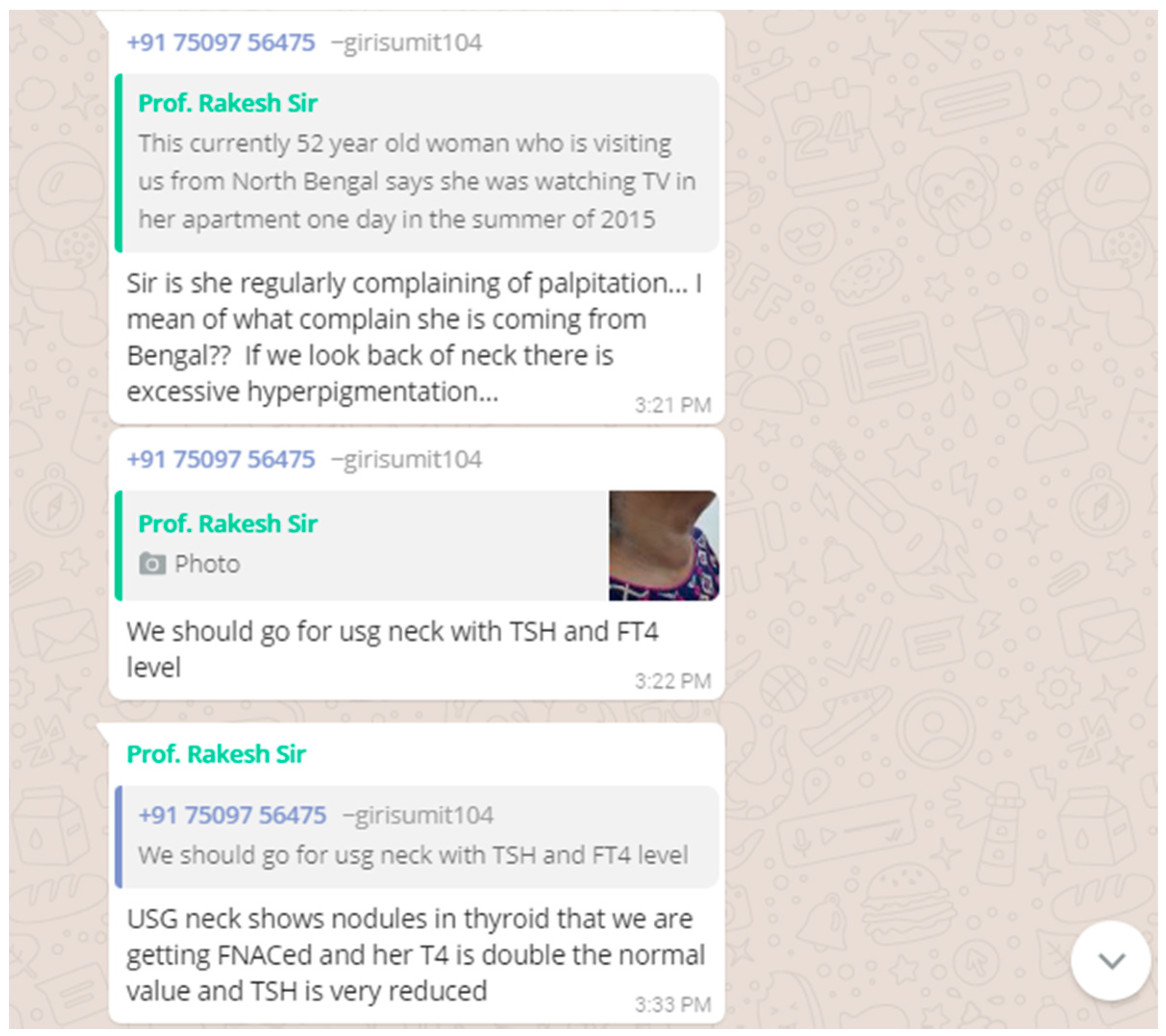
A 52 year old woman, who was visiting us from North Bengal said she was watching TV in her apartment one day in the summer of 2015 and suddenly noticed that the TV was swinging followed by swinging walls and even the stairs of her apartment when she quickly started running down to safety and amidst all this commotion she noticed a buzz of people shouting earthquake! Earthquake!! She remembers that is the first time she had experienced severe palpitations since then which she complains of repeatedly experiencing over the years. However, she didn’t notice the swelling in front of her neck that we noticed. We didn’t notice any protruding eyes or tremors etc. USG neck showed nodules in the thyroid that was sent for fine-needle aspiration cytology (FNAC) and her T4 was double the normal value and TSH was very low.
Toxic adenoma is a toxic thyroid nodule which produces excessive thyroid hormones. They are managed either by prolonged thionamide therapy, surgery or radioiodine ablation. In this non-diabetic patient, a non-velvety, hyperpigmented area involving skin folds was noticed at the back of the neck that was very atypical of acanthosis nigricans. In view of thyroid nodule and hyperpigmentation in the neck, paraneoplastic acanthosis nigricans was brought into consideration. Later, FNAC report was available which showed features of benign thyroid lesion with foci of mild atypia.
3.3.1. After the Discussion, the Following Questions about the Patient Were Raised
3.3.2. If FNAC Is Positive, We Would Know What to Do Next, But What Are the Chances of False Negativity after FNAC?
This made us look up specificity and sensitivity of FNAC in detecting thyroid malignancy. A study showed FNAC is more specific (98%) than sensitive (80%) in detecting malignancy [23]. Here if FNAC is more specific than sensitive so what should we do if the FNAC is negative? There is still 20% chance of its being malignant.
3.3.3. What Is the Frequency of Having to Obtain Excision/Incision Biopsies in Such Situations?
The decision to go for excision biopsy primarily for histopathological examination (HPE) to rule out malignancy is often a judgement call. In this context from a precision medicine perspective would a liquid biopsy be feasible or help? A feasibility study was conducted by detecting BRAF(V600E) circulating tumour DNA (ctDNA) in the plasma of patients with thyroid nodules to distinguish between benign and malignant nodules. Results showed that BRAF(V600E) ctDNA could distinguish between the two [24].
3.3.4. Based on FNAC Findings, How Would You Decide between Surgery and Medical Management in This Patient?
The problem with both radioiodine and surgery is that the patient would become hypothyroid eventually and end up in a lifelong dose of maintenance thyroxine. The problem with carbimazole is that we may not be sure of the response.
3.3.5. Analogy-Driven Analysis: Retrospective Notes from the Dugout
3.4. Precision Medicine through a Hematoncologists Narrative Lens (in Relatively High-Resource Settings)
In a frigid Wisconsin morning last December, I received a phone call from my friend, whose brother was in the battle for his life against Multiple Myeloma. This patient’s disease had progressed after multiple lines of chemotherapy including two stem cell transplants in the span of 4 years. My friend was desperate for a ray of hope to help his brother. He had exhausted all available options, and enrollment in a clinical trial was the only potential solution for his disease. As a myeloma focussed physician, I was aware of a clinical trial that was exploring an experimental agent for the subtype of myeloma he had; myeloma with chromosomal translocation 11 and 14; t(11:14). With ample caution, I routed them towards relevant myeloma clinical trials in their area. Fast forward a few months, my friend called me again, but this time excitement was palpable through the phone. His brother was enrolled in a clinical trial using bcl 2-inhibitor (venetoclax)—a drug active against a particular subtype of multiple myeloma with t(11:14) [25]. This result, a remission, was shared by others and this would open a new era of precision medicine in the therapeutic armamentarium of multiple myeloma.
Analogy-Driven Analysis: Retrospective Notes from the Dugout
4. Discussion
4.1. Current Narratives in Precision Oncogenomics
4.2. Age-Old Precision Medicine
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christensen, C.M.; Grossman, J.H.; Hwang, J. The innovator’s prescription. In Soundview Executive Book Summaries; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Institute for Precision Medicine. Available online: http://ipm.weill.cornell.edu/about/definition(2015) (accessed on 14 May 2018).
- Thomas, B.; UST Global Health Group. The New Healthcare Ecosystem: 5 Emerging Relationships. Available online: https://www.beckershospitalreview.com/hospital-management-administration/the-new-healthcare-ecosystem-5-emerging-relationships.html (accessed on 28 January 2018).
- Peter, A. How Does Accuracy Differ from Precision in Scientific Measurements? Socratic Questions. Available online: https://socratic.org/questions/how-does-accuracy-differ-from-precision-in-scientific-measurements (accessed on 1 March 2018).
- Nagashree, B.; Manchukonda, R.S. Prescription audit for evaluation of present prescribing trends in a rural tertiary care hospital in South India: An observational study. Int. J. Basic Clin. Pharmacol. 2016, 5, 2094–2097. [Google Scholar]
- Krishna, P. Medical education. Health Millions 1992, 18, 42–44. [Google Scholar]
- Shankar, P.R.; Chandrasekhar, T.S.; Mishra, P.; Subish, P. Initiating and strengthening medical student research: Time to take up the gauntlet. Kathmandu Univ. Med. J. 2006, 4, 135–138. [Google Scholar]
- Behnke, L.M.; Solis, A.; Shulman, S.A.; Skoufalos, A. A targeted approach to reducing overutilization: Use of percutaneous coronary intervention in stable coronary artery disease. Popul. Health Manag. 2013, 16, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, S.; Chalkidou, K.; Doust, J.; Elshaug, A.G.; Glasziou, P.; Heath, I.; Nagpal, S.; Saini, V.; Srivastava, D.; Chalmers, K.; et al. Evidence for overuse of medical services around the world. Lancet 2017, 390, 156–168. [Google Scholar] [CrossRef]
- Patil, D.; Lanjewar, C.; Vaggar, G.; Bhargava, J.; Sabnis, G.; Pahwa, J.; Phatarpekar, A.; Shah, H.; Kerkar, P. Appropriateness of elective percutaneous coronary intervention and impact of government health insurance scheme—A tertiary centre experience from Western India. Indian Heart J. 2017, 69, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Podder, V. A Large Force of Health System—The Medical Students: Have They Been Utilized Adequately? Available online: https://blogs.bmj.com/case-reports/2017/12/12/a-large-force-of-health-system-the-medical-students-have-they-been-utilized-adequately/ (accessed on 27 January 2018).
- Price, A.; Chandra, S.; Bera, K.; Biswas, T.; Chatterjee, P.; Wittenberg, R.; Mehta, N.; Biswas, R. Understanding clinical complexity through conversational learning in medical social networks: Implementing user-driven health care. In Handbook of Systems and Complexity in Health; Springer: New York, NY, USA, 2013; pp. 767–793. [Google Scholar]
- Biswas, R.; Sturmberg, J.P.; Martin, C.M. The User Driven Learning Environment. In User-Driven Healthcare and Narrative Medicine: Utilizing Collaborative Social Networks and Technologies; IGI Global: Hershey, PA, USA, 2011; pp. 229–241. [Google Scholar]
- Purkayastha, S.; Price, A.; Biswas, R.; Ganesh, A.J.; Otero, P. From Dyadic Ties to Information Infrastructures: Care-Coordination between Patients, Providers, Students and Researchers: Contribution of the Health Informatics Education Working Group. Yearb. Med. Inform. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R. Clinical audit and lifelong reflective practice as game changers to integrate medical education and practice. J. Fam. Med. Prim. Care 2015, 4, 476. [Google Scholar] [CrossRef] [PubMed]
- Price, A.I.; Djulbegovic, B.; Biswas, R.; Chatterjee, P. Evidence-based medicine meets person-centred care: A collaborative perspective on the relationship. J. Eval. Clin. Pract. 2015, 21, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Chatterjee, P.; Biswas, R. Comparative effectiveness research collaboration and precision medicine. Ann. Neurosci. 2015, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Tamrakar, N.; Price, A.; Biswas, R. Medical Students Meet User Driven Health Care for Patient Centered Learning in Clinical Medicine. Int. J. User Driven Healthc. (IJUDH) 2014, 4, 7–17. [Google Scholar] [CrossRef]
- Price, A.; Biswas, T.; Biswas, R. Person-centered healthcare in the information age: Experiences from a user driven healthcare network. Eur. J. Pers. Cent. Healthc. 2013, 1, 385–393. [Google Scholar] [CrossRef]
- Case Based Blended Learning Ecosystem Narratives. Available online: http://cbblenarratives.blogspot.com/2018/05/cbble-narratives.html (accessed on 30 March 2018).
- Raz, R. Urinary tract infection in postmenopausal women. Korean J. Urol. 2011, 52, 801–808. [Google Scholar] [CrossRef] [PubMed]
- BMJ Case Report Student Electives. Available online: https://promotions.bmj.com/jnl/bmj-case-reports-student-electives-2/ (accessed on 30 March 2018).
- Basharat, R.; Bukhari, M.H.; Saeed, S.; Hamid, T. Comparison of fine needle aspiration cytology and thyroid scan in solitary thyroid nodule. Pathol. Res. Int. 2011, 2011, 754041. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B. Detection of Circulating Thyroid Tumor DNA in Patients with Thyroid Nodules; Electronic Thesis and Dissertation Repository: London, ON, Canada, 2015. [Google Scholar]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t (11; 14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J. Uncertainty in the era of precision medicine. N. Engl.J. Med. 2016, 375, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Saußele, S.; Krauß, M.-P.; Hehlmann, R.; Lauseker, M.; Proetel, U.; Kalmanti, L.; Hanfstein, B.; Fabarius, A.; Kraemer, D.; Berdel, W.E.; et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: Results of the randomized CML Study IV. Blood 2015, 126, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Han, G.; Schalper, K.A.; Carvajal-Hausdorf, D.; Pelekanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.; LoRusso, P.; Rimm, D.L. Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Falzon, M.; Blackhall, F.; Spicer, J.; Nicolson, M.; Chaudhuri, A.; Middleton, G.; Ahmed, S.; Hicks, J.; Crosse, B.; et al. Randomized prospective biomarker trial of ERCC1 for comparing platinum and nonplatinum therapy in advanced non-small-cell lung cancer: ERCC1 trial (ET). J. Clin. Oncol. 2016, 35, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.-J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N.; et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Menon, S.P.; Dy, G.K. Alterations in genes other than EGFR/ALK/ROS1 in non-small cell lung cancer: Trials and treatment options. Cancer Biol. Med. 2016, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Do, K.; O’Sullivan Coyne, G.; Chen, A.P. An overview of the NCI precision medicine trials—NCI MATCH and MPACT. Chin. Clin. Oncol. 2015, 4. [Google Scholar] [CrossRef]
- Bhatt, J.R.; Klotz, L. Overtreatment in Cancer–Is It a Problem? Taylor &Francis: Didcot, UK, 2016. [Google Scholar]
- Klotz, L. Cancer overdiagnosis and overtreatment. Curr. Opin. Urol. 2012, 22, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Esserman, L.J.; Thompson, I.M.; Reid, B.; Nelson, P.; Ransohoff, D.F.; Welch, H.G.; Hwang, S.; Berry, D.A.; Kinzler, K.W.; Black, W.C.; et al. Addressing overdiagnosis and overtreatment in cancer: A prescription for change. Lancet Oncol. 2014, 15, e234–e242. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R. User Driven Health Care. Available online: http://userdrivenhealthcare.blogspot.com/2016/08/role-of-patient-information.html?m=0 (accessed on 27 January 2018).
- Andritsos, P.; Jurisica, I.; Glasgow, J.I. Case-Based Reasoning for Biomedical Informatics and Medicine. In Springer Handbook of Bio-/Neuroinformatics; Springer: New York, NY, USA, 2014; pp. 207–221. [Google Scholar]
- Pantazi, S.V.; Arocha, J.F.; Moehr, J.R. Case-based medical informatics. BMC Med. Inform. Decis. Mak. 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.C.; Weisner, T.S.; Subramanian, S.; Duan, N.; Niedzinski, E.J.; Kravitz, R.L. Harnessing experience: Exploring the gap between evidence-based medicine and clinical practice. J. Eval. Clin. Pract. 2008, 14, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Dussart, C.; Pommier, P.; Siranyan, V.; Grelaud, G.; Dussart, S. Optimizing clinical practice with case-based reasoning approach. J. Eval. Clin. Prac. 2008, 14, 718–720. [Google Scholar] [CrossRef] [PubMed]
- BMJ Elective Case Log. Available online: http://bmjcaselogvivek.blogspot.com/ (accessed on 30 May 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podder, V.; Dhakal, B.; Shaik, G.U.S.; Sundar, K.; Sivapuram, M.S.; Chattu, V.K.; Biswas, R. Developing a Case-Based Blended Learning Ecosystem to Optimize Precision Medicine: Reducing Overdiagnosis and Overtreatment. Healthcare 2018, 6, 78. https://doi.org/10.3390/healthcare6030078
Podder V, Dhakal B, Shaik GUS, Sundar K, Sivapuram MS, Chattu VK, Biswas R. Developing a Case-Based Blended Learning Ecosystem to Optimize Precision Medicine: Reducing Overdiagnosis and Overtreatment. Healthcare. 2018; 6(3):78. https://doi.org/10.3390/healthcare6030078
Chicago/Turabian StylePodder, Vivek, Binod Dhakal, Gousia Ummae Salma Shaik, Kaushik Sundar, Madhava Sai Sivapuram, Vijay Kumar Chattu, and Rakesh Biswas. 2018. "Developing a Case-Based Blended Learning Ecosystem to Optimize Precision Medicine: Reducing Overdiagnosis and Overtreatment" Healthcare 6, no. 3: 78. https://doi.org/10.3390/healthcare6030078
APA StylePodder, V., Dhakal, B., Shaik, G. U. S., Sundar, K., Sivapuram, M. S., Chattu, V. K., & Biswas, R. (2018). Developing a Case-Based Blended Learning Ecosystem to Optimize Precision Medicine: Reducing Overdiagnosis and Overtreatment. Healthcare, 6(3), 78. https://doi.org/10.3390/healthcare6030078





