The Incidence of Contrast-Induced Nephropathy Among Low-Risk Cancer Patients with Preserved Renal Function on Active Treatment Undergoing Contrast-Enhanced Computed Tomography: A Single-Site Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Data Collection and Management
2.3. Ethical Considerations
2.4. Statistical Analysis
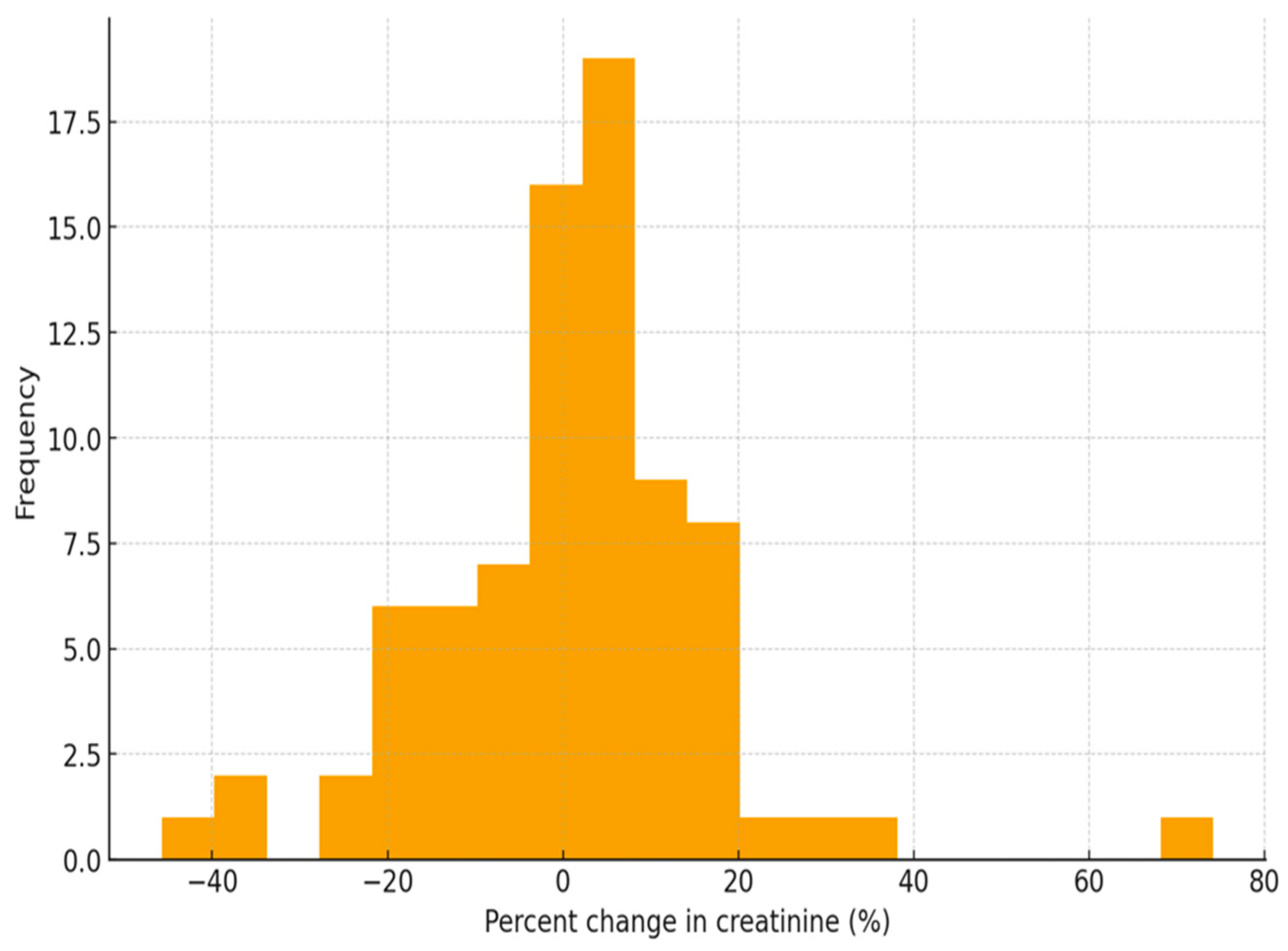
3. Results
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehran, R.; Nikolsky, E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int. 2006, 69, S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Shams, E.; Mayrovitz, H.N. Contrast-induced nephropathy: A review of mechanisms and risks. Cureus 2021, 13, e14842. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaperumal, Y.; Sivadasan, S.; Aiyalu, R. Contrast-induced nephropathy: An overview. Dr. Sulaiman Al Habib Med. J. 2023, 5, 118–127. [Google Scholar] [CrossRef]
- Inamdar, A.; Shinde, R.K. The diagnostic impact of contrast-enhanced computed tomography (CECT) in evaluating lymph node involvement in colorectal cancer: A comprehensive review. Cureus 2024, 16, e61832. [Google Scholar] [CrossRef] [PubMed]
- Sonhaye, L.; Kolou, B.; Tchaou, M.; Amadou, A.; Assih, K.; N’Timon, B.; Adambounou, K.; Agoda-Koussema, L.; Adjenou, K.; N’Dakena, K. Intravenous contrast medium administration for computed tomography scan in emergency: A possible cause of Contrast-Induced nephropathy. Radiol. Res. Pract. 2015, 2015, 805786. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.; Chaisson, K.; Horton, S.; Petrin, C.; Marshall, E.; Bowden, S.; Scott, L.; Conley, S.; Stender, J.; Kent, G.; et al. Reducing acute kidney injury due to contrast material: How nurses can improve patient safety. Crit. Care Nurse 2017, 37, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Tumlin, J.; Bapat, B.; Zyczynski, T. Economic burden of contrast-induced nephropathy: Implications for prevention strategies. J. Med. Econ. 2007, 10, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chen, P.; Wang, K.; Li, H.; Chen, S.; Liu, J.; He, Y.; Song, F.; Liu, Y.; Chen, J.Y. Contrast-Induced nephropathy and Long-Term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology 2018, 70, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ministry of National Guard Health Affairs. King Abdulaziz Medical City in Jeddah. n.d. Available online: https://mngha.med.sa/english/MedicalCities/Jeddah/Pages/default.aspx (accessed on 15 October 2025).
- Gray, J.; Grove, S. Burns and Grove’s the Practice of Nursing Research: Appraisal, Synthesis, and Generation of Evidence; Elsevier: St. Louis, MO, USA, 2021; ISBN -13: 978-0323673174. [Google Scholar]
- Moriya, H.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Yamano, M.; Suzuki, H.; Ohtake, T.; Hidaka, S.; Kobayashi, S. Impact of contrast-induced nephropathy on long-term renal function after coronary angiography and contrast-enhanced computed tomography. Cardiol. Cardiovasc. Med. 2022, 6, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Stata. Statistical Software for Data Science. Available online: https://www.stata.com/ (accessed on 15 October 2025).
- Hong, S.I.; Ahn, S.; Lee, Y.S.; Kim, W.Y.; Lim, K.S.; Lee, J.H.; Lee, J.L. Contrast-induced nephropathy in patients with active cancer undergoing contrast-enhanced computed tomography. Support. Care Cancer 2015, 24, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Mosaddegh, R.; Mohammadi, F.; Valipour, A.M.; Hosseini, S.M.; Naghshbandi, M.; Yarahmadi, M.; Faradonbeh, N.A. The incidence of Contrast-Induced nephropathy following computed tomography and associated risk factors. Radiol. Res. Pract. 2025, 2025, 7484380. [Google Scholar] [CrossRef] [PubMed]
- Salagierski, M.; Barwiński, F. Evidence-based medicine and the misconception of contrast-induced kidney disease. Cent. Eur. J. Urol. 2022, 75, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, S.; Yoo, H.; Kim, K.; Kim, Y.; Park, S.; Jang, H.R.; Kim, D.K.; Huh, W.; Kim, Y.G.; et al. Risk prediction for contrast-induced nephropathy in cancer patients undergoing computed tomography under preventive measures. J. Oncol. 2019, 2019, 8736163. [Google Scholar] [CrossRef] [PubMed]
- Obed, M.; Gabriel, M.M.; Dumann, E.; Barbosa, C.V.; Weißenborn, K.; Schmidt, B.M.W. Risk of acute kidney injury after contrast-enhanced computerized tomography: A systematic review and meta-analysis of 21 propensity score–matched cohort studies. Eur. Radiol. 2022, 32, 8432–8442. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Hsieh, C.C.; Chang, T.T.; Li, C.Y. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am. J. Roentgenol. 2019, 213, 728–735. [Google Scholar] [CrossRef] [PubMed]


| Demographic Variables | Total | % |
|---|---|---|
| Age | ||
| 18–40 | 15 | 18.75% |
| 41–60 | 31 | 38.75% |
| 61–100 | 34 | 42.50% |
| Gender | ||
| Female | 33 | 41.25% |
| Male | 47 | 58.75% |
| eGFR level | ||
| Normal (≥60 mL/min) | 64 | 80.00% |
| Abnormal (<60 mL/min) | 16 | 20.00% |
| Type of visit | ||
| Outpatient (OP) | 37 | 46.25% |
| Inpatient (IP) | 43 | 53.75% |
| Diagnosis | ||
| Breast cancer | 19 | 23.75% |
| Colon cancer | 9 | 11.25% |
| Lung cancer | 9 | 11.25% |
| Esophageal cancer | 4 | 5.00% |
| Pancreatic cancer | 4 | 5.00% |
| Ewing sarcoma | 3 | 3.75% |
| Lymphoma | 3 | 3.75% |
| Rectal cancer | 3 | 3.75% |
| Renal cell carcinoma | 3 | 3.75% |
| Neoplasm of the liver | 2 | 2.50% |
| Neoplasm of the rectum | 2 | 2.50% |
| Prostate cancer | 2 | 2.50% |
| Sarcoma | 2 | 2.50% |
| Anal cancer | 1 | 1.25% |
| Bladder cancer | 1 | 1.25% |
| Brain tumor | 1 | 1.25% |
| Duodenal cancer | 1 | 1.25% |
| Gallbladder adenoacanthoma | 1 | 1.25% |
| Gastric cancer | 1 | 1.25% |
| Hodgkin lymphoma | 1 | 1.25% |
| Nasopharynx neoplasm | 1 | 1.25% |
| Neoplasm of the endometrium | 1 | 1.25% |
| Neuroendocrine carcinoma | 1 | 1.25% |
| Osteosarcoma | 1 | 1.25% |
| Skin cancer | 1 | 1.25% |
| Small bowel cancer | 1 | 1.25% |
| Stomach cancer | 1 | 1.25% |
| Testis cancer | 1 | 1.25% |
| Predictor | Coefficient | p-Value |
|---|---|---|
| Age | +0.05% per year | 0.41 |
| Male sex | 1.80% | 0.29 |
| Baseline creatinine | −0.03% per µmol/L | 0.18 |
| Abnormal GFR (vs. normal) | 4.70% | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subahi, A.; Alhazmi, N.; Lardi, M.; Alkathiri, F.; Bokhari, L.; Alqahtani, S.; Abourokba, N.; Alshamrani, K. The Incidence of Contrast-Induced Nephropathy Among Low-Risk Cancer Patients with Preserved Renal Function on Active Treatment Undergoing Contrast-Enhanced Computed Tomography: A Single-Site Experience. Healthcare 2026, 14, 115. https://doi.org/10.3390/healthcare14010115
Subahi A, Alhazmi N, Lardi M, Alkathiri F, Bokhari L, Alqahtani S, Abourokba N, Alshamrani K. The Incidence of Contrast-Induced Nephropathy Among Low-Risk Cancer Patients with Preserved Renal Function on Active Treatment Undergoing Contrast-Enhanced Computed Tomography: A Single-Site Experience. Healthcare. 2026; 14(1):115. https://doi.org/10.3390/healthcare14010115
Chicago/Turabian StyleSubahi, Ahmad, Nada Alhazmi, Maryam Lardi, Fatimah Alkathiri, Layan Bokhari, Sultanah Alqahtani, Nesreen Abourokba, and Khalid Alshamrani. 2026. "The Incidence of Contrast-Induced Nephropathy Among Low-Risk Cancer Patients with Preserved Renal Function on Active Treatment Undergoing Contrast-Enhanced Computed Tomography: A Single-Site Experience" Healthcare 14, no. 1: 115. https://doi.org/10.3390/healthcare14010115
APA StyleSubahi, A., Alhazmi, N., Lardi, M., Alkathiri, F., Bokhari, L., Alqahtani, S., Abourokba, N., & Alshamrani, K. (2026). The Incidence of Contrast-Induced Nephropathy Among Low-Risk Cancer Patients with Preserved Renal Function on Active Treatment Undergoing Contrast-Enhanced Computed Tomography: A Single-Site Experience. Healthcare, 14(1), 115. https://doi.org/10.3390/healthcare14010115






